Abstract
Understanding the impact of future climates on crop performance is essential for sustainable agricultural production. In the current research, the development and biological behavior of soybean plants during gradual desiccation of the soil (from the 100% of pot water holding capacity to the gs of plant decreased to 10% of that of the control plants) at ambient [CO2] (a[CO2], 400 ppm) and elevated [CO2] (e[CO2], 800 ppm) were investigated. The results showed that plants grown under e[CO2] conditions had remarkably higher photosynthetic rate (An) but lower stomatal conductance (gs) and transpiration rate (E) compared to plants at a[CO2] conditions, which led to an enhanced water use efficiency at both stomatal (WUEi) and leaf levels (WUEleaf). In addition, the e[CO2]-grown soybeans showed a stunted gs response to progressive soil drying, coinciding with a decrease in the susceptibility of gs to the ABA signaling, though they tended to maintain a better leaf water status under drought than the a[CO2]-grown plants. Although the leaf nitrogen concentration (Nleaf) and the total plant N content were notably lower at the e[CO2] condition, the specific leaf N content (SLN) was similar at different [CO2] conditions. Compared to soybean grown under e[CO2], the greater number of nodules at e[CO2] treatment would lead to an enhanced N-fixation, yet, it did not improve the N nutrition of the plants. Nevertheless, by sustaining the SLN, the soybean plants enhanced An when growing at e[CO2], particularly under dry conditions. This knowledge is essential for sustaining soybean production in future climate change scenarios.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The average atmosphere CO2 concentration has gone up from 280 ppm to 410 ppm over the past 250 years (Tausz-Posch et al. 2020). IPCC (2014) predicted atmospheric [CO2] might exceed 700 ppm towards the late 21st generation. In the future climate, rising ambient [CO2], temperatures as well as changing precipitation patterns are predicted to have a farreaching impact on agricultural production (Shao et al. 2015). Plant growth and progression are directly influenced by elevated [CO2] (e[CO2]) (Wei et al. 2020). Likewise, drought stress can affect plant water relations (Bencze et al. 2014), cell membrane integrity (Hessini et al. 2009), photosynthesis and other physiological processes (Liu et al. 2018; Zheng et al. 2020). Therefore, a deeper knowledge of the integrated effect of plants to e[CO2] environment combined with drought conditions could help to maximize crop yield in future climate scenarios.
Elevated [CO2] often has a beneficial impact on plant development (Dong et al. 2020). Previous research shows that e[CO2] increases the net photosynthetic rate (An) while reducing the stomatal conductance (gs) (Ainsworth and Rogers 2007; Hu et al. 2022). The decrease in gs curtails the transpiration rates (E), while the high An results in more photo-assimilates and biomass accumulating (Wei et al. 2020), thus improving water use efficiency (WUE) of plants (Tausz-Posch et al. 2015; Yan et al. 2017). Therefore, it is essential to understand how e[CO2] affects stomatal behavior and leaf gas exchange. In tomato plants, the decrease of gs under e[CO2] was closely related to the endogenous ABA level (Fang et al. 2019). Ethylene compensates for induced stomatal closure during gradual desiccation of the soil (Liang et al. 2023). Hsu et al. (2018) discovered that e[CO2] triggered stomatal closure by regulating OST1/SnRK2 kinases. On the other hand, hydraulic signals also modulate plants’ gs response to e[CO2]. Reduced gs and E may help to maintain a higher leaf water potential (Ψl) at e[CO2] (Wullschleger et al. 2010). Bunce (1996) found that although gs and E of plants at e[CO2] were lower, hydraulic conductance could also be reduced, and therefore Ψl was not always higher. Some reports have indicated that e[CO2] decreases hydraulic conductance by down-regulating aquaporin expression (Fang et al. 2019), while others suggest that e[CO2] may enhance hydraulic conductance (Li et al. 2004).
Water deficit reduces gs hereby increase stomatal limitation to CO2 diffusion (Bousba et al. 2009). Research has demonstrated that e[CO2] maintains a high WUE mainly by enhancing An while reducing gs and E, thereby mitigating the negative impacts of aridity (Tausz-Posch et al. 2015; Wall et al. 2001). Plant water use efficiency increases under the interaction of elevated CO2 and drought stress (Silveira et al. 2023). Contrastingly, other studies have also shown that the effect of drought on gs is more profound than that of e[CO2]. Leakey et al. (2006) conducted an experiment investigating the interaction impacts of drought and e[CO2], reporting that decreased gs caused by drought tended to be greater than that caused by e[CO2]. Li et al. (2013) pointed out that e[CO2] did not alleviate the suppressive effect of drought on soybean seed yield, although it increased An and WUE.
Soybeans are the 4th major agricultural crop worldwide and an important economic crop in China (Ainsworth et al. 2012). Shaanxi province belongs to Loess Plateau spring soybean production area. In the last few years, owing to the changing weather conditions, the temperature on the Loess Plateau is on the rise, precipitation is gradually decreasing, freshwater resources for irrigation are limited (Li et al. 2011), and droughts are probably persist in the coming climate. Wang et al. (2018) considered that e[CO2] enhances soybean stress resistance mainly by improving their photosynthetic capacity and water use efficiency. This mitigating effect was also seen in peanut, tomato and wheat crops (Laza et al. 2021, Li et al. 2019, Yang et al. 2020). Rhizomes in soybean roots are able to fix atmospheric nitrogen into ammonia for plant uptake (Minguillon et al. 2022). Due to its ability of biological nitrogen fixation, soybean responds to e[CO2] and drought may be differently from other C3 plants. Rhizobia could consume 4–11% of the carbohydrates produced by photosynthesis (Kaschuk et al. 2009, Soba et al. 2021). On the one side, drought can limit photosynthesis and reduce carbohydrate availability in legume rhizomes, thereby limiting N2 fixation (Parvin et al. 2019). On the other side, e[CO2] may promote the growth in soybeans under drought by providing more photo assimilates for nodule growth and hence N fixation. It is well understood that the impact of e[CO2] on plant performance is very much dependent on the N availability, and e[CO2] grown environment often leads to a reduced N concentration in most plant species, restricting the CO2-fertilization effect on plants (Aranjueloa et al. 2014; Gojon et al. 2023), particularly under stress environment. While this is true in most of the C3 species, it could be different for legumes such as soybean due to its ability to fix N. Therefore, it is important to research the interaction of e[CO2] and aridity stimuli in soybean growth and physiology as such knowledge is essential for the future production of soybeans in a sustainable climate.
