Abstract
In recent decades, the demand for vegetables has increased significantly due to the blooming global population. Climate change has affected vegetable production by increasing the frequencies and severity of abiotic and biotic stresses. Among the abiotic stresses, drought and salinity are the major issues that possess severe threats on vegetable production. Many vegetables (e.g., carrot, tomato, okra, pea, eggplant, lettuce, potato) are usually sensitive to drought and salt stress. The defence mechanisms of plants against salt and drought stress have been extensively studied in model plant species and field crops. Better understanding of the mechanisms of susceptibility of vegetables to drought and salt stresses will help towards the development of more tolerant genotypes as a long-term strategy against these stresses. However, the intensity of the challenges also warrants more immediate approaches to mitigate these stresses and enhance vegetable production in the short term. Therefore, this review enlightens the updated knowledge of responses (physiological and molecular) against drought and salinity in vegetables and potentially effective strategies to enhance production. Moreover, we summarized different technologies such as seed priming, genetic transformation, biostimulants, nanotechnology, and cultural practices adopted to enhance vegetable production under drought and salinity stress. We propose that approaches of conventional breeding, genetic engineering, and crop management should be combined to generate drought and salt resistance cultivars and adopt smart cultivation practices for sustainable vegetable production in a changing climate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate change is increasingly threatening agricultural production via increasing incidents and severity of two types of unfavorable conditions, i.e., biotic (e.g. insects, diseases) and abiotic (e.g. drought, flooding, salinity, heat, frost, nutrients imbalance). Drought and salinity are two major abiotic factors that affect plant growth, development, and ultimately its yield (Niu et al. 2014; Khalid et al. 2019; Zhang et al. 2022a, b). Plants developed several defense mechanisms that cope with the stress to maintain their metabolism and growth (Khalid et al. 2019). Plants such as vegetable crops are capable of surviving under different environmental stresses by natural acclimation and adaptation mechanisms, but these abilities may not be sufficient to cope with the swift climate changes (Dhankher and Foyer 2018). How plants respond to abiotic stresses depend on the species, stress intensity, stress duration, phenological stage of the plant, and the parts of the plant (tissue or organ) involved in the responsive mechanisms. Abiotic stresses cause changes in plant physiology and metabolism which can be reversible or irreversible (Seymen 2021). These factors affect the vegetable crops which are usually susceptible to abiotic stresses (Shannon and Grieve 1998; Walter et al. 2013; Devi and Arumugam 2019; Parkash and Singh 2020). To fulfill the future demand for vegetables globally, we urge to develop new cultivation techniques or tolerant genotypes to tackle the pressing drought and salinity issues (Pathak et al. 2018).
Vegetables are a major constituent of the human diet, as they are a rich source of antioxidants, vitamins, minerals, and dietary fibers (Slavin and Lloyd 2012). Vegetables are also consumed for their unique taste, texture, and religious importance. Global vegetable production increased 65% from 446 Mt in 2000 to 1128 Mt in 2019. However, there are still over 770 million undernourished people out of the close to 8 billion global population (FAO 2021). Scientists and growers are investing efforts to increase the production and nutritional value of vegetables under stressful conditions (Gruda et al. 2019). The magnitude of drought and salt stress depends on various environmental factors, such as the occurrence and distribution of solar radiation, evapotranspiration needs, and the ability to retain soil moisture (Khalid et al. 2019). Therefore, sustainable breeding technologies and agricultural management practices should be developed to monitor drought and salt stress in order to minimize their damage to vegetable crops.
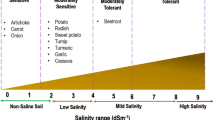
Drought and salinity not only affect the production, but also the quality of vegetables. Most vegetables are sensitive to salt with salinity threshold at electrical conductivity (EC) ~ 2.5 dS m−1 (Behera et al. 2022) and sensitive to drought at volumetric water content of ~ 20% (Prakash and Singh 2020; Razi and Muneer 2021). Drought- and salinity-induced osmotic, ionic and oxidative stresses lead to the closure of plant stomata in the short-term to result in a decrease in size of plants in the long-term (Safdar et al. 2019) (Fig. 1). Stomatal closure results in reduced CO2 uptake (Chen et al. 2005; Liu et al. 2014; Cai et al. 2017), limiting carboxylation and lowering internal CO2 levels, resulting in increased photorespiration (Fig. 1). The production of reactive oxygen species is also enhanced in the plants when exposed to drought and salt stress conditions which lead to oxidative damage to cellular organelles (Fig. 1).
