Abstract
The quality of mountain-cultivated ginseng (Panax ginseng Meyer; MCG) was closely related to the terpenoids metabolism which was significantly affected by harvest months and cultivation years. In this study, the metabolisms of terpenoids and carbohydrates in the MCG harvested at different months and cultivation years were elucidated using a transcriptomic approach. Based on the RNA-Seq analysis, 42 and 41 genes related to terpenoids metabolism were identified in the MCG of different harvest months (August, September, and October) and cultivation years (5, 10, and 15 years), respectively. In August, the biosyntheses of terpineol, valencene, germacrene, solavetivone, and brassinolide were more active, and those of valencene and brassinolide were less active than in September and October, while those of gibberellin (GA), campesterol, and strigol gradually became active from September through October in the 10 years’ MCG. Terpenoids metabolisms in MCG were repressed in October, except for the biosyntheses of neomenthol, stigmasterol, and abscisic acid. Besides, one of the reasons why MCG does not like high temperature or is not suitable for high temperature survival were explained. By comparing the difference in terpenoids metabolism in MCG harvested in September) of different cultivation years, it was found that the biosyntheses of neomenthol, germacrene, GA, and brassinolide were more active in the 5th year. In the 10th year, only the biosyntheses of terpineol, solavetivone, and campesterol were activated. Surprisingly, all these pathways associated with terpenoids metabolisms became inhibited at the 15th year. In addition, in the process of carbohydrates metabolisms, the growth environment has greater influence, whereas there is little correlation between cultivation years and carbohydrates metabolisms. These findings will deepen our understanding of the complicated but important biosynthesis and regulation of terpenoids in the plant species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant secondary metabolites play various roles mainly including defense against pathogens, pests, and herbivores; response to environmental stresses; and mediation of organismal interactions. (Ahanger et al. 2020). Plant secondary metabolism is dynamic and is affected by many factors. Thus, the relationship between secondary metabolism of plants and its influencing factors has become a research hotspot. Seasonal changes, including the changes in light, temperature, precipitation, and other environmental factors, significantly affect the changes of plant secondary metabolism. The contents of phenolic compounds of wine grapes such as catechin, epicatechin, and anthocyanins had slightly increased in September (Tassoni et al. 2019). Xu et al. (2018) obtained the metabolome and transcriptome profiles of tea leaves harvested at five different months, and the content of sesquiterpene was the lowest when harvested in April, increased markedly in June, and declined in August, September, and October. Cheng et al. (2019) studied the difference of carotenoids pathway during the two growing seasons in grapes, indicating that expression levels of the majority of genes involved in the carotenoids metabolic pathway in winter grapes were generally upregulated compared to those in summer grapes. In addition to seasonal changes, cultivation years are also considered as one of the key factors affecting the secondary metabolism of plants. For example, Chen et al. (2019) manifested that the levels of monoterpenoids of Chamaecyparis formosensis showed opposite trends at the 5th and 15th years. Flavonoids of Dendrobium huoshanense were accumulated mainly at the 3rd year of cultivation (Yuan et al. 2022).
Plant secondary metabolites can be categorized into three chemically distinct groups: terpenoids, phenolics, and nitrogen-containing compounds. Among them, terpenoids are the largest group (Huang et al. 2021a, b), which can be further classified by the number of C5 units they contain, including monoterpenoids (C10), sesquiterpenoids (C15), diterpenoids (C20), triterpenoids (C30), tetraterpenoids (C40), and polyterpenoids ([C5]n, n > 8). Many plants contain mixtures of volatile monoterpenoids and sesquiterpenoids, called essential oils, that are well-known in possessing insect repellent properties (Papanastasiou et al. 2020). Certain terpenoids have well-characterized functions in plant growth and development. For example, abscisic acid and gibberellin, important groups of plant hormones, are sesquiterpenoids and diterpenoids, respectively. Brassinosteroids, another plant hormones with terpenoid structure, also have functions of plant growth regulation. Limonoids, a group of nonvolatile triterpenoids, are the source of bitter substances in citrus fruits (Huang et al. 2021a, b). The red, orange, and yellow carotenoids are tetraterpenes which act as accessory pigments in photosynthesis and protect photosynthetic apparatus from photooxidation (Son et al. 2021).
In addition to the above effects on growth as well as defense of plants, some terpenoids are also beneficial to human health. Ginsenoside in ginseng (Panax ginseng Meyer) is a type of representative triterpenoids possessing various pharmacological effects including antioxidant (He et al. 2022) and anti-inflammatory (Yao et al. 2019). Ginseng root has been used in medicine and healthcare for thousands of years in the world (Fang et al. 2022). To satisfy the increasing consumption of ginseng product, mountain-cultivated ginseng (MCG) has been widely planted in recent decades. MCG is a type of semi-wild ginseng cultivated by artificially sowing seeds or planting seeds in natural or artificial forests. Generally, the harvest season for ginseng is autumn in the northeast of China, concentrated in the three months around September. There are great differences in duration of light, average temperature, and precipitation between these three months, which make the content of secondary metabolites of ginseng, especially terpenoids, different at different harvest months. Currently, research on the seasonal changes in terpenoids metabolism of ginseng focuses mainly on field-cultivated ginseng (FCG) which is usually planted in gardens or fields. For example, the contents of ginsenosides Rb1, Ro, Rc, and Rb2 were gradually increased from August to October (Shan et al. 2014), and the Re was higher than the Rg1 in contents in May and June, but lower than the Rg1 from August to October (Liu et al. 2017). The research status of influence of cultivation years on terpenoids metabolism is similar to that of harvest months. The total saponins content of FCG increases with the extension of growth years until the 4th year during 2–6 years cultivation (Wang et al. 2006), and the contents of ginsenosides such as Rb1 and Rg1 may be first increased in early stage of development and then decreased (Shi et al. 2007). However, the relationship at a molecular level between terpenoids metabolism in MCG and seasonal changes and cultivation years is still unclear.
In this study, the changes in terpenoids metabolism in MCG at different harvest months and cultivation years were compared on the basis of transcriptome analysis. The biosyntheses of terpenoids in MCG at different harvest months and cultivation years, such as monoterpenoids, sesquiterpenoids, diterpenoids, triterpenoids, and carotenoids, were significantly different. Surprisingly, the carbohydrates metabolism in MCG was also greatly affected by the harvest months, while the years of cultivation had less impact. These data provide an overview of the biosyntheses of terpenoids and carbohydrates in MCG at different harvest months and cultivation years, and preliminarily elucidated the relationship between metabolisms of terpenoids and carbohydrates, and growth environment and development of plants.