This research aims to explore the impacts of e[CO2] on leaf water relations and gas exchange, and plant N nutrition of soybean subjected to gradual desiccation of the soil. It was hypothesized that, soybean plants subjected to the fertilization effect of elevated CO2 would have increased rhizomatous nitrogen fixation, which might have altered the plant’s N status and mitigated the adverse effects of e[CO2] on leaf N concentrations. Furthermore biological nitrogen fixation in soybean may cause plants under e[CO2] to alter their response to drought.
Materials and methods
Experimental material and site
The study was conducted from 22 March to 28 May 2021 in two [CO2] controlled growth chambers with a[CO2] (400 ppm) and e[CO2] (800 ppm) [CO2], respectively, at the Northwest A&F University, Yangling, Shaanxi province. Each of the growth chambers had an area of 3.8 m × 2.6 m. Carbon dioxide concentration in the chambers was measured every 6 s with a CO2 Transmitter Series GMT220 (Vaisala Group, Helsinki, Finland). Both chambers were maintained at 60% relative humidity and 25/18 ± 2 °C day/night temperature (T), the photosynthetic photon flux density was controlled at about 500 µmol m−2 s−1 from 7:00 to 19:00 h. S1 (in the supplementary figures) shows the average of daily [CO2] and T in the chambers during this study.
Soybean seeds (variety “Shanning 17”) were sterilized and sown in 6 L pots with 6.5 kg of air dried clay loam soil per pot. Five seeds were sown in each pots. One week after emergence, thinning was done and plants with similar size (one plant per pot) were kept. In addition, 10 ml soybean rhizobia solution (strain: “HH103”) was inoculated in each pot when the first true leaf appeared. The soil had a pH of 8.2, total C and N content of 12.5 and 0.35 g kg−1, total P and K content of 0.84 and 18.56 g kg−1. To ensure adequate nutrient supply, additional 0.5 g N, 0.8 g P and 0.8 g K were added to each pot. Additionally, 1 cm layer of perlite was covered on the surface of soil before the start of drought treatment to reduce evaporation.
Treatments
All plants were supplied with sufficient water (i.e. 90% pot water-holding capacity) for their growth until 15 May, 2021. Pot water-holding capacity was determined according to Liu et al. (2019). In each growth chamber, four plants were randomly selected as controls and maintained with adequate water supply, other plants were subjected to progressive drought by stopping irrigation. The endpoint of the progressive drought was determined when the stomatal conductance (gs) of the drought treatment decrease to 10% of the gs of the well-watered treatment based on the daily measurement of the leaf gas exchange rates after onset of the drought treatment (see "Leaf gas exchange" Section). Harvests of the drought-treated plants were taken every other day during the progressive soil drying (five times in total with four replicates each).
Measurements
Soil water status
During the progressive drying treatment, the pots were weighed at 9:00 am every day to calculate the daily water consumption. The fraction of transpirable soil water (FTSW) is a measure of soil moisture status in the pot. It was calculated as:
where TTSW is total transpirable soil water, which is obtained using the pot weight at 100% WHC (i.e., 8.45 kg) minus the weight at final harvest (i.e., 6.85 kg). WTn and WTf (i.e., 6.85 kg) are the pot weight of the drought stressed pots at a given date and at final the harvest, respectively. S2 (in the supplementary figures) shows the changes of FTSW under the two [CO2] growth conditions during the experimental period.
Leaf gas exchange
An, gs and E were measured using a portable photosynthetic system (LiCor-6800, LI-Cor, NE, USA) every day at 10:00–12:00 h during the progressive drying treatment (i.e., from 16 May to 28 May). Measurements were made by placing fully expanded upper canopy leaves in leaf chambers (four replicates per treatment) with a leaf chamber temperature of 25 °C, a photon flux density of 1400 µmol m−2 s−1, CO2 concentrations of 400 and 800 ppm, respectively. Based on the above data, the intrinsic water use efficiency (WUEi) was calculated as An/gs, the instantaneous water use efficiency (WUEleaf) was calculated as An/E.
Leaf water relation and ABA concentration
Gas exchange parameters were measured at the beginning of each destructive sampling, then the leaf was removed and frozen in liquid nitrogen, then kept in a refrigerator at − 80 °C for later analysis of abscisic acid (ABA). The method of ABA determination was as follows: Grinding of fresh leaves in liquid nitrogen, 40 mg of the sample was admitted into a 1.5 ml Eppendorf tube. And 1.0 ml milli-Q water was admitted into the test tube and shaken overnight at 4 °C for the extraction of ABA, extracts were then centrifuged for 5 min. After centrifugation, 0.7 ml supernatants were taken as the test solution and [ABA]leaf was determined by the ELISA method (Enzyme Linked Immuno Sorbent Assay). ABA-ELISA kits (The cross-reactivity of anti-ABA McAb with ABA analogs is < 3.5%) were purchased from China Agricultural University.