Effect of drought and salt stress on vegetables and its physiological and molecular response mechanism. The water deficit and accumulation of toxic ions leads to physiological changes in plants, i.e., closure of stomata, decrease in leaf traits, and carbon dioxide, which ultimately enhance the photorespiration and decrease in photosynthesis. At the molecular level, plants responded to drought and salinity with changes in expression of genes, proteins, and metabolites such as the production of ROS, which leads to oxidative damage in the cell and lipid carboxylation
Despite the abundance of research in vegetables in the past few decades, there are still some unfilled knowledge gaps on the responses of vegetables to drought and salinity. This review aims to present the latest knowledge on two major abiotic stresses, drought and salinity in modern agriculture, and the responses of plants to these stresses. We review physiological and molecular responses to drought and salinity in vegetables and potentially effective strategies to enhance production. Moreover, we summarized different technologies such as seed priming, genetic transformation, biostimulants, nanotechnology, and cultural practices adopted to enhance vegetable production under drought and salinity stress For reviews on the mechanisms of drought and salinity tolerance in model plants and crops, the readers are referred to (Cattivelli et al. 2008; Munns et al. 2020; Van et al. 2020).
Vegetable production and drought stress
Physiological response
Water is the main constituent of plants as it is required by many vital functions. However, due to climate change, water scarcity is a critical global challenge nowadays especially to agriculture (Khalid et al. 2019). When plants are exposed to drought stress, stomatal closure was induced to retain water in the plant by decreasing leaf transpiration. However, stomatal closure also results in declined photosynthesis and gas exchange. Water use efficiency can be derived by comparing biomass accumulation to transpiration because they are tightly coupled. Genetic analyses showed that a large part of the variation in water use efficiency is controlled by genes in several species, but with low heritability (Chen et al. 2011). Indeed, water use efficiency varies with evaporative demand, time of day, seasons, soil types and crop species. Therefore, breeding plants for high water use efficiency has most often resulted in slow-growing plants that are uninteresting from an agronomical perspective (Blum 2009). For instance, it was shown that increase water use efficiency lead to a 15% yield increase under water deficit condition, but this yield increase was declined with precipitation and nullified with rainfall of 400 mm (Condon et al. 2004).
Protective cell responses to ABA-mediated hydraulic and non-hydraulic signals support a fundamental role of ABA in plant drought signaling (Chen et al. 2017; Xue et al. 2017; Munns et al. 2020). Under drought stress, the photosynthetic rate is slowed down because the captured light cannot be completely converted into chemical binding energy. Meanwhile, the excess energy leads to photoinhibition, that is, a decrease in the maximum quantum yield (Fv/Fm) of the PSII reaction center. Several mechanisms mitigate the negative effects of photoinhibition, such as non-photochemical quenching, photorespiration via Mehler reactions, non-radiative energy dissipation, and chlorophyll content regulation. Fv/Fm values can be used not only as an indicator of water deficit stress conditions but also to distinguish tolerant and sensitive genotypes to drought stress. For example, when drought tolerant genotypes of tomato were subjected to water deficit stress, the PSII activity was not decreased and thus had higher photosynthetic activity compared to sensitive genotypes (Chatterjee and Solankey 2015).
The production of reactive oxygen species (ROS) leads to oxidative damage to the chloroplast, thereby reducing carboxylation. Reducing leaf size also limits carboxylation. Low control of acyclic electron transport inhibits ATP synthesis. These events together lead to a significant reduction in plant photosynthesis. When the plant is exposed to water deficit conditions, the ability to tolerate water deficit stress and maintain water potential is also reduced. Vegetables usually contain more than 90% of water because of their succulent nature. Many physiological and biochemical processes that are involved in plant growth and development are affected by drought stress conditions (Bahadur et al. 2011). Water deficiency during critical growth stages (e.g. flowering, and fruit set stages) of vegetables can severely affects the yield and quality of vegetables. Examples of vegetable affected by water deficiency at some critical growth stages are shown in Fig. 2.
