Abstract
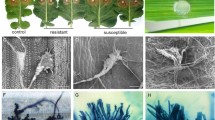
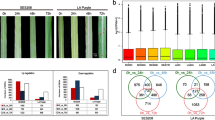
Brassica napus is one of the most important oilseed crops in the world, yet there are many challenges to its potential production. Sclerotinia sclerotiorum, which causes Sclerotinia stem rot, is a major fungal disease that reduces seed yield and oil quality. Research has been conducted to understand the genetic basis of resistance to Sclerotinia stem rot, with the majority of studies based on the identification of single or few resistant genes for disease control. The aim of this study was to identify genes involved in horizontal resistance to achieve a stable resistance response by investigating the key pathways adopted by the resistant lines in response to Sclerotinia stem rot. Therefore, we conducted comparative transcriptomic and metabolomic profiling to identify overlapping pathways in gene expression and biosynthesis. The resistance response was activated with the induction of the biosynthesis of amino acids and the biosynthesis of secondary metabolites in the resistant line. Early induction of phenylpropanoid biosynthesis at 24 hours post inoculation and arginine biosynthesis at three time points, i.e., 24, 48, and 96 hours post inoculation, induced specificity in resistant line. Glucosinolate biosynthesis, flavonoid biosynthesis, and the alanine, aspartate, and glutamate metabolism pathways were induced in the resistant line in our transcriptomics and metabolomics study. The genes for ethylene, salicylic acid, and jasmonic acid were also highly induced in the resistant line compared to the susceptible line based on the annotation of biotic stress genes using MapMan analysis. Our results may allow for the achievement of horizontal resistance and provide insight to breeders developing resistant varieties.









Similar content being viewed by others
References
Aliferis KA, Faubert D, Jabaji S (2014) A metabolic profiling strategy for the dissection of plant defense against fungal pathogens. PLoS ONE 9:e111930
Bar-Even A, Noor E, Lewis NE, Milo R (2010) Design and analysis of synthetic carbon fixation pathways. Proc Natl Acad Sci 107:8889
Barrientos-Moreno L, Molina-Henares MA, Pastor-García M, Ramos-González MI, Espinosa-Urgel M (2019) Arginine biosynthesis modulates pyoverdine production and release in Pseudomonas putida as part of the mechanism of adaptation to oxidative stress. J Bacteriol 201:e00419-e0454
Castro-Moretti FR, Gentzel IN, Mackey D, Alonso AP (2020) Metabolomics as an emerging tool for the study of plant-pathogen interactions. Metabolites 10:52
Chalhoub B, Denoeud F, Liu S, Parkin IA, Tang H, Wang X, Chiquet J, Belcram H, Tong C, Samans B (2014) Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science 345:950–953
De La Torre F, Cañas RA, Pascual MB, Avila C, Cánovas FM (2014) Plastidic aspartate aminotransferases and the biosynthesis of essential amino acids in plants. J Exp Bot 65:5527–5534
Derbyshire MC, Denton-Giles M (2016) The control of sclerotinia stem rot on oilseed rape (Brassica napus): current practices and future opportunities. Plant Pathol 65:859–877
Ducat DC, Silver PA (2012) Improving carbon fixation pathways. Curr Opin Chem Biol 16:337–344
Friedt W, Tu J, Fu T (2018) Academic and economic importance of Brassica napus rapeseed. In: Liu S, Snowdon R, Chalhoub B (eds) The Brassica napus genome. Springer, Cham, pp 1–20
Girard IJ, Tong C, Becker MG, Mao X, Huang J, De Kievit T, Fernando WGD, Liu S, Belmonte MF (2017) RNA sequencing of Brassica napus reveals cellular redox control of Sclerotinia infection. J Exp Bot 68:5079–5091
Halkier BA, Gershenzon J (2006) BIOLOGY AND BIOCHEMISTRY OF GLUCOSINOLATES. Annu Rev Plant Biol 57:303–333
Hildebrandt TM, Nunesnesi A, Araújo WL, Braun HP (2015) Amino acid catabolism in plants. Mol Plant 8:1563–1579
Hong J, Yang L, Zhang D, Shi J (2016) Plant metabolomics: an indispensable system biology tool for plant science. Int J Mol Sci 17:767
Kanehisa M (2016) KEGG bioinformatics resource for plant genomics and metabolomics. Methods Mol Biol 1374:55–70
Kanehisa M, Furumichi M, Tanabe M, Sato Y, Morishima K (2016) KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res 45:D353–D361
Kostyn K, Czemplik M, Kulma A, Bortniczuk M, Skała J, Szopa J (2012) Genes of phenylpropanoid pathway are activated in early response to Fusarium attack in flax plants. Plant Sci 190:103–115
Maramorosch K, Loebenstein G (2009) Plant disease resistance: natural, non-host innate or inducible. In: Schaechter M (ed) Encyclopedia of microbiology, 3rd edn. Academic Press, Oxford, pp 589–596