Materials and methods
Plant materials
Five ginsengs (P. ginseng Meyer cv. Ermaya) samples were collected from August to October. Among these, 5- and 15-year-old MCG were collected in September, and 10-year-old MCG were collected in August, September, and October. The ages of ginseng plants were determined based on local cultivation records. The sampling area was located in a mountain area (125°45’16.49"E, 41°6’44.32"N) in Kuandian County, Liaoning province, China, where the average annual temperature and precipitation were 7.13 °C and 700.03 mm, respectively. The monthly average temperatures, rainfalls and daylengths of the sampling plot from August to October in the year of harvest were shown in Table 1. After cleaning with distilled water on the spot, ginseng roots (n = 3) were immediately frozen in liquid nitrogen and sent to the lab at Beijing Forestry University for further analyses.
RNA extraction, library construction, and sequencing
Isolation, library construction, and sequencing of RNA were conducted according to our previous report (Fan et al. 2019). Briefly, total RNAs were isolated from the roots of all samples using Trizol reagent (Thermo Fisher Scientific Inc., Waltham, MA, USA) according to the manufacturer’s protocol. To avoid interference by proteins and polysaccharides, RNA concentration and quality should meet the standards of TruSeq RNA sample preparation guide (Illumina Inc., San Diego, CA, USA), which displayed a ratio of absorbance at 260 nm to that at 280 nm (R260/280) between 1.8 and 2.0, and the RNA integrity number was larger than 8.0. Qualified RNA from each sample was then used for cDNA library construction through the TruSeq RNA sample preparation Kit. Subsequently, these libraries were sequenced via Illumina Hiseq4000 platform (Illumina Inc., San Diego, CA, USA) using a Truseq SBS Kit v3-HS 200 cycles Kits (Illumina Inc., San Diego, CA, USA). The sequencing raw reads are available from the NCBI Sequence Read Archive database (http://www.ncbi.nlm.nih.gov/sra/) under the accession number of PRJNA532810 and PRJNA532951.
Transcriptome mapping and functional annotation
Adapter and low-quality sequences were removed from the raw reads after sequencing using SeqPrep (https://github.com/jstjohn/SeqPrep) and Sickle (https://github.com/najoshi/sickle) software. According to our previous research (Fan et al. 2019), the obtained clean reads were mapped to a known ginseng genome (Ginseng Genome Database, http://ginsengdb.snu.ac.kr/data.php) (Jayakodi et al. 2018) by sequence alignment using HISAT2 (http://ccb.jhu.edu/software/hisat2/index.shtml) (Kim et al. 2015) and assembled as genes using StringTie (https://ccb.jhu.edu/software/stringtie/index.shtml) (Pertea et al. 2015). Next, all genes were functionally annotated via GO (Gene Ontology) (http://www.geneontology.org/), COG (Clusters of Orthologous Groups of proteins) (http://www.ncbi.nlm.nih.gov/COG/), and KEGG (Kyoto Encyclopedia of Genes and Genomes) (http://www.genome.jp/kegg/) databases.
Gene expression profiling
The expression of the annotated unigenes was profiled by the values of fragments per kilobase of transcript per million mapped reads (FPKM) using the featureCounts (http://bioinf.wehi.edu.au/featureCounts/) and RSEM (http://deweylab.github.io/RSEM/) software. The DESeq R package (https://bioconductor.org/packages/release/bioc/html/DESeq2.html) was performed to analyze the differentially expressed genes (DEGs). Genes with a p value < 0.05 and |log2 fold change| ≥ 1 were considered to be DEGs. Subsequently, KEGG pathways enrichment analyses were performed based on these DEGs using the KOBAS (http://kobas.cbi.pku.edu.cn/home.do) software. KEGG pathways with p < 0.05 were considered to be significantly enriched.
Statistical analysis
Experiments on all determinations were carried out in triplicate, and results were presented as means ± stand deviation (SD). The statistical analysis was performed using SPSS software (v 20.0; SPSS Inc., Chicago, IL, USA). Confidence levels for statistical significance were set at p < 0.05.
Results
Validation of the transcriptomic data
To explore genes expression changes altered with harvest months and cultivation years or ages, transcriptomic analysis was first performed among the three 10-year-old MCG samples harvested in August, September, and October. Considering the results and harvest habits of the farmer, 5-, 10-, and 15-year-old MCGs harvested in September were then selected to explore the difference in genes expressions between different cultivation years. Statistical analysis results of transcriptome data of the five samples were shown in Table 2. To validate the transcriptional profiles, these data were subjected to sequence alignment with a reference genome mentioned above. The proportions of total reads mapped to the reference sequence (mapped reads in Table 2) in each sample were all greater than 94%. In addition, the numbers and proportions of clean reads with multiple and unique locations on the reference genome were obtained (Table 2), and these results show that the transcriptome data were suitable for analysis.
Next, to further validate the transcriptome data, GO, KEGG, and COG databases of all genes were exploited to analyze the gene annotation of the reads mapped to the reference genome. GO analysis (Fig. 1) annotated 33,144 genes that were further assigned into three main categories. In the first category “biological process” (Fig. 1A), the top three terms were “metabolic process”, “cellular process”, and “single-organism process”. The fourth term “response to stimulus” may be related to secondary metabolites. The dominant terms in the second category “cellular component” (Fig. 1B) were “cell”, “cell part”, and “organelle”. In the third main category “molecular function” (Fig. 1C), the dominant terms were “catalytic activity”, “binding”, and “transporter activity”. And the term “antioxidant activity” ranked eighth.
For the COG analysis, total 21,625 genes were assigned to 24 functional classifications (Fig. 2A). Among these classifications, “signal transduction mechanisms” was the largest group, followed by “general function prediction only” and “posttranslational modification, protein turnover, chaperones”. Particularly, 793 and 294 genes were annotated to “secondary metabolites biosynthesis, transport and catabolism” and “defense mechanism”, respectively. A total of 14,981 genes were assigned through KEGG analysis (Fig. 2B), among which the pathway category “Translation” covered the most genes. It was worth noting that many pathways were associated with metabolism, such as “carbohydrate metabolism”, “amino acid metabolism”, “lipid metabolism”, “metabolism of terpenoids and polyketides”. In addition, the pathways of “drug resistance: antimicrobial”, “biosynthesis of other secondary metabolites”, and “environmental adaptation”, which related to secondary metabolites, were also annotated. A large number of genes were accurately annotated, providing support for subsequent experiments.
Changes of terpenoids metabolisms in MCG harvested at different months
Biosynthesis of the terpenoids backbone
To reveal the variation in terpenoids biosynthesis in MCG at different harvest months, 16 DEGs (p value < 0.05 and |log2 fold change| ≥ 1) involved in isopentenyl diphosphate (IPP) metabolism were selected based on the KEGG database (Fig. 3A). In the mevalonate pathway, four enzymes genes, including, hydroxymethylglutaryl-CoA synthase (HMGS), hydroxymethylglutaryl-CoA reductase (HMGCR), mevalonate kinase (mvaK1), and diphosphomevalonate decarboxylase (mvaD), were analyzed. As shown in Fig. 4A, except for upregulation the mvaK1 genes that continued to be upregulated (August vs. September, p = 0.699; September vs. October, p = 0.0413) until October, the genes encoding other enzymes showed upregulation at September and then the trend of upregulation was gradually reduced at October. There was a similar finding in the non-mevalonate pathway. In the biosynthesis of IPP with glyceraldehyde 3-phosphate as the precursor, only the 4-hydroxy-3-methylbut-2-enyl diphosphate reductase (ispH) genes were continuously downregulated (August vs. September, p = 0.42; September vs. October, p = 0.0427) at the following two months, and the other genes were upregulated at September and downregulated at October. In the downstream pathway of IPP, the genes encoding farnesyl diphosphate synthase (FDPS) and geranylgeranyl diphosphate synthase (GGPS) had the highest expression levels at October and September, respectively, compared with those at the other two months. The results showed that the processes of biosynthesis and transformation of IPP in MCG were most active at September, and the activities were inhibited at October.