Molecular response
Water scarcity forces the plants to close their stomata which increases the production of ROS, i.e., singlet oxygen (O2•−), hydrogen peroxide (H2O2), hydroxyl radical (OH•), superoxide radical (1O2) in cellular organelles. The increase in ROS production will cause oxidative stress which ultimately affects plant growth and production. Subcellular compartments, such as the chloroplast, mitochondria, and peroxisomes, are sites of major metabolic activities and ROS generation (Mittler 2002). The Mehler reaction in chloroplasts, electron transfer in mitochondria, and photorespiration in peroxisomes are the main metabolic activities, leading to cellular ROS accumulation. The balance between production and elimination of intracellular ROS must be tightly regulated and/or metabolized efficiently. This balance is necessary to minimize potential damage to cellular components by ROS, as well as to maintain growth, metabolism, development, and overall plant productivity (Moller and sweetlove 2010). To cope with the destructive consequences of ROS in cellular organelles, plants produce different antioxidative enzymes including superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), ascorbate peroxidase (APX), glutathione reductase (GR), and osmolytes (proline, glycine betaine, etc.) (Mittler 2002; Khalid et al. 2021). SODs are the frontline fighters against the ROS, responsible for the conversion of 1O2 into H2O2. CATs play a critical role to convert H2O2 into H2O. APXs help in the conversion of H2O2 into H2O using ascorbate as a specific electron donor (Razi and Muneer 2021) (Fig. 3). Vegetables enhance the production of antioxidant enzymes and osmolytes when they are exposed to drought stress conditions. The tolerant genotypes tend to have higher levels of SOD, POD, CAT, APx, GR, proline, and glycine betaine as compared to the sensitive genotypes. However, this trend may vary among different vegetables. This may underlie the observation of higher levels of H2O2 accumulation and lipid peroxidation in drought-sensitive vegetables. In tomato plants, antioxidant activity has been reported to increase when plants are exposed to drought conditions (Zhou et al. 2019). To mitigate the drought stress and enhance the activity of different antioxidative enzymes, researchers have introduced nano-organic fertilizers (Ahanger et al. 2021), foliar application of minerals (Farzane et al. 2021), and grafting techniques (Sanzhez-Rodriguez et al. 2012). Drought-tolerant eggplant and sweet pepper genotypes with strong antioxidant activities were found to tolerate drought stress efficiently at the seedling stage (Maham and Muhammad 2019; Abdelaal et al. 2020a; Kopta et al. 2020; Alabdullah et al. 2021; Mahmood et al. 2021; Semida et al. 2021). Cucumber seedlings also showed a similar result when exposed to drought stress conditions. They increased the production of SOD, POD, CAT, when exposed to water deficit conditions (Jing et al. 2009; Fan et al. 2017).
A model of different cellular organelles (chloroplast, mitochondria, and peroxisomes) where ROS are generated and scavenged by antioxidative enzymes under water deficit conditions. The production of ROS is carried out by electron transport chain via PSI and PSII (Mehler reaction) and conversion of O2·− into H2O2 with the help of SOD in chloroplast. In mitochondria, complex 1 and 2 of electron transport chain are involved in the production of ROS. In peroxisome, xanthine and fatty acids are involved in the production of H2O2. O2·− Superoxide ion, PSI II Photosystem I, II, H2O2 Hydrogen peroxide, SOD Superoxide dismutase, APX Ascorbate peroxidase, CAT Catalase, NADH Nicotinamide adenine dinucleotide, ROS Reactive oxygen species
When plants are exposed to water deficit conditions, roots are the first part that sense the water loss and communicate water deficit as a stress signal to shoot through the xylem. Abscisic acid (ABA) is the main chemical signal that integrates into the roots and moves towards shoots and leaves to regulate stomata under water deficit conditions (Osakabe et al. 2014; Malcheska et al. 2017) or rapidly biosynthesized in leaves in response to stomatal closure (Zhang et al. 2018). ABA helps the regulation of plant growth and development by inducing stomata closure (He et al. 2018) and triggering a complex cascade of signaling pathways and expression of drought responsive genes under the drought stress condition (Chen et al. 2017; Xue et al. 2017).