Martínez G, Regente M, Jacobi S, Del Rio M, Pinedo M, De La Canal L (2017) Chlorogenic acid is a fungicide active against phytopathogenic fungi. Pestic Biochem Physiol 140:30–35
Moselhy SS, Asami T, Abualnaja KO, Al-Malki AL, Yamano H, Akiyama T, Wada R, Yamagishi T, Hikosaka M, Iwakawa J, Okada K, Mori M, Kumosani TA (2016) Spermidine, a polyamine, confers resistance to rice blast. J Pestic Sci 41:79–82
Obermeier C, Hossain MA, Snowdon R, Knüfer J, Von Tiedemann A, Friedt W (2013) Genetic analysis of phenylpropanoid metabolites associated with resistance against Verticillium longisporum in Brassica napus. Mol Breeding 31:347–361
Qasim MU, Zhao Q, Shahid M, Samad RA, Ahmar S, Wu J, Fan C, Zhou Y (2020) Identification of QTLs containing resistance genes for Sclerotinia Stem Rot in Brassica napus using comparative transcriptomic studies. Front Plant Sci 11:776
Qiu X-M, Sun Y-Y, Ye X-Y, Li Z-G (2020) Signaling role of glutamate in plants. Front Plant Sci. https://doi.org/10.3389/fpls.2019.01743
Ranjan A, Westrick NM, Jain S, Piotrowski JS, Ranjan M, Kessens R, Stiegman L, Grau CR, Conley SP, Smith DL, Kabbage M (2019) Resistance against Sclerotinia sclerotiorum in soybean involves a reprogramming of the phenylpropanoid pathway and up-regulation of antifungal activity targeting ergosterol biosynthesis. Plant Biotechnol J 17:1567–1581
Seifbarghi S, Borhan MH, Wei Y, Coutu C, Robinson SJ, Hegedus DD (2017) Changes in the Sclerotinia sclerotiorum transcriptome during infection of Brassica napus. BMC Genom 18:266
Suharti WS, Nose A, Zheng S-H (2016) Metabolite profiling of sheath blight disease resistance in rice: in the case of positive ion mode analysis by CE/TOF-MS. Plant Prod Sci 19:279–290
Sun H, Song N, Ma L, Li J, Ma L, Wu J, Wu J (2017) Ethylene signalling is essential for the resistance of Nicotiana attenuata against Alternaria alternata and phytoalexin scopoletin biosynthesis. Plant Pathol 66:277–284
Wei L, Jian H, Lu K, Filardo F, Yin N, Liu L, Qu C, Li W, Du H, Li J (2016) Genome-wide association analysis and differential expression analysis of resistance to Sclerotinia stem rot in Brassica napus. Plant Biotechnol J 14:1368–1380
Wen B, Mei Z, Zeng C, Liu S (2017) metaX: a flexible and comprehensive software for processing metabolomics data. BMC Bioinform 18:183
Winter G, Todd CD, Trovato M, Forlani G, Funck D (2015) Physiological implications of arginine metabolism in plants. Front Plant Sci. https://doi.org/10.3389/fpls.2015.00534
Wu J, Zhao Q, Yang Q, Liu H, Li Q, Yi X, Cheng Y, Guo L, Fan C, Zhou Y (2016) Comparative transcriptomic analysis uncovers the complex genetic network for resistance to Sclerotinia sclerotiorum in Brassica napus. Sci Rep 6:19007
Yang B, Srivastava S, Deyholos MK, Kav NNV (2007) Transcriptional profiling of canola (Brassica napus L.) responses to the fungal pathogen Sclerotinia sclerotiorum. Plant Sci 173:156–171
Zeier J (2013) New insights into the regulation of plant immunity by amino acid metabolic pathways. Plant Cell Environ 36:2085–2103
Zhang H, Stephanopoulos G (2016) Co-culture engineering for microbial biosynthesis of 3-amino-benzoic acid in Escherichia coli. Biotechnol J 11:981–987
Zhang Y, Shi H, Liang S, Ning G, Xu N, Lu J, Liu X, Lin F (2015) MoARG1, MoARG5,6 and MoARG7 involved in arginine biosynthesis are essential for growth, conidiogenesis, sexual reproduction, and pathogenicity in Magnaporthe oryzae. Microbiol Res 180:11–22
Zhao J, Buchwaldt L, Rimmer SR, Sharpe A, Mcgregor L, Bekkaoui D, Hegedus D (2009) Patterns of differential gene expression in Brassica napus cultivars infected with Sclerotinia sclerotiorum. Mol Plant Pathol 10:635–649
Funding
This research was financially supported by the funding from the Ministry of Science and Technology of China (2017YFE0104800) and the Natural Science Foundation of China (31671725).
Author information
Authors and Affiliations
Contributions
MQ and YZ designed the study. MQ, MS and QZ performed the experiments. GC, HH, GL and CF helped in data analysis. MQ and YZ wrote the manuscript. YZ supervised the project. All the authors read and revised the manuscript.
Corresponding author
Additional information
Communicated by Daolong Dou.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qasim, M.U., Zhao, Q., Shahid, M. et al. Overlapping pathways involved in resistance against Sclerotinia stem rot in Brassica napus revealed through transcriptomic and metabolomic profiling. Plant Growth Regul 102, 297–312 (2024). https://doi.org/10.1007/s10725-023-00998-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-023-00998-y