Terpenoids metabolisms in MCG at different harvest months. (A) Terpenoids backbone biosynthesis, (B) Biosynthesis of the carotenoids, (C) Biosynthesis of the triterpenoids, (D) Biosynthesis of the sesquiterpenoids, (E) Biosynthesis of the monoterpenoids, and (F) Biosynthesis of the diterpenoids. (-)-α-TS, (-)-α-terpineol synthase; (+)-ND, (+)-neomenthol dehydrogenase; ABA2, xanthoxin dehydrogenase; CCD8, carlactone synthase; crtB, 15-cis-phytoene synthase; crtZ, β-carotene 3-hydroxylase; CYP51, sterol 14-demethylase; CYP710A, sterol 22-desaturase; CYP85A2, brassinosteroid-6-oxidase 2; DET2, steroid 5-α-reductase; DHCR24, δ-24-sterol reductase; DXR, 1-deoxy-D-xylulose-5-phosphate reductoisomerase;DXS, 1-deoxy-D-xylulose-5-phosphate synthase; FDPS, farnesyl diphosphate synthase; FNTA, α-protein farnesyltransferase; FOLK, farnesol kinase; GA20ox, gibberellin 20-oxidase; GA2ox, gibberellin 2-oxidase.GA3ox1, gibberellin 3-β-dioxygenase; GERD, (-)-germacrene D synthase; GGPS, geranylgeranyl diphosphate synthase; HMGCR, hydroxymethylglutaryl-CoA reductase; HMGS, hydroxymethylglutaryl-CoA synthase; HVS, vetispiradiene synthase; ispE, 4-diphosphocytidyl-2-C-methyl-D-erythritol kinase; ispF, 2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase; ispG, (E)-4-hydroxy-3-methylbut-2-enyl-diphosphate synthase; ispH, 4-hydroxy-3-methylbut-2-enyl diphosphate reductase; KAO, ent-kaurenoic acid hydroxylase; lcyB, lycopene β-cyclase; mvaD, diphosphomevalonate decarboxylase; mvaK1, mevalonate kinase; mvaK2, phosphomevalonate kinase; NCED, 9-cis-epoxycarotenoid dioxygenase; PCYOX1, prenylcysteine oxidase; RCE1, prenyl protein peptidase;SMO1, methylsterol monooxygenase 1; SMO2, methylsterol monooxygenase 2; SMT1, sterol 24-C-methyltransferase; SQLE, squalene monooxygenase; TPS1, valencene/7-epi-alpha-selinene synthase; VDE, violaxanthin de-epoxidase; ZEP, zeaxanthin epoxidase
In the farnesyl cycle (Figs. 3 and 4 A), the genes encoding α-protein farnesyltransferase (FNTA; August vs. September, p = 0.791; September vs. October, p = 0.037), prenyl protein peptidase (RCE1; August vs. September, p = 0.033; September vs. October, p = 0.04), and prenylcysteine oxidase (PCYOX1; August vs. September, p = 0.813; September vs. Octobe, p = 0.037) were downregulated at October compared to those at August and September, indicating that the process of farnesyl diphosphate (FPP) binding protein transport was prevented. Meanwhile, the encoding genes of NAD+-dependent farnesol dehydrogenase (FLDH) had a high expression at August and September, accelerating the conversion of farnesol to farnesal. The excessive accumulation of farnesal may slow its release from complex, leading to the inhibition of this cycle in MCG.
Biosynthesis of the carotenoids
In the carotenoids biosynthesis pathway in MCG harvested at different months, 8 DEGs were screened (Figs. 3B and 4B). During the biosynthesis of β-carotene from geranylgeranyl diphosphate (GGPP), genes encoding 15-cis-phytoene synthase (crtB; August vs. September, p = 0.0273; September vs. October, p = 0.00184) were continuously downregulated from August to October, and the gene encoding lycopene β-cyclase (lycB) was highly expressed continuously at September and October. By comparing FPKM, it indicated that the biosynthesis of β-carotene maintained active during this period. Intriguingly, the subsequent transformation of β-carotene showed different inclinations at the last two months. At September, carlactone synthase (CCD8) and xanthoxin dehydrogenase (ABA2) genes were highly expressed, and violaxanthin de-epoxidase (VDE) and 9-cis-epoxycarotenoid dioxygenase (NCED) genes were highly expressed at October, suggesting that the β-carotene in MCG tended to biosynthesize strigol and xanthoxin at September and October, respectively.
Biosynthesis of the triterpenoids
A total of 9 DEGs were identified in the biosynthesis pathway of triterpenoids in MCG (Figs. 3 and 4 C). In the biosynthesis of squalene formed by FPP and then converted to 24-methylenelophenol and campesterol, all genes had a high expression level at September. These findings indicated that the biosynthesis of campesterol was activated only at September. From campesterol to brassinolide, steroid 5-α-reductase (DET2; August vs. September, p = 0.0464; September vs. October, p = 0.0109) genes were upregulated only at September, and the levels of brassinosteroid-6-oxidase 2 (CYP85A2) genes had a low expression at September, suggesting that 6-deoxocathasterone generated from campesterol could not be transferred to brassinolide, thus the biosynthesis of brassinolide was intervened since September. Surprisingly, the genes encoding sterol 22-desaturase (CYP710A) that catalyze the formation of stigmasterol from 24-methylenelophenol were highly expressed at September and October. Thus, it is likely that squalene favored to synthesize stigmasterol instead of campesterol during this period.
Biosynthesis of the other terpenoids
In the sesquiterpenoids biosynthesis pathway (Figs. 3D and 4D), of which 3 DEGs were identified, there are three main transformation directions of FPP: the synthesis of germacrene D by (-)-germacrene D synthase (GERD); the synthesis of valencene by valencene/7-epi-alpha-selinene synthase (TPS1); and the synthesis of solavetivone by vetispiradiene synthase (HVS). The encoding genes of these three enzymes were low expressed continuously, indicating that the biosynthesis of sesquiterpenoids in MCG was inhibited during August through October. Furthermore, the expressions of GERD and TPS1 were decreased at October, and the month of change was later than that of HVS, which means that the biosyntheses of solavetivone in MCG were first interfered compared to germacrene D and valencene.