Before the final harvest, gas exchange parameters were measured, followed by leaf water potential (Ψl, MPa) and RWC and ABA measurements on leaves selected at the same leaf position as those used for photosynthesis measurements. The Ψl was determined by pressure chamber at noon (Soil Moisture Equipment, Santa Barbara, CA, USA), the relative water content (RWC) of the leaves were calculated following the protocol of Liu et al. (2019). In detail, one leaflet was excised from the plant and its fresh weight (FW) was determined immediately. Then, the leaflet was put on distilled water with the cutting ending submerged in the water at 20 °C for 2 h under dim illumination to avoid respiratory losses. After blotting the turgid weight (TW) was determined. Dry weight (DW) was measured after drying the leaflet to constant weight at 70 °C. Then the RWC was calculated as:
Plant morphological and physiological traits
Since the evaporation from the soil was negligible, the sum of the change in soil moisture and the amount of irrigation during the experiment were used to calculate the water consumption of the potted plants (WU, cm3). At each sampling time, the plant samples were divided into leaves, stems, and roots, then they were dried at 70 °C to constant weight (about 48 h). Plant water use efficiency (WUEp, kg m−3) was calculated as:
Where the biomass refers to the total biomass produced by a plant during the experimental treatment, which was obtained by subtracting the dry mass at the beginning from the dry mass at the end of the processing. Ep refers to the total amount of water transpired by the plant in the same period, which was WU in this study.
The dried plant samples were ground into fine powder in a mortar and pestle. To-tal carbon (C) and total nitrogen (N) in the samples (%) were determined by elemental analyzer (Vario Max CN; Elemnetar Corp., Germany) and the amount of N per plant (Plant N content, g plant-1) is determined by multiplying the plant N concentration by the dry weight. Leaf N concentration ([N]leaf, g kg−1) converted from proportional N (%). The specific leaf nitrogen content (SLN, g N m−2) was calculated as the ratio of leaf N concentration to the specific leaf area (SLA, cm2 g−1) at different growth stages.
The leaf area (LA, cm2) of plants was determined by leaf area meter (LICOR 3100). The specific leaf area (SLA, cm2 g−1) was calculated as the ratio of LA to leaf dry mass. The nitrogen balance index (NBI) was determined by Plant polyphenol chlorophyll optical leaf clip meter (DUALEX SCIENTIFIC TM).
Statistical analysis
The responses of gs, An, E, WUEi and WUEleaf to gradual desiccation of the soil were characterized by a linear-plateau model (Wei et al. 2020):
where y represents gs, An, E, WUEi or WUEleaf, respectively; yinitial means the initial values of gs, An, E, WUEi and WUEleaf, respectively, at which the plants were unaffected by water deficit, c is the FTSW threshold at which y started to diverge from yinitial for gs, An, E, WUEi and WUEleaf. The parameters yinitial, a and c were determined by PROC NLIN fitting using PC SAS 9.4 software (SAS Institute Inc., Cary, NC, USA, 2002–2012). T test was performed for each parameter determined by linear-plateau regression between two [CO2] conditions using Med Calc statistical software 19.0.7, and P value was obtained.
All the figures and PCA analysis were done by Origin 2022 software (OriginLab Corp., USA). All data were analyzed by SPSS 22 software (two-way ANOVA) (IBM Corp., 2021), the mean values of all treatments were compared using Duncan’s multiple test (P = 0.05). The quantitative relationships between gs and [ABA]leaf / Ψl were analyzed by linear regressions.
Results
Effects of CO2 and soil water deficits on leaf gas exchange
Under the condition of sufficient water supply, An of soybean leaves at e[CO2] condition increased 85.7% remarkably compared to that at a[CO2] (Fig. 1a; Table 1). With the decrease of soil moisture, the FTSW decreased continuously, the An of plants at e[CO2] started to decrease when the FTSW was 0.43, while the An at a[CO2] declined when the FTSW was 0.42. Compared with a[CO2], An of e[CO2] decreased more sharply when FTSW < 0.42. At the initial stage of soil desiccation, leaf stomatal conductance was 38.4% inferior at e[CO2] than that at a[CO2] (P < 0.05, Fig. 1b; Table 1). With the decrease in FTSW, gs of soybean leaves at e[CO2] tended to decline later than plants at e[CO2] (i.e., FTSW = 0.44 vs. 0.48, not significant). Likewise, the initial transpiration rate (E) of leaves at a[CO2] was 46.6% higher than those at e[CO2] (P < 0.05, Fig. 1c; Table 1). As FTSW further decreased, E of the plants grown at e[CO2] tended to decline later than those at a[CO2] (i.e., FTSW = 0.44 vs. 0.48, not significant).
Changes of net photosynthetic rate (An ) (a), stomatal conductance (gs) (b), and transpiration rate (E) (c) intrinsic water use efficiency (WUEi, An/gs) (d) and leaf water use efficiency (WUEleaf , An/Tr) (e) of soybean leaves grown under ambient (400 ppm) and elevated (800 ppm) atmospheric CO2 concentrations during progressive soil drying. Fraction of transpirable soil water (FTSW) indicates the degree of dryness of the soil (n = 4)
Effects of CO2 and water stress on plant water relations
Aridity treatment had remarkable effect on leaf RWC and Ψl (Table 2). Under a[CO2], the RWC of drought-stressed soybean leaves was 32.2% lower than that of well-watered leaves. Under e[CO2], the RWC of the drought-stressed treatment was 23.6% lower than that of well-watered treatment. Compared to a[CO2]-treated plants, RWC in e[CO2]-treated plants decreased less pronouncedly by drought stress in relation to the well-watered controls, yet there were no obvious correlations between the CO2 and water treatment. The level of CO2 in the growth environment had no remarkable impact on plant water relation characteristics (Table 2). Plant water use (WU) under drought was significantly less than that under well-watered condition. WU of plants at e[CO2] was notably less than that at a[CO2] (P = 0.02).