Under water deficit conditions, plant undergoes different defence strategies and production of cuticular wax is one example. Plant cuticular wax acts as barrier against water deficit conditions (Shepherd and Griffiths 2006; Xue et al. 2017). The cuticle is synthesized by the epidermal cells. As a barrier, it prevents water loss from non-stomatal channels (Xue et al. 2017; Kosma and Jenks 2007) reported that ABA was responsible for the upregulation of 10 cuticle-related genes. The increase in cuticular wax production has been observed in tomatoes (Al-Abdallat et al. 2014) and cucumbers (Wang et al. 2015), contributing to the decrease in non-stomatal transpirations and hence enhanced tolerance against water deficit conditions. By increasing cuticular wax production, SlSHN1 overexpression reduced tomato cuticular permeability and improved drought tolerance (Al-Abdallat et al. 2014). A major component of cuticular wax in tomatoes is n-alkanes, which are synthesized in both tomato leaves and fruit by SlCER1s and SlCER3s. Overexpression of SlCER1–1 resulted in accumulation of n-alkanes in tomato leaves and fruits, which enhance their drought tolerance and postharvest shelf life of fruit (Wu et al. 2022; Liu et al. 2022a, b) found that ectopic expression of orange CsECR increases the content of total wax and aliphatic wax fractions in the transgenic tomato plants as well as decreased the cuticle permeability in fruits and leaves.
Aquaporins are critical to the maintenance of hydraulic conductance in roots, maintenance of osmotic homeostasis, the expansion of the tissue structure, the efficiency of water usage, the viability of seeds, the response and recovery after drought stress (Tyerman et al. 2021). A variety of factors play a role in aquaporin regulation and expression, including pH, cations, ROS, stoichiometry, and phytohormones at various stages, including genes, transcripts, and proteins (Patel and Mishra 2021). As a result of drought stress, guard cell-specific aquaporin genes are expressed differently, which alters the stomatal conductance. The overexpression of aquaporin genes PIP1, PIP2, and TIP increase gs, whereas PIP knocked-down mutants have a decrease in gs (Ahmed et al. 2021). For instance, ectopically expressing MdPIP1; 3 increased fruit size and enhanced drought tolerance of tomatoes (Wang et al. 2017).
Natural selection has led to plants evolving diverse stress adaptation mechanisms, which include modifying root system architectures to obtain water and nutrients in response to water deficit (Kulkarni et al. 2017). The root system architecture is determined by the angle of root growth, the number and length of primary and lateral roots, and the density and length of root hairs (Gérard et al. 2017; Pagès 2021). Drought stresses significantly alter root system architecture, resulting in the generation of lateral roots and root hairs (Koevoets et al. 2016). It has been shown that phytohormone homeostasis plays a critical role during root initiation and development under normal conditions as well as under abiotic stress conditions (Ranjan et al. 2021). Among the phytohormones that regulate root system architecture under stress, conditions are ABA, auxins, cytokinins, ethylene, and jasmonic acid. A recent report showed that root architecture was significantly enhanced in tomato plants under water deficit conditions when melatonin was applied exogenously (Altaf et al. 2022).
A wide variety of aspects of plant development and stress tolerance are regulated by microribonucleic acids (miRNAs) of 21–24 nucleotides length, which negatively modulate target genes through transcription cleavage and translational inhibition (Deng et al. 2022). It has now been demonstrated that drought stress-induced phytohormone signaling and gene expression have a significant influence on miRNA-mediated root growth and branching regulation, and ultimately determine RSA under stressed conditions (Bakhshi et al. 2016). Transcription factors or genes that are targeted by miRNAs control root growth and patterning. As an example, miR160 regulates the expression of transcription factors ARF10 and ARF16, which are critical in primary root development (Wang et al. 2005a, b). For instance, in tomato, over-expression of microRNA169 enhanced drought tolerance (Zhang et al. 2011) and miR1916 was reported to be as a negative regulator in drought stress resistance (Chen et al. 2019).