GPP can biosynthesize (+)-neomenthol and (-)-α-terpineol by (+)-neomenthol dehydrogenase ((+)-ND) and (-)-α-terpineol synthase ((-)-α-TS), respectively. In this study (Figs. 3E and 4D), expression of the DEG encoding (+)-ND was the highest at October, while that of the DEG encoding (-)-α-TS was the opposite compared to those at the other months. These findings indicate that the biosynthesis of (-)-α-terpineol was intervened, and (+)-neomenthol was more easily biosynthesized by GPP in October.
The difference in the diterpenoids biosynthesis pathway (Figs. 3F and 4D) in MCG harvested at different months was reflected primarily in the gibberellin (GA) biosynthesis pathway. Three DEGs encoding gibberellin synthases, ent-kaurenoic acid hydroxylase (KAO), gibberellin 20-oxidase (GA20ox) and gibberellin 3-β-dioxygenase (GA3ox1), showed a high expression at September, while they were subsequently repressed at October. These results showed that the biosynthesis of GA was more active at September than October. Moreover, the expressions of gibberellin 2-oxidase (GA2ox) were gradually increased during these last two months. The role of GA2ox is to eliminate GA activity, which was consistent with the above results.
Changes of terpenoids metabolisms in MCG harvested at different ages
Biosynthesis of the terpenoids backbone
In the present study, 14 DEGs involved in IPP metabolism in MCG harvested at different ages were screened based on the KEGG database (Figs. 5 and 6 A). In the mevalonate pathway, acetyl-CoA C-acetyltransferase (ACAT), HMGS, HMGCR, and phosphomevalonate kinase (mvaK2) were identified in turn. Among them, the genes encoding the first three enzymes at the upstream of this pathway were highly expressed in 10- and 15-year-old MCG compared with those in 5-year-old MCG, whereas mvaK2 genes (5 years vs. 10 years, p = 0.501; 10 years vs. 15 years, p = 0.016) were downregulated only in 15-year-old MCG. In the non-mevalonate pathway, most of the genes were significantly upregulated in 10-year-old MCG compared to those in the other two ages. Next, the genes encoding GGPS (5 years vs. 10 years, p = 0.012; 10 years vs. 15 years, p = 0.882) were upregulated with the increase of ages of MCG. For the farnesyl cycle, the expression of the first three DEGs were increased in 10-year-old MCG and then were decreased in 15-year-old MCG, and the expression of β-protein farnesyltransferase (FNTB) genes in 10- and 15-year-old MCG was a little lower than that in 5-year-old MCG with no significant difference between them. The above results indicated that both the biosynthesis and transformation of IPP and the farnicalization of proteins were weakened with ages.
Terpenoids metabolism in MCG at different cultivation ages. (A) Terpenoids backbone biosynthesis, (B) Biosynthesis of the carotenoids, (C) Biosynthesis of the triterpenoids, (D) Biosynthesis of the sesquiterpenoids, (E) Biosynthesis of the monoterpenoids, and (F) Biosynthesis of the diterpenoids. (-)-α-TS, (-)-α-terpineol synthase; (+)-ND, (+)-neomenthol dehydrogenase; AAO3, abscisic-aldehyde oxidase; ACAT, acetyl-CoA C-acetyltransferase; CAS, cycloartenol synthase; CCD8, carlactone synthase; crtB, 15-cis-phytoene synthase; crtISO, prolycopene isomerase; CYP51, sterol 14-demethylase; CYP734A1, PHYB activation tagged suppressor 1; CYP85A2, brassinosteroid-6-oxidase 2; CYP90B1, steroid 22-α-hydroxylase; CYP90D1, 3-epi-6-deoxocathasterone-23-monooxygenase; DHCR24, δ-24-sterol reductase; DHCR7, 7-dehydrocholesterol reductase; DXR, 1-deoxy-D-xylulose-5-phosphate reductoisomerase; DXS, 1-deoxy-D-xylulose-5-phosphate synthase; FK, δ-14-sterol reductase; FNTB, β-protein farnesyltransferase; GA20ox, gibberellin 20-oxidase; GA2ox, gibberellin 2-oxidase. GA3ox1, gibberellin 3-β-dioxygenase; GERD, (-)-germacrene D synthase; GGPS, geranylgeranyl diphosphate synthase; HMGCR, hydroxymethylglutaryl-CoA reductase; HMGS, hydroxymethylglutaryl-CoA synthase; HVS, vetispiradiene synthase; ispD, 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase; ispE, 4-diphosphocytidyl-2-C-methyl-D-erythritol kinase; ispG, (E)-4-hydroxy-3-methylbut-2-enyl-diphosphate synthase; KAO, ent-kaurenoic acid hydroxylase; lcyB, lycopene β-cyclase; mvaK2, phosphomevalonate kinase; NCED, 9-cis-epoxycarotenoid dioxygenase; PCYOX1, prenylcysteine oxidase; RCE1, prenyl protein peptidase; SMO1, methylsterol monooxygenase 1; SMO2, methylsterol monooxygenase 2; SMT2, 24-methylenesterol-C-methyltransferase; SQLE, squalene monooxygenase; STE24, STE24 endopeptidase; VDE, violaxanthin de-epoxidase; ZEP, zeaxanthin epoxidase
Biosynthesis of the carotenoids
Totally 7 DEGs were screened in the biosynthesis pathway of carotenoids (Figs. 5B and 6B). There were no differences (p > 0.05) in genes expression between 5- and 10-year-old MCGs during the biosynthesis of β-carotene from GGPP. Compared to the 5-year-old MCG, genes encoding 15-cis-phytoene synthase (crtB) and prolycopene isomerase (crtISO) were low expressed, and genes encoding lcyB were highly expressed in the 15-year-old MCG. These findings showed that although the process of lycopene formed by GGPP was inhibited, the biosynthesis of β-carotene formed by lycopene was activated in the 15-year-old MCG. Subsequently, the expression levels of genes of the 10- and 15-year-old MCGs in the biosynthesis of violaxanthin converted by β-carotene were similar, which were lower than those of the 5-year-old MCG. In the biosynthesis of abscisic acid, NCED (5 years vs. 10 years, p = 0.00141; 10 years vs. 15 years, p = 0.00729) and abscisic-aldehyde oxidase (AAO3; 5 years vs. 10 years, p = 0.03; 10 years vs. 15 years, p = 0.043) genes were upregulated only in 10-year-old MCG. Furthermore, there was no difference (p > 0.05) between the two groups in the genes involved in the biosynthesis of strigol formed by β-carotene, suggesting that the biosynthesis of abscisic acid was intervened by longer cultivation years of MCG, while the biosynthesis of strigol was not affected.