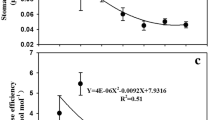
Under well-watered condition, the ABA concentration of soybean leaf ([ABA]leaf) was slightly higher at e[CO2] than that at a[CO2] (Fig. 2a). As the FTSW gradually decreases, the [ABA]leaf increased exponentially under both CO2 concentrations, and at a same FTSW level the [ABA]leaf at e[CO2] was slightly greater than that at a[CO2] (P > 0.05) (Table 2; Fig. 2a). When FTSW > 0.3, the gs of soybean leaves grown under the two different CO2 concentrations dropped in a linear fashion as [ABA]leaf increased, and the output of analysis of co-variance (ANCOVA) showed that the slope of the regression line was less steep at e[CO2] than at a[CO2] (Fig. 2b).
Changes in ABA concentration (a) and the relationship between leaf ABA concentration and stomatal conductance (gs) of soybean plants (b) grown under a[CO2] and e[CO2] during progressive soil drying. Fraction of transpirable soil water (FTSW) indicates the degree of dryness of the soil. Error bars indicate standard error of the means (SE) (n = 3) ∗Indicates the regression lines was statistically significantly at P < 0.05 level
At sufficient soil moisture conditions, the initial WUEi (An/gs) of soybean leaves at e[CO2] was about 2.54-fold greater than those at a[CO2] (Fig. 1d), and the initial WUEleaf (An/E) of soybean leaves at e[CO2] was 2.55 times greater than that under a[CO2] (Fig. 1e). With the gradual decline of FTSW, WUEi and WUEleaf remained the initial value at first, and when FTSW decreased to 0.30–0.45, they began to increase linearly. There was no significant difference between the FTSW threshold (the time when WUE began to increase) of plants grown under the two different CO2 concentrations. Compared with the soybean plants grown at a[CO2] condition, WUEp of plants at e[CO2] was significantly improved (P < 0.05, Table 2).
Effects of CO2 and drought on plant biomass and nutrition
Soybean leaf dry matter (LDM) was mainly influenced by both [CO2] and water treatments. In arid condition, the LDM of the e[CO2] plants was 66.0% higher than that of the a[CO2] plants, while under well-watered condition, the LDM of the e[CO2] plants was 31.6% higher than that of the a[CO2] (Table 2). Compared with plants grown un-der drought stress, the leaf area (LA) of plants under well-watered condition was 41.4% and 48.1% greater at a[CO2] and e[CO2], respectively (Table 2). The specific leaf area (SLA) was notably influenced by CO2 growth environment (Fig. 3b). In contrast to the a[CO2] treatment, e[CO2] treatment significantly decreased the SLA of plants by 13.7% and 33.0% at well-watered and drought-stressed conditions, correspondingly. Water and [CO2] both had significantly effect on the number of root nodules (NN). In contrast to the a[CO2] treatment, e[CO2] significantly improved the number of root nodules by 20.2% and 50.0% under well-watered and drought condition, respectively. Drought stress significantly decreased NN under either CO2 growth environment (Table 2).
Elevated [CO2] notably reduced the leaf nitrogen concentration ([N]leaf) (P < 0.05). In contrast to the a[CO2] treatment, e[CO2] plants decreased 21.4% and 23.4% [N]leaf, respectively, under well-watered and drought conditions (Fig. 3a). There was a significant interaction between [CO2] and water treatment on specific leaf nitrogen content (SLN) (Fig. 3b). The SLN was similar between well-watered and drought-stressed treatments at a[CO2], but at e[CO2] SLN was notably higher under drought. Elevated [CO2] significantly decreased the leaf nitrogen balance index (NBI). The NBI is the ratio of chlorophyll to flavonoids. NBI values decrease when nitrogen deficiency occurs, it can be used to evaluate plant nitrogen status. In contrast to a[CO2] treatment, e[CO2] treatment significantly decreased the NBI of by 26.8% and 13.6% under well-watered and drought-stressed environments, respectively. [CO2] and water treatments significantly affected plant N content. Compared to a[CO2] treatment, e[CO2] decreased plant N content by 26.6% and 12.3% at well-watered and drought-stressed environments, respectively. In contrast to well-watered treatment, drought stress reduced plant N content by 25.0% and 10.3% under a[CO2] and e[CO2], respectively. Moreover, elevated CO2 significantly decreased the effect of drought on N content in soybean plants due to the interaction of [CO2] and water treatments (Fig. 3c).
PCA and correlation analysis among physiological parameters
Principal component analysis (PCA) shows the association among the physiological parameters of soybean plants exposed to the four treatments (Fig. 4). The results showed that PC1 and PC2 explained 47.9% and 24.0% of the total variance, separately. Among them, parameters ABA, Ψl, gs and LA, WU contribute the most to PC1, while SLA and [N]leaf contribute the most to PC2. The irrigation treatment was mainly influenced by PC1, and the [CO2] treatment was mainly influenced by PC2 (Fig. 4). Therefore, the water treatment affected mainly on parameters including WU, Ψl and ABA, while the CO2 treatment mainly affected parameters of SLA, [N]leaf and NBI. In addition, PCA analysis showed that the irrigation treatment separated the parameters into different clusters, while the clusters of the [CO2] treatment were not as clearly separated. And under drought conditions, the e[CO2] treatment was more clearly distinguished from the a[CO2] treatment. The PCA plots also reveal that the number of root nodules was directly related to RWC, An, gs, LA and WU, while it was adversely related to ABA and Ψl. The NBI was directly related to plant N content and SLA, while negatively correlated with WUEi and WUEp. The [N]leaf was directly related to SLA and adversely related to LDM.