In different species, some novel genes are identified that cause changes in physiological and morphological traits under drought stress. For example, root length and numbers depend on the activity of many genes and the expression of dominant alleles of those genes, while root thickness depends on the expression of recessive alleles (Kumar et al. 2012). Genes involved in solute accumulation (e.g., the mtlD gene responsible for mannitol accumulation, or the P5CS gene for increased proline accumulation) help to balance the reduction in plant water potential and encode different enzymes required for the synthesis of these molecules (Abebe et al. 2003). In vegetables, overexpression of these genes resulted in specific responses to drought stress: ABF4 transcription factor genes are not only important in tolerating drought stress in potato but also increase tuber quality and yield (Muñiz García et al. 2018). SlGRAS4 transcription factor gene is involved in increasing the sensitivity of stomata to ABA (Liu et al. 2021), thereby reducing water loss. AVP1 gene is involved in root growth (Park et al. 2005). NADP-Me gene was involved in the reduction of stomatal conductance and the improvement of water use efficiency (Laporte et al. 2002); Wilty gene was involved in the wilting process of tomato leaves under drought stress (Kumar et al. 2012).
Vegetable production and salinity
Physiological response
Soil salinization can be divided into primary salinization and secondary salinization. The saline soil generally contains large amounts of cations such as Na+, Ca2+, Mg2+, and lower amounts of K+ and Fe2+ while the most common anions are Cl−, SO42−, NO3− and HCO3−. Most saline soil (primary salinization) is formed through natural processes such as weathering (rock), salt accumulation from rainfall, and deposition of windblown salt. Secondary salinization is the result of human activities, such as the use of poor-quality water and fertilizers, and improper practice of agricultural management, together inducing soil salinization. Currently, 7% of the world’s land surface (~ 1 Bha) contains salinized soil (Hopmans et al. 2021; Shahid et al. 2018). This includes approximately ~ 70 Mha of irrigated land which occupies around one-third of the total irrigated land in the Mediterranean Basin. Water quality has become a limiting factor for agriculture due to the overuse of salt water in irrigated land and coastal areas (Petretto et al. 2019; Zhu 2001; Tyerman et al. 2019).
Plant salinity tolerance is a multigenic trait, regulated by multiple genes and associated mechanisms. Bahmani et al. (2015) outlined a myriad of cellular components related to salinity tolerance from very upstream signaling and hormone regulation to cellular protection and ion homeostasis against salinity. These components work interactively to maintain cellular activities under salinity stress via three mechanisms: osmotic modulation, antioxidative regulation, and ion homeostasis. Increasing the level of NaCl in soil solution affects plant water uptake due to osmotic stress (Munns 2002; Munns et al. 2020; Shabala et al. 2020). Ultimately, this osmotic stress has a flow-on effect, resulting in a reduction of the rate of cell expansion in growing tissues and the stomatal opening, as well as a reduction in the amount of nutrient diffusion in the leaves. Also, plants are less likely to be able to fully exploit light absorbed by photosynthetic pigments when stomatal closure is induced by osmosis or when Na+ is accumulated in the cytosol under saline conditions (Shabala et al. 1998; Tavakkoli et al. 2011).
Salts dissolved in soil solutions are in close contact with roots and affect plant growth because osmosis reduces water uptake by plants, thereby reducing water potential in leaves and tissues (Passioura and Munns 1984). Excessive salt concentrations in plant tissues can affect growth and productivity as they hinder several key processes such as germination, photosynthesis, nutrient balance, and redox balance (Parihar et al. 2015). For example, salinity affects germination because it reduces the osmotic potential of the germination medium for seed adsorption and alters the activities of enzymes involved in nucleic acid and protein metabolism (Parihar et al. 2015). The salinity stress effect on seed germination varies by species, variety, and salinity. In general, there is a negative correlation between salinity and germination rate, such as in carrots (Bolton and Simon 2019), cucumbers (Baghbani et al. 2013), sweet peppers (Chartzoulakis and Klapaki 2000), eggplants (Hannachi and Labake 2018) and tomatoes (Singh et al. 2012). The effect of salinity on plant growth has two consecutive stages (Parihar et al. 2015). In the first stage, saline conditions do not significantly alter plant growth, as Na+ and Cl− that enter the xylem are collected in the vacuole, while the meristem continues to grow through the phloem. At this stage, only reduced leaf and root development have been observed (Munns 1993). In the second stage, as the amount of salt accumulated in plant tissues exceeds the storage capacity of the vacuoles, the concentrations in the cytoplasm increase, and the activity of many enzymes is severely inhibited (Munns 2005).