Biosynthesis of the triterpenoids
A total of 12 DEGs were identified in the biosynthesis of triterpenoids (Figs. 5 and 6 C). Among the 8 DEGs that encode enzymes in catalyzing FPP to squalene and campesterol, the expression level of most genes reached the peak in the 10th year. Then, in the pathway from campesterol to brassinolide, the genes encoding steroid 22-α-hydroxylase (CYP90B1), 3-epi-6-deoxocathasterone-23-monooxygenase (CYP90D1) and CYP85A2 exhibited lower expression levels in the 10- and 15-year-old MCG when compared to those in the 5-year-old MCG. These results show that the biosynthesis of campesterol maintained a high activity and the biosynthesis of brassinolide was inhibited in the 10-year-old MCG, and both the biosynthesis of campesterol and brassinolide were intervened in the 15-year-old MCG.
Biosynthesis of the other terpenoids
In sesquiterpenoids and monoterpenoids biosynthesis pathways, 4 DEGs exhibited different expression trends (Fig. 5D and E, and 6D). The expression levels of genes encoding GERD and (+)-ND decreased with ages of MCG, indicating that the biosyntheses of germacrene D and (+)-neomenthol were attenuated in the 15-year-old MCG. HVS genes (5 years vs. 10 years, p = 0.0328; 10 years vs. 15 years, p = 0.932) were upregulated in the 10- and 15-year-old MCG compared with the 5-year-old MCG; however, there was no difference (p > 0.05) between 10- and 15-year-old MCG. (-)-α-TS genes showed the highest expression level in the 10-year-old MCG, suggesting the accumulation of solavetivone and (-)-α-terpineol might continuously increase. In the pathway of diterpenoids biosynthesis (Figs. 5F and 6D), except for the high expression of the genes encoding GA3ox1 and no changes of the GA20ox genes, other genes were downregulated in the 15-year-old MCG compared to those in the 5-year-old MCG, indicating that the biosynthesis of GA was more active in the 5-year-old MCG than in the 10- and 15-year-old MCG.
Changes of carbohydrate metabolism in MCG
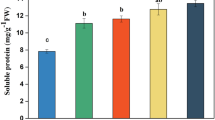
As shown in Figs. 7 and 8 A, 20 DEGs related to carbohydrate metabolism were identified at different harvest months. In the process of starch degradation, the expression of BMY genes in October were higher than those at the other two months, indicating that starch trended to degraded into glucose with the increase of months. The low expression of genes associated with sucrose degradation and biosynthesis indicated that the harvest months had a weak effect on sucrose. In the process of glucose metabolism, most DEGs had a high expression level with the increase of months. Most of all, the rate limiting enzyme genes, pyruvate kinase (PK), can directly affect the biosynthesis of pyruvate. These results indicate that the harvest months of MCG accelerated carbohydrate metabolism to degrade pyruvate into the tricarboxylic acid cycle. There is no obvious rule for the changes of carbohydrate metabolisms in different cultivation years of MCG (Figs. 7B and 8B), and the key rate limiting enzyme genes, 6-phosphofructokinase (PFK), hexokinase (HK), and PK had a low expression level, indicating that there is little correlation between the cultivation years monitored and carbohydrate metabolisms.
Carbohydrate metabolism in MCG at different harvest months (A) and cultivation years (B). ALDO, fructose-bisphosphate aldolase; AMY, alpha-amylase; BMY, beta-amylase. ENO, enolase; PK, pyruvate kinase; FBP, fructose-1,6-bisphosphatase I; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; GPI, glucose-6-phosphate isomerase; HK, hexokinase; INV, beta-fructofuranosidase; PFK, 6-phosphofructokinase; PGK, phosphoglycerate kinase; SPS, sucrose-phosphate synthase; SUS, sucrose synthase
Discussion
Ginseng root contains considerable amounts of natural products with nutritional value, such as terpenoids and polysaccharides. Terpenoids represent the largest family of over 22,000 individual compounds endowed with physiological, defense, and structural functions in plants. Researchers have focused on the health benefits and economic value of the terpenoids contained in ginseng, but little is known about their role in plant metabolism and regulation (Zhou and Pichersky 2020). All terpenoids begin with two universal five-carbon isoprene-like building blocks, IPP and its isomer dimethylallyl diphosphate (DMAPP). In plants, IPP and DMAPP are synthesized in at least two different ways, the mevalonic acid (MVA) with acetyl-CoA as precursor and the methylerythritol phosphate (MEP) pathways with glyceraldehyde 3-phosphate as precursor. While the MVA pathway distributes between cytoplasm, endoplasmic reticulum, and peroxisomes, the MEP pathway locates exclusively in plastids. IPP and DMAPP combine to form a larger amount of terpenoids. First, they react to give geranyl diphosphate (GPP), the 10-carbon precursor of nearly all monoterpenoids. GPP can then link to IPP to form FPP and GGPP, precursors of nearly all the sesquiterpenoids and diterpenoids, respectively. Finally, FPP and GGPP can dimerize to give triterpenoids and tetraterpenes, respectively (Henry et al. 2018). Typical terpenoids, including gibberellin, abscisic acid, brassinolide, and so on, are susceptible to seasonal changes and years of cultivation. However, few studies have reported the relationship between terpenoids metabolism in ginseng root, especially those cultivated in the mountainous, and harvest months and cultivation years. To fill this gap, the above transcriptome analysis was conducted to explore the varying levels of terpenoids metabolism in MCG with different harvest months and cultivation years.
The harvest month affected the terpenoids metabolisms in MCG and its quality
In this study, the synthesis pathways of germacrene and valencene were more active in August than in September and October, indicating that metabolism of sesquiterpenoids was significantly affected by seasonal changes. It has been reported that the content of germacrene decreased gradually from autumn to winter (Victorio et al. 2018). Kpoviessi et al. (2011) found that the content of valencene in Sclerocarya birrea leaves was higher in summer than in winter. These findings were consistent with ours (Figs. 3D and 4D). For solavetivone, which is a sesquiterpenoids with strong antifungal activity (Yokose et al. 2004), the change trend at different harvest months was similar to those of germacrene and valencene. In addition, the genes encoding synthetases of neomenthol and terpineol were upregulated and downregulated only in October compared to August and September in the MCG, respectively. These findings indicate that monoterpenes always respond to seasonal changes slightly later than sesquiterpenes. In a previous study, it has been confirmed that the contents of neomenthol in Mentha × piperita L (Grulova et al. 2015) and terpineol in Zanthoxylum clava-herculis (Eiter et al. 2010) increased and decreased in autumn and winter, respectively, which were also consistent with our findings (Figs. 3E and 4D).