Discussion
Physiological response of soybean plants to e[CO2]
Improvement of An in soybean by elevated [CO2] treatment was stronger than in other crops, which may be an additional gain due to nitrogen fixation in soybean. In addition, the photosynthetic capacity of soybeans under e[CO2] was improved by increased leaf thickness. In this research, e[CO2] improved the WUE of soybean leaves due to an increased An and decreases in gs and E, consistent with earlier finding in soybean plants (Wang et al. 2018). Many research indicates that e[CO2] often results in an increase in An (Fan et al. 2020; Yang et al. 2020), which is due to that elevated [CO2] facilitates the carboxylation of Rubisco while depressing the photorespiration (Bowes 1991). The increase of An in soybean leaves (Ye et al. 2019) upon growing at e[CO2] is consistent with the findings in other species, but unlike non-nitrogen fixing crops, the stimulating effect of e[CO2] on An to a much larger extend than that in other crops (85.7% in soybean vs. 35–60% in other crops). This could have been related to the N-fixation role played by soybean root nodules as the number of root nodules (NN) was significant increased by e[CO2] (Table 2). Coincided with this, the PCA revealed that the An was most strongly correlated with the NN (Fig. 4). The increased An at e[CO2] could provide more carbon to root supporting nodulation and nodule growth, contributing to enhanced N fixation thus better plant N nutrition. In this study, e[CO2] did not affect leaf area (LA), while significantly increased leaf dry mass (LDM) (Table 2), resulting in a significant reduction in SLA, which indicates an increase in the thickness of the leaf. Similar increases in leaf thickness were also observed in experiments with oilseed rape and soybean under e[CO2] environment by Uprety and Mahalaxmi (2000) and Ainsworth et al. (2002). Increased leaf thickness could improve leaf light absorption and better light distribution inside the leaf, improving the photosynthetic capacity (Brodrick et al. 2013). This would have contribute to the significant increase in photosynthetic capacity of soybean when grown at e[CO2] environment. The increase in leaf photosynthetic rate and leaf thickness was generally coupled with an improvement in leaf N concentration (Marenco et al. 2017), but Nleaf was significantly lower at e[CO2] environment. Zhu et al. (2009) suggest that this may be a result of increased protease activity in the leaves caused by elevated CO2, which enables the remobilization. The reduction in Nleaf caused a reduction in Rubisco content, which in turn led to a down-regulation of photosynthetic rate, resulting in photo acclimation, but this was not found in the present research (Aranjuelo et al. 2005).
Response of soybean leaf stomata to Progressive drought under e[CO2]
Soybeans grown at e[CO2] had a delayed gs response to progressive soil drying compared to the a[CO2] treatment, coinciding with a reduced sensitivity of gs to the ABA signaling, though they tended to maintain a better leaf water status under drought than the a[CO2]-grown plants.
In this study, gs of soybean leaf was remarkably reduced by e[CO2] (Fig. 1b; Table 1). The e[CO2]-induced stomatal closure is thought to be a response by guard cells membrane channels to the increase in intercellular CO2 concentration (Maurel et al. 2016), on the other hand, it has been considered as the decrease in stomatal size and density (Xu et al. 2016). The decrease in gs reduces evapotranspiration and allows the plant to maintain a better water status. As can be seen in S2 (in the supplementary figures), the FTSW at e[CO2] treatment were consistently greater than that at a[CO2], indicating that the reduced gs and E had lowered plant water consumption thus soil water depletion during the progressive drought. In addition, along with the decrease of FTSW, gs of the soybean leaves at e[CO2] began to decrease gradually when FTSW below 0.44, this FTSW threshold was slightly lower than that under a[CO2]. Such delayed stomatal closure during soil drying under e[CO2] has also been identified in tomato plants (Liu et al. 2019, Li et al. 2019). This suggests that the reduction in gs under e[CO2] leads to a reduction in plant water consumption, conserving soil water and improving plant growth at drought conditions (Aranjueloa et al. 2014, Bowes 1991). In contrast, Gray et al. (2016) reported that did not protect soybean plants from drought stress. The discrepancy among different studies could be due to the diverse cultivars tested or the varied environmental conditions applied in the experiments.
Research has indicated that ABA, as an early chemical signal, can induce stomatal closure at moderate drought stress (Zhang et al. 2018). Besides, it has also been shown that ABA is involved in the stomatal CO2 response (Raschke 1975). In the adequate water supply environment, the ABA concentration of soybean leaf was slightly higher at e[CO2] than that at a[CO2] (Table 2). With the intensification of drought, and the Nleaf grew exponentially as the FTSW decreased. Similar trends were found for plants at both [CO2] conditions, though at a given FTSW level, a slightly higher ABA concentration was shown in the e[CO2] plants (Fig. 2a). Moreover, regression analysis revealed a negative correlation between ABA concentration and gs, and the ANCOVA showed that the slope of the regression line was less steep at e[CO2] than at a[CO2] (Fig. 2b). In line with this, Yan et al. (2017) found similar results in tomato plants. This suggests that the gs of soybean leaf become less sensitive to ABA signaling at e[CO2], in agreement with results obtained for non-nitrogen-fixing crops such as tomato and wheat (Li et al. 2019; Wei et al. 2020). Numerous field trials had also been carried out in Illinois investigating the interactive effects of droughts and e[CO2] on soybean growth and physiology, and in particular, the study on the stomatal response of ABA to e[CO2] pointed out that e[CO2] enhanced the stomata sensibility to ABA (Gray et al. 2016). Obviously, this disagrees with our findings in the present study, which probably attributed to the different environmental conditions and soybean varieties used among different studies. Nonetheless, such uncertainties merit further studies.