In general, the synthesis pathway of GA will be inhibited, and thus the growth rate of plants will also be accordingly weakened from summer to autumn. The expression of GA2ox which deactivates GA hydroxyl group (He et al. 2019) was upregulated from September to October in MCG, which also verified this view well (Figs. 3F and 4D). Intriguingly, the expressions of GA20ox and GA3ox1 with the function of promoting GA synthesis in MCG were highest in September, indicating that the GA synthesis was more active in September than in both August and October, which may be caused by the temperature. The temperature in the northeast of China is usually high in August every year, and the growth of MCG was inhibited to respond the high temperature via downregulating the biosynthesis of GA. This also explains one of the reasons why MCG does not like high temperature or is not suitable for high temperature survival. In September, the biosynthesis of GA was upregulated with the decrease of temperature, resulting in the MCG growth again. When the temperature drops below 12℃ in October, GA biosynthesis decreases again and MCG stops growing in preparation for winter dormancy (Derkx and Karssen 1994). Strigolactones, a derivative of strigol, have been widely reported that it has the function of inducing the development of the lateral root and promoting the elongation of the root hair (Brewer et al. 2013). The biosynthesis of strigol in MCG was activated when GA promoted its growth in September (Figs. 3B and 4B). Meanwhile, the biosynthesis of abscisic acid in October was also enhanced to accelerate the MCG to become dormancy, which is consistent with the study of Ma et al. (2018) on abscisic acid promoting plant dormancy.
It is well known that brassinolide can improve the tolerance of plants to high temperature (Li et al. 2018) and stimulate the division and elongation of plants cell (Fridman and Savaldi-Goldstein 2013). In this study, the biosynthesis of brassinolide formed by campesterol in MCG was activated to resist the damage by high temperature in August, and was inhibited when the stem and leaves begin to wither in September (Figs. 3 and 4 C). Surprisingly, squalene flowed to the direction of stigmasterol biosynthesis instead of campesterol in October. One of the reasons was that a low level of brassinolide could enhance the cold tolerance of plants (Kim et al. 2010), which meant that the decreased brassinolide biosynthesis in October contributed to adapting to the cold environment. On the other hand, sterol on cell membrane was transformed into stigmasterol with stronger molecular cohesion to improve the cold tolerance of plants by reducing the fluidity of membrane lipid in low temperature stress (Dufourc 2008). Piispanen and Saranpaa (2004) proved that the level of (3β)-Stigmast-5-en-3ol, one of the stigmasterol homologues, was unambiguously increased from July (20℃) to November (-4℃) in the silver birch in Finland, which is consistent with our findings. All the above findings indicated that the harvest month affects the terpenoids metabolisms in MCG and its quality.
Age affected the terpenoids metabolisms in MCG and its quality
On the basis of the transcriptome analysis, the biosynthesis of terpenoids in MCG was significantly affected by the cultivation years or ages, except that there was no significant difference in the biosynthesis of abscisic acid. As shown in Figs. 5E and 6D, the level of (+)-ND was higher in 5-year-old MCG, and (-) α-TS had a higher level in 10- and 15-year-old MCG. These findings indicate that the content of neomenthol was decreased with increasing years of cultivation, while that of terpineol was the opposite. Meanwhile, the change trend of the genes encoding germacrene synthase in MCG was similar to that of neomenthol. Rini et al. (2012) found that the content of terpineol was increased in 10- and 15-year-old Leucadendron linn. leaf compared to the 5th year using GC/MS analysis. Petronilho et al. (2013) identified 100 terpenoids in Callistemon citrinus of different cultivation years, and found that the monoterpenoids and sesquiterpenoids, represented by neomenthol and germacrene, respectively, were accumulated in plants with low cultivation years. These findings are consistent with ours. The expression of HVS that can synthesize solavetivone was high in 10- and 15-year-old MCG in this study (Figs. 5D and 6D), leading to that 40% of the IPP generated through the MVA pathway was used for the biosynthesis of sterols under normal growth conditions. Whereas, the biosynthesis of sterols is blocked when plants are stressed by pathogens, and half of IPP used for sterol biosynthesis is converted to sesquiterpenoids (Takahashi et al. 2007). In view of the fact that the species of pathogenic fungi in the soil where ginseng was planted was increased with the growth of cultivation years (Xiao et al. 2016), we speculate that the high expressions of HVS in 10- and 15-year-old MCG were possibly associated with the adaptability of MCG to pathogens.
Both GA and brassinolide can promote the growth and development of plants (Sheng et al. 2022). In the 5-year-old MCG (Fig. 5C F 6 C and 6D), the biosyntheses of GA and brassinolide were more active than those of the other two groups, which corresponded to the characteristics of vigorous growth in plants of young ages. Chen et al. (2018) reported that the content of campesterol in 13-, 15-, and 20-year-old ginseng was reduced with ages based on a metabolomic approach. Similarly, the expressions of a large number of genes related to biosynthesis of campesterol in 15-year-old MCG were significantly reduced compared to those in 10-year-old MCG (Figs. 5 and 6 C), indicating that the biosynthesis of campesterol was gradually restrained during 10 to 15 years of cultivation. These findings indicated that age affects the terpenoids metabolisms in MCG and its quality.
The harvest month affected the carbohydrates metabolisms in MCG
Carbohydrates metabolisms involve the energy metabolism, homeostasis, and the linkage of various physiological functions, which is of great significant to plants. In the previous study, the polysaccharides of ginseng root have been well explored (Sun et al. 2022). However, the understanding of the changes of carbohydrates metabolisms in MCG with harvest month and cultivation year is lacking. In the current study (Fig.s 7 and 8), the delay in harvest months of MCG accelerated carbohydrates metabolisms to produce specific substrates of the tricarboxylic acid cycle, such as pyruvate, which facilitates access to energy for an organism. Ouyang et al. (2019) found that starch decreased from November towards January in all four cultivars, which is consistent with our results. Whereas, there is a little correlation between cultivation years or ages and carbohydrates metabolisms. These findings showed that the seasonal growth environment has greater influence on carbohydrates metabolisms.
Conclusions
To sum up, the biosyntheses of terpenoids, such as monoterpenoids, sesquiterpenoids, diterpenoids, triterpenoids, and carotenoids, in MCG at different harvest months and at different ages were significantly different. Specifically, the biosyntheses of terpineol, valencene, germacrene, solavetivone, and brassinolide were more active in August. The biosynthesis of valencene and brassinolide were inhibited, and the biosyntheses of GA, campesterol, and strigol gradually became active in September. Terpenoids metabolism was repressed in October, except the biosyntheses of neomenthol, stigmasterol, and abscisic acid. Meanwhile, one of the reasons why MCG does not like high temperature or not suitable for high temperature survival were explained. For MCG at different ages, it was found that the biosyntheses of neomenthol, germacrene, GA, and brassinolide at the 5th year were more active. In the 10-year-old MCG, only the biosyntheses of terpineol, solavetivone, and campesterol were activated. Surprisingly, all the above pathways associated with terpenoids metabolisms were inhibited in the 15-year-old MCG. In the process of carbohydrates metabolisms, the seasonal growth environment had greater influence. Whereas, there was little correlation between cultivation years or ages and carbohydrates metabolisms. Understanding the relationships between terpenoids metabolisms in MCG, and harvest months and/or cultivation years will improve our understanding of the complicated but important biosynthesis and regulation of terpenoids in plants.