Response of soybean growth to drought and e[CO2] environment
Dilution of plant nitrogen concentration at e[CO2] was not altered by the increase in nitrogen fixation capacity, i.e. increase in NN. The CO2 fertilization effect in previous studies was that increased photosynthesis generally promotes increased plant growth when [CO2] is elevated (Zheng et al. 2020). However, sufficient N is required for plants to fully utilize the increased carbon supply at e[CO2], plants that are not capable of biological N fixation are often limited by N availability (Ainsworth et al. 2007). The reduction in NBI at e[CO2] in this study suggests that soybean leaves remain N-limited under elevated CO2 as other non-N-fixing crops, so the extra N fixed by enhanced N fixation must be available to plant growth. Many studies about the influence of e[CO2] on the N trophic have shown that N concentration decreases in the presence of atmospheric CO2 enrichment (Liu et al. 2019; Wang et al. 2021). This is likely to be because the “dilution effect” caused the large accumulation of non-structural carbohydrates and biomass under e[CO2]. Similar results were obtained in this study, with elevated CO2 significantly reduced [N]leaf. However, unlike other non-nitrogen fixing crops, SLN is not affected by e[CO2] (Liu et al. 2019; Zheng et al. 2020). This could be attributed to the presence that biological N fixation provides additional N to the plants, alleviating N limitation under e[CO2] condition. Soybean plants thus have both a greater carbon sink capacity and the ability to match their N and carbon supplies at e[CO2] (Ainsworth et al. 2007). Analysis of the N content of the whole soybean plants revealed that both e[CO2] and DS reduced plant N content, but the drought had a smaller effect on plant N content in elevated CO2 environment (Fig. 3c). Jin et al. (2019) also showed that legumes are influenced by dilution effects to a lesser extent and can satisfy the N requirements for e[CO2] conditions by enhancing N fixation. In this research, the results showed that N density in the leaves remained dilutive at e[CO2], suggesting that N fixation in soybean is inadequate to alleviate the dilutive impact of e[CO2] on leaf N concentrations; but the drought impact on [N]leaf was mitigated at e[CO2], with differences between the various water treatments reduced.
Through the previous analysis we found that compared to the a[CO2] treatment, the greater number of nodules of the e[CO2] plants would lead to an enhanced N-fixation, which, however, did not improve N nutrition of the plants. Nevertheless, by sustaining the SLN, the soybean plants were able to enhance An when growing at e[CO2], particularly under dry conditions. PCA plots illustrate that there were more root nodules, better leaf water status and more dry matter in the WW treated plants. While under drought, soybean plants grown under e[CO2] had greater WUEp and SLN compared to the a[CO2], indicating that the drought stress were alleviated by elevated CO2. In addition, PCA plots reveal that the effect of drought was greater than that of e[CO2] (Fig. 4). This may be due to the fact that under severe drought stress, leaves were mainly subject to hydraulic regulation and were more affected by water deficits (Fan et al. 2020).
Conclusions
Elevated [CO2]-treated plants had significantly greater An compared to a[CO2]-treated plants, but lower gs and E, resulting in enhanced WUEi and WUEleaf. In addition, soybeans grown at e[CO2] had a delayed gs response to progressive soil drying compared to the a[CO2] treatment, coinciding with a reduced sensitivity of gs to the ABA signaling, though they tended to maintain a better leaf water status under drought than the a[CO2]-grown plants. Although the leaf nitrogen concentration and the total plant N content were markedly lower in plants grown at e[CO2], there was no remarkable difference in SLN between the two [CO2] treatments. Compared to the e[CO2] treatment, the greater number of nodules of the e[CO2] plants would lead to an enhanced N-fixation, which, however, did not improve N nutrition of the plants. Nevertheless, by sustaining the SLN, the soybean plants were able to enhance An when growing at e[CO2], particularly under dry conditions. This provides a theoretical basis for how to better utilize the nitrogen fixation of soybeans in future climates, and also provides a scientific basis for future exploration of water-saving irrigation of soybeans in drought and CO2 rich climate conditions.
References
Ainsworth EA, Rogers A (2007) The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant Cell Environ 30(3):258–270
Ainsworth EA, Davey PA, Bernacchi CJ, Dermody OC, Heaton EA, Moore DJ, Morgan PB, Naidu SL, Yoo Ra HS, Zhu XG, Curtis PS, Long SP (2002) A meta-analysis of elevated CO2 effects on soybean (Glycine max) physiology, growth and yield. Glob Change Biol 8:695–709
Ainsworth EA, Rogers A, Leakey AD, Heady LE, Gibon Y, Stitt M, Schurr U (2007) Does elevated atmospheric [CO2] alter diurnal C uptake and the balance of C and N metabolites in growing and fully expanded soybean leaves? J Exp Bot 58(3):579–591
Ainsworth EA, Yendrek CR, Skoneczka JA, Long SP (2012) Accelerating yield potential in soybean: potential targets for biotechnological improvement. Plant Cell Environ 35(1):38–52
Aranjuelo I, Zita G, Hernandez L, Perez P, Martinez-Carrasco R, Sanchez-Diaz M (2005) Response of nodulated alfalfa to water supply, temperature and elevated CO2: photosynthetic downregulation. Physiol Plant 123:348–358
Aranjueloa I, Arrese-Igorb C, Molero G (2014) Nodule performance within a changing environmental context. J Plant Physiol 171:1076–1090
Bencze S, Bamberger Z, Janda T, Balla K, Varga B, Bedo Z, Veisz O (2014) Physiological response of wheat varieties to elevated atmospheric CO2 and low water supply levels. Photosynthetica 52(1):71–82
Bousba R, Ykhlef N, Djekoun A (2009) Water use efficiency and flat leaf photosynthetic in response to water deficit of durum wheat (Triticum durum Desf). World J Agric Sci 5:609–616
Bowes G (1991) Growth at elevated CO2: photosynthetic responses mediated through rubisco. Plant Cell Environ 14(8):795–806
Brodrick R, Bange MP, Milroy SP, Hammer GL (2013) Physiological determinants of high yielding ultra-narrow row cotton: canopy development and radiation use efficiency. Field Crop Res 148:86–94
Bunce J (1996) Growth at elevated carbon dioxide concentration reduces hydraulic conductance in alfalfa and soybean. Glob Change Biol 2:155–158
Dong J, Gruda N, Li X, Tang Y, Zhang P, Duan Z (2020) Sustainable vegetable production under changing climate: the impact of elevated CO2 on yield of vegetables and the interactions with environments-a review. J Clean Prod 253:119920
Fan X, Cao X, Zhou H, Hao L, Dong W, He C, Xu M, Wu H, Wang L, Chang Z, Zheng Y (2020) Carbon dioxide fertilization effect on plant growth under soil water stress associates with changes in stomatal traits, leaf photosynthesis, and foliar nitrogen of bell pepper (Capsicum annuum L). Environ Exp Bot 179:104203
Fang L, Abdelhakim LOA, Hegelund JN, Li S, Liu J, Peng X, Li X, Wei Z, Liu F (2019) ABA-mediated regulation of leaf and root hydraulic conductance in tomato grown at elevated CO2 is associated with altered gene expression of aquaporins. Hortic Res 6:104
Gojon A, Cassan O, Bach L, Lejay L, Martin A (2023) The decline of plant mineral nutrition under rising CO2: physiological and molecular aspects of a bad deal. Trends Plant Sci 28(2):185–198
Gray SB, Dermody O, Klein SP, Locke AM, McGrath JM, Paul RE, Rosenthal DM, Ruiz-Vera UM, Siebers MH, Strellner R, Ainsworth EA, Bernacchi CJ, Long SP, Ort DR, Leakey ADB (2016) Intensifying drought eliminates the expected benefits of elevated carbon dioxide for soybean. Nat Plants 2(9):1–8
Hessini K, Martinez JP, Gandour M, Albouchi A, Soltani A, Abdelly C (2009) Effect of water stress on growth, osmotic adjustment, cell wall elasticity and water-use efficiency in Spartina alterniflora. Environ Exp Bot 67(2):312–319
Hsu Po K, Takahashi Y, Munemasa S, Merilo E, Laanemets K, Waadt R, Pater d, Kollist H, Schroeder JI (2018) Abscisic acid-independent stomatal CO2 signal transduction pathway and convergence of CO2 and ABA signaling downstream of OST1 kinase. Proc Natl Acad Sci USA 115(42):9971–9980
Hu S, Chen W, Tong K, Wang Y, Jing L, Wang Y, Yang L (2022) Response of rice growth and leaf physiology to elevated CO2 concentrations: a meta-analysis of 20-year face studies. Sci Total Environ 807:151017
Jin J, Armstrong R, Tang C (2019) Impact of elevated CO2 on grain nutrient concentration varies with crops and soils - a long-term FACE study. Sci Total Environ 651:2641–2647
Kaschuk G, Kuyper TW, Leffelaar PA, Hungria M, Giller KE (2009) Are the rates of photosynthesis stimulated by the carbon sink strength of rhizobial and arbuscular mycorrhizal symbioses? Soil Biol Biochem 41(6):1233–1244
Laza Haydee E, Baker Jeffrey T, Yates C, Mahan James R, Burow Mark D, Puppala N, Gitz Iii, Dennis C, Emendack Yves Y, Layland N, Ritchie Glen L, Chen J, Rowland D, Tissue David T and Payton, Paxton R (2021) Effect of elevated CO2 on peanut performance in a semi-arid production region. Agricultural and Forest Meteorology 308–309: 108599.
Leakey ADB, Uribelarrea M, Ainsworth EA, Naidu SL, Rogers A, Ort DR, Long SP (2006) Photosynthesis, productivity, and yield of maize are not affected by open-air elevation of CO2 concentration in the absence of drought. Plant Physiol 140(2):779–790
Li F, Kang S, Zhang J (2004) Interactive effects of elevated CO2, nitrogen and drought on leaf area, stomatal conductance, and evapotranspiration of wheat. Agric Water Manage 67(3):221–233
Li Z, Wenzhao L, Fenli Z (2011) Assessing the site-specific impacts of climate change on hydrology, soil erosion and crop yields in the Loess Plateau of China. Clim Change 105:223–242
Li D, Liu H, Qiao Y, Wang Y, Cai Z, Dong B, Shi C, Liu Y, Li X, Liu M (2013) Effects of elevated CO2 on the growth, seed yield, and water use efficiency of soybean (Glycine max (L.) Merr.) under drought stress. Agric Water Manage 129:105–112
Li X, Kristiansen K, Rosenqvist E, Liu F (2019) Elevated CO2 modulates the effects of drought and heat stress on plant water relations and grain yield in wheat. J Agron Crop Sci 205(4):362–371
Liang K, Chen X, Liu F (2023) Crosstalk between ABA and ethylene in regulating stomatal behavior in tomato under high CO2 and progressive soil drying. J Exp Bot. https://doi.org/10.1093/jxb/erad309
Liu B, Cheng X, Liu J, Pu H (2018) Investigation into particle emission characteristics of partially premixed combustion fueled with high n-butanol-diesel ratio blends. Fuel 223:1–11
Liu J, Hu T, Fang L, Peng X, Liu F (2019) CO2 elevation modulates the response of leaf gas exchange to progressive soil drying in tomato plants. Agric Meteorol 268:181–188