References
Ahanger MA, Bhat JA, Siddiqui MH, Rinklebe J, Ahmad P (2020) Integration of silicon and secondary metabolites in plants: a significant association in stress tolerance. J Exp Bot 71(21):6758–6774. https://doi.org/10.1093/jxb/eraa291
Brewer PB, Koltai H, Beveridge CA 2013 diverse roles of strigolactones in plant development. Mol Plant 6(1):18–28. https://doi.org/10.1093/mp/sss130
Chen J, Yuan Y, Ran X, Guo N, Dou D (2018) Metabolomics analysis based on a UPLC-Q-TOF-MS metabolomics approach to compare Lin-Xia-Shan-Shen and garden ginseng. RSC Adv 8(53):30616–30623. https://doi.org/10.1039/c8ra04823a
Chen YJ, Lin CY, Hsu HW, Yeh CY, Chen YH, Yeh TF, Chang ST (2019) Seasonal variations in emission rates and composition of terpenoids emitted from Chamaecyparis formosensis (Cupressaceae) of different ages. Plant Physiol Biochem 142:405–414. https://doi.org/10.1016/j.plaphy.2019.08.002
Cheng G, Zhou S, Zhang J, Huang X, Bai X, Xie T, Guo R, Liu J, Yu H, Xie L (2019) Comparison of transcriptional expression patterns of phenols and carotenoids in ‘Kyoho’ grapes under a two-crop-a-year cultivation system. PLoS ONE 14(1):e0210322. https://doi.org/10.1371/journal.pone.0210322
Derkx MPM, Karssen CM (1994) Are seasonal dormancy patterns in Arabidopsis thaliana regulated by changes in seed sensitivity to light, nitrate and Gibberellin? Ann Bot 73(2):129–136. https://doi.org/10.1006/anbo.1994.1015
Dufourc EJ (2008) The role of phytosterols in plant adaptation to temperature. Plant signal Behave 3(2):133–134. https://doi.org/10.4161/psb.3.2.5051
Eiter LC, Fadamiro H, Setzer WN (2010) Seasonal variation in the leaf essential oil composition of Zanthoxylum clava-herculis growing in Huntsville, Alabama. Nat Prod Commun 5(3):457–460. https://doi.org/10.1177/1934578x1000500323
Fan H, Li K, Yao F, Sun L, Liu Y (2019) Comparative transcriptome analyses on terpenoids metabolism in field- and mountain-cultivated ginseng roots. BMC Plant Bio 19(1):82. https://doi.org/10.1186/s12870-019-1682-5
Fang X, Wang M, Zhou X, Wang H, Wang H, Xiao H (2022) Effects of growth years on ginsenoside biosynthesis of wild ginseng and cultivated ginseng. BMC Genomics 23(1):325. https://doi.org/10.1186/s12864-022-08570-0
Fridman Y, Savaldi-Goldstein S (2013) Brassinosteroids in growth control: how, when and where. Plant Sci 209:24–31. https://doi.org/10.1016/j.plantsci.2013.04.002
Grulova D, De Martino L, Mancini E, Salamon I, De Feo V (2015) Seasonal variability of the main components in essential oil of Mentha × piperita L. J Sci food Agr 95(3):621–627. https://doi.org/10.1002/jsfa.6802
He H, Liang G, Lu S, Wang P, Liu T, Ma Z, Zuo C, Sun X, Chen B, Mao J (2019) Genome-wide identification and expression analysis of GA2ox, GA3ox, and GA20ox are related to Gibberellin oxidase genes in grape (VitisVinifera L). Genes 10(9):680. https://doi.org/10.3390/genes10090680
He B, Chen D, Zhang X, Yang R, Yang Y, Chen P, Shen Z (2022) Oxidative stress and ginsenosides: an update on the Molecular Mechanisms. Oxid Med Cell Longev 9299574. https://doi.org/10.1155/2022/9299574
Henry LK, Thomas ST, Widhalm JR, Lynch JH, Davis TC, Kessler SA, Bohlmann J, Noel JP, Dudareva N (2018) Contribution of isopentenyl phosphate to plant terpenoid metabolism. Nat plants 4(9):721–729. https://doi.org/10.1038/s41477-018-0220-z
Huang LM, Huang H, Chuang YC, Chen WH, Wang CN, Chen HH (2021a) Evolution of terpene synthases in Orchidaceae. Int J Mol Sci 22(13):6947. https://doi.org/10.3390/ijms22136947
Huang S, Dong T, Xion B, Qiu X, Wang Z (2021b) Variation in the content and composition of limonoids in fruits of four pomelo varieties during fruit development: the natural debittering process in pomelo fruits. J Food Compost Anal 100(2):103928. https://doi.org/10.1016/j.jfca.2021b.103928
Jayakodi M, Choi BS, Lee SC, Kim NH, Park JY, Jang W, Lakshmanan M, Mohan S, Lee DY, Yang TJ (2018) Ginseng genome database: an open-access platform for genomics of Panax ginseng. BMC Plant Bio 18(1):62. https://doi.org/10.1186/s12870-018-1282-9
Kim SY, Kim BH, Lim CJ, Lim CO, Nam KH (2010) Constitutive activation of stress-inducible genes in a brassinosteroid-insensitive 1 (bri1) mutant results in higher tolerance to cold. Physiol Plant 138(2):191–204. https://doi.org/10.1111/j.1399-3054.2009.01304.x
Kim D, Langmead B, Salzberg SL (2015) HISAT: a fast spliced aligner with low memory requirements. Nat methods 12(4):357–360. https://doi.org/10.1038/nmeth.3317
Kpoviessi DSS, Gbaguidi FA, Kossouoh C, Agbani P, Yayi-Ladekan E, Sinsin B, Moudachirou M, Accrombessi GC, Quetin-Leclercq J (2011) Chemical composition and seasonal variation of essential oil of Sclerocarya birrea (A. Rich.) Hochst subsp birrea leaves from Benin. J Med Plants Res 5(18):4640–4646. https://doi.org/10.5897/JMPR.9000348
Li X, Wei JP, Ahammed GJ, Zhang L, Li Y, Yan P, Zhang LP, Han WY (2018) Brassinosteroids Attenuate Moderate High Temperature-Caused decline in Tea Quality by enhancing Theanine Biosynthesis in Camellia sinensis L. Front Plant Sci 9:1016. https://doi.org/10.3389/fpls.2018.01016
Liu Z, Wang CZ, Zhu XY, Wan JY, Zhang J, Li W, Ruan CC, Yuan CS (2017) Dynamic changes in Neutral and Acidic Ginsenosides with different cultivation years and Harvest Seasons: identification of Chemical characteristics for Panax ginseng Quality Control. Molecules 22(5):734. https://doi.org/10.3390/molecules22050734
Ma Y, Cao J, He J, Chen Q, Li X, Yang Y (2018) Molecular mechanism for the regulation of ABA homeostasis during Plant Development and stress responses. Int J Mol Sci 19(11):3643. https://doi.org/10.3390/ijms19113643
Ouyang L, Leus L, De Keyser E, Van Labeke MC (2019) Seasonal changes in cold hardiness and carbohydrate metabolism in four garden rose cultivars. J Plant Physiol 232:188–199. https://doi.org/10.1016/j.jplph.2018.12.001