Marenco RA, Camargo MAB, Antezana-Vera SA, Oliveira MF (2017) Leaf trait plasticity in six forest tree species of central Amazonia. Photosynthetica 55:679–688
Maurel C, Verdoucq L, Rodrigues O (2016) Aquaporins and plant transpiration. Plant Cell Environ 39(11):2580–2587
Minguillon S, Matamoros MA, Duanmu D, Becana M (2022) Signaling by reactive molecules and antioxidants in legume nodules. New Phytol 236:815–832
Parvin S, Uddin S, Tausz-Posch S, Fitzgerald G, Armstrong R, Tausz M (2019) Elevated CO2 improves yield and N2 fixation but not grain N concentration of faba bean (Vicia faba L.) subjected to terminal drought. Environ Exp Bot 165:161–173
Raschke K (1975) Simultaneous requirement of carbon dioxide and abscisic acid for stomatal closing in Xanthium strumarium L. Planta 125(3):243–259
Shao Y, Wu J, Ye J, Liu Y (2015) Frequency analysis and its spatiotemporal characteristics of precipitation extreme events in China during 1951–2010. Theoret Appl Climatol 121(3):775–787
Silveira AMF, Coelho Netto RA, Marenco RA (2023) Biomass allocation in Ceiba pentandra (Malvaceae) under water stress and high CO2 concentration. Scientia Forestalis 51:e3955
Soba D, Aranjuelo I, Gakiere B, Francoise Gilard, Perez-Lopez U, Mena-Petite A, Munoz-Rueda A, Lacuesta M, Sanz-Saez A (2021) Soybean inoculated with one bradyrhizobium strain isolated at elevated [CO2] show an impaired C and N metabolism when grown at ambient [CO2]. Front Plant Sci 12:656961
Tausz-Posch S, Dempsey RW, Seneweera S, Norton RM, Fitzgerald G, Tausz M (2015) Does a freely tillering wheat cultivar benefit more from elevated CO2 than a restricted tillering cultivar in a water-limited environment? Eur J Agron 64:21–28
Tausz-Posch S, Tausz M, Bourgault M (2020) Elevated [CO2] effects on crops: advances in understanding acclimation, nitrogen dynamics and interactions with drought and other organisms. Plant Biology 22(S1):38–51
Uprety DC, Mahalaxmi V (2000) Effect of elevated CO2 and nitrogen nutrition on photosynthesis, growth and carbon–nitrogen balance in Brassica juncea. J Agron Crop Sci 184:271–276
Wall GW, Brooks TJ, Adam NR, Cousins AB, Webber AN (2001) Elevated atmospheric CO2 improved Sorghum plant water status by ameliorating the adverse effects of drought. New Phytol 152(2):231–248
Wang A, Lam SK, Hao X, Li FY, Zong Y, Wang H, Li P (2018) Elevated CO2 reduces the adverse effects of drought stress on a high-yielding soybean (Glycine max (L.) Merr.) cultivar by increasing water use efficiency. plant Physiol Biochem 132:660–665
Wang C, Sun Y, Chen HYH, Ruan H (2021) Effects of elevated CO2 on the C:N stoichiometry of plants, soils, and microorganisms in terrestrial ecosystems. CATENA 201:105219
Wei Z, Fang L, Li X, Liu J, Liu F (2020) Effects of elevated atmospheric CO2 on leaf gas exchange response to progressive drought in barley and tomato plants with different endogenous ABA levels. Plant Soil 447:431–446
Wullschleger SD, Tschaplinski TJ, Norby RJ (2010) Plant water relations at elevated CO2-implications for water-limited environments. Plant, Cell Environ 25(2):319–331
Xu Z, Jiang Y, Jia B, Zhou G (2016) Elevated CO2 response of stomata and its dependence on environmental factors. Front Plant Sci 7(657):1–15
Yan F, Li X, Liu F (2017) ABA signaling and stomatal control in tomato plants exposure to progressive soil drying under ambient and elevated atmospheric CO2 concentration. Environ Exp Bot 139:99–104
Yang X, Zhang P, Wei Z, Liu J, Hu X, Liu F (2020) Effects of CO2 fertilization on tomato fruit quality under reduced irrigation. Agric Water Manage 230:105985
Ye Z, Duan S, Kang H (2019) Comparison of water use efficiency for glycine max leaves under different CO2 concentration. J Nucl Agric Sci 33(5):1006–1015
Zhang FP, Sussmilch F, Nichols DS, Cardoso AA, Brodribb TJ, McAdam SAM (2018) Leaves, not roots or floral tissue, are the main site of rapid, external pressure-induced ABA biosynthesis in angiosperms. J Exp Bot 69(5):1261–1267
Zheng Y, He C, Guo L, Hao L, Cheng D, Li F, Peng Z, Xu M (2020) Soil water status triggers CO2 fertilization effect on the growth of winter wheat (Triticum aestivum). Agric For Meteorol 291:108097
Zhu C, Zhu J, Zeng Q, Liu G, Xie Z, Tang H, Cao J, Zhao X (2009) Elevated CO2 accelerates flag senescence in wheat due to ear photosynthesis which causes greater ear nitrogen sink capacity and ear carbon sink limitation. Funct Plant Biol 36:291–299
Acknowledgements
This work was supported by the National Natural Science Foundation of China (51909220). Bingjing Cui appreciates the Chinese Scholarship Council (CSC) for supporting her study at the Faculty of Science, University of Copenhagen, Denmark.
Funding
Open access funding provided by Royal Library, Copenhagen University Library. Funding was supported by National Natural Science Foundation of China (Grant No. 51909220).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors have not disclosed any competing interests.
Additional information
Communicated by Honglang Duan.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cui, B., Liu, J., Zhang, M. et al. CO2 elevation modulates the growth and physiological responses of soybean (Glycine max L. Merr.) to progressive soil drying. Plant Growth Regul 103, 139–150 (2024). https://doi.org/10.1007/s10725-023-01092-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-023-01092-z