Papanastasiou SA, Ioannou CS, Papadopoulos NT (2020) Oviposition-deterrent effect of linalool - a compound of citrus essential oils - on female Mediterranean fruit flies, Ceratitis capitata (Diptera: Tephritidae). Pest Manag Sci 76(9):3066–3077. https://doi.org/10.1002/ps.5858
Pertea M, Pertea GM, Antonescu CM, Chang TC, Mendell JT, Salzberg SL (2015) StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol 33(3):290–295. https://doi.org/10.1038/nbt.3122
Petronilho S, Rocha SM, Ramirez-Chavez E, Molina-Torresb J, Rios-Chavez P (2013) Assessment of the terpenic profile of Callistemon citrinus (curtis) skeels from Mexico. Ind Crop Prod 46:369–379. https://doi.org/10.1016/j.indcrop.2013.02.012
Piispanen R, Saranpää P (2004) Seasonal and within-stem variations of neutral lipids in silver birch (Betula pendula) wood. Tree physiol 24(9):991–999. https://doi.org/10.1093/treephys/24.9.991
Rini P, Ohtani Y, Ichiura H (2012) Antioxidant, anti-hyaluronidase and antifungal activities of Melaleuca leucadendron Linn. Leaf oils. J Wood Sci 58(5):429–436. https://doi.org/10.1007/s10086-012-1270-x
Shan SM, Luo JG, Huang F, Kong LY (2014) Chemical characteristics combined with bioactivity for comprehensive evaluation of Panax ginseng C.A. Meyer in different ages and seasons based on HPLC-DAD and chemometric methods. J Pharmaceut Biomed 89:76–82. https://doi.org/10.1016/j.jpba.2013.10.030
Sheng J, Li X, Zhang D (2022) Gibberellins, brassinolide, and ethylene signaling were involved in flower differentiation and development in Nelumbo nucifera. Hortic Plant J 8(2):243–250. https://doi.org/10.1016/j.hpj.2021.06.002
Shi W, Wang Y, Li J, Zhang H, Ding L (2007) Investigation of ginsenosides in different parts and ages of Panax ginseng. Food Chem 102(3):664–668. https://doi.org/10.1016/j.foodchem.2006.05.053
Son M, Hart SM, Schlau-Cohen GS (2021) Investigating carotenoid photophysics in photosynthesis with 2d electronic spectroscopy. Trends Chem 3(9). https://doi.org/10.1016/j.trechm.2021.05.008
Sun J, Zhong X, Sun D, Cao X, Yao F, Shi L, Liu Y (2022) Structural characterization of polysaccharides recovered from extraction residue of ginseng root saponins and its fruit nutrition preservation performance. Front Nutr 9:934927. https://doi.org/10.3389/fnut.2022.934927
Takahashi S, Yeo YS, Zhao Y, O’Maille PE, Greenhagen BT, Noel JP, Coates RM, Chappell J (2007) Functional characterization of premnaspirodiene oxygenase, a cytochrome P450 catalyzing regio- and stereo-specific hydroxylations of diverse sesquiterpene substrates. J Biol Chem 282(43):31744–31754. https://doi.org/10.1074/jbc.M703378200
Tassoni A, Zappi A, Melucci D, Reisch BI, Davies PJ (2019) Seasonal changes in amino acids and phenolic compounds in fruits from hybrid cross populations of american grapes differing in disease resistance. Plant Physiol Biochem 135:182–193. https://doi.org/10.1016/j.plaphy.2018.11.034
Victorio CP, de Azevedo AC, Da Silveira EGP, Marcelo da CS, Sato A, Gama PE, Bizzo HR, Arruda RCO (2018) Leaf essential oils and volatiles, histochemistry and micromorphology of Neomitranthes obscura (DC.) N. Silveira (Myrtaceae) growing in sandy coastal plains of Rio de Janeiro. Biochem Sys Ecol 78:66–76. https://doi.org/10.1016/j.bse.2018.03.010
Wang Y, Pan JY, Xiao XY, Lin RC, Cheng YY (2006) Simultaneous determination of ginsenosides in Panax ginseng with different growth ages using high-performance liquid chromatography-mass spectrometry. Phytochem Anal 17(6):424–430. https://doi.org/10.1002/pca.944
Xiao C, Yang L, Zhang L, Liu C, Han M (2016) Effects of cultivation years and modes on microbial diversity in the rhizosphere soil of Panax ginseng. J Ginseng Res 40(1):28–37. https://doi.org/10.1016/j.jgr.2015.04.004
Xu Q, He Y, Yan X, Zhao S, Zhu J, Wei C (2018) Unraveling a crosstalk regulatory network of temporal aroma accumulation in tea plant (Camellia sinensis) leaves by integration of metabolomics and transcriptomics. Environ Exp Bot 149:81–94. https://doi.org/10.1016/j.envexpbot.2018.02.005
Yao F, Xue Q, Li K, Cao X, Sun L, Liu Y (2019) Phenolic Compounds and Ginsenosides in Ginseng shoots and their antioxidant and anti-inflammatory capacities in LPS-Induced RAW264.7 mouse macrophages. Int J Mol Sci 20(12):2951. https://doi.org/10.3390/ijms20122951
Yokose T, Katamoto K, Park S, Matsuura H, Yoshihara T (2004) Anti-fungal sesquiterpenoid from the root exudate of Solanum abutiloides. Biosci Biotech Bioche 68(12):2640–2642. https://doi.org/10.1271/bbb.68.2640
Yuan Y, Zuo J, Zhang H, Li R, Yu M, Liu S (2022) Integration of Transcriptome and Metabolome provides New Insights to Flavonoids Biosynthesis in Dendrobium huoshanense. Front Plant Sci 13:850090. https://doi.org/10.3389/fpls.2022.850090
Zhou F, Pichersky E (2020) More is better: the diversity of terpene metabolism in plants. Curr Opin Plant Biol 55:1–10. https://doi.org/10.1016/j.pbi.2020.01.005
Acknowledgements
This work was financially supported by the special funds for Forestry Public Welfare Scientific Research Projects [No. 201404718], China.
Author information
Authors and Affiliations
Contributions
Jing Sun and Hang Fan, conceptualization, methodology, investigation, writing - original draft; Dandan Sun, investigation, software; Xinyu Zhong, investigation; Liren Xu, software; Kangxin Hou, investigation; Xiaohong Zhou, validation; Donglin Fu, validation; Lingling Shi and Yujun Liu; conceptualization, writing - review & editing, supervision.
Corresponding authors
Ethics declarations
Conflict of interest
The authors state that there is no conflict of interest.
Additional information
Communicated by Kalina Ananieva.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sun, J., Fan, H., Sun, D. et al. Dynamic changes in terpenoids metabolisms of mountain-cultivated ginseng harvested at different months and ages. Plant Growth Regul 101, 473–487 (2023). https://doi.org/10.1007/s10725-023-01035-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-023-01035-8