Molecular response
Under salt stress, plants are mainly affected by disturbance of rhizosphere osmotic potential, which is caused by higher salt levels. The accumulation of ions in cellular compartments and organelles can reach toxic levels, hindering many physiological processes, and leading to plant death. Salinity-induced stomatal closure reduces the diffusion of CO2 into the stomata, reducing the rate of photosynthesis, transpiration, and carbohydrate accumulation (Munns 2002). When plants encounter reduced intercellular carbon dioxide content, this often accelerates ROS accumulation due to photorespiration (Gupta et al. 2016). Although ROS are considered signaling molecules that play an important role in plant defense mechanisms (Mittler 2017), they can also adversely affect cellular metabolism and photosynthesis mechanisms. To protect cellular systems from ROS, plant defense mechanisms produce several oxidative scavenging enzymes (Khalid et al. 2020). When salt accumulates in chloroplasts, it reduces chlorophyll content, affects the photosynthetic transport system, and inhibits the activity of photosystem II. To overcome salinity, plants regulate and sequester toxic ions and produce osmotic substances (e.g. proline, betaine) that help maintain osmotic pressure (Khalid et al. 2020). Under saline conditions, the activity of these antioxidant enzymes, including SOD, CAT, and POD, and the concentrations of inactive compounds, including glutathione and ascorbic acid, are increased. For example, tomato plants showed an increase in antioxidative enzymes when exposed to salt stress conditions, but the tolerant genotype appeared to have more antioxidative enzymes as compared to the sensitive ones (Gharsallah et al. 2016). Similar observations have been also reported for eggplants and sweet peppers, in which salt-tolerant genotypes tend to have more antioxidant enzymes as compared to the sensitive ones (Wu et al. 2012; Fikret et al. 2013; Abdelaal et al. 2020b). Similar findings have been highlighted in salt-tolerant cucumber and potato cultivars that were exposed to salt stress (Zhu et al. 2004; Rahnama and Ebrahimzadeh 2005; Aghaei et al. 2009; Furtana et al. 2010). Increases in enzymatic activity and concentrations of bioactive compounds increase tolerance in plants and can be used to estimate plant salt tolerance.
The accumulation of toxic ions in plant cellular organelles leads to the disturbance in ion homeostasis. Transporters regulating Na+ and Cl− concentrations in mitochondria and chloroplasts are largely unknown and could be a major source of energy cost (Munns et al. 2020; Chen et al. 2021; Shabala et al. 2020; Jiang et al. 2021). Exclusion of Na+ from roots, regulation of rhizome transport, cellular compartmentalization of Na+, and maintenance of cytoplasmic osmotic balance are important mechanisms of salt tolerance (Van Zelm et al. 2020). Plants have evolved ways to eliminate Na+ ions from the cytoplasm to maintain low levels of ionic Na/H antiporters and transport Na+ in exchange for H+ ions. This involves the transfer of Na+ ions by Na/H ion antiporters to apoplast at the plasma membrane, while Na/H ion antiporter maintains Na+ ion separation in the vacuole (Fig. 4) (Hussain et al. 2018; Abdelaziz et al. 2019). Similarly, high-affinity potassium transpoter1 (HKT1) type transporters play a vital role in maintaining the Na+ and K+ ion homeostasis and decreasing sodium toxicity in plants. They exclude the Na+ ions from the xylem stream and roots to keep the shoots safe from toxic ions (Maser et al. 2002; Horie et al. 2005; Møller et al. 2009). The HKT1 transporters mediate Na+ ion movement in tomatoes (Almeida et al. 2014; Romero-Aranda et al. 2020) and cucumbers grafted with pumpkin (Sun et al. 2018). In eggplants, the weak induction of HKT1 in roots has demonstrated higher Na+ ion accumulation in stems and leaves (Assaha et al. 2015).
The diagram illustrates the salinity induced-Ca2+ and ROS signaling, and important transporters involved in Na+/K+ homeostasis in plant cells. The hydraulic sensor firstly senses turgor pressure via reducing water potential (Ψw). The hydraulic sensor then triggers transient Ca2+, ABA synthesis or both to activate short- and long-distance ROS and ABA signaling. SOS (Salt Overly Sensitive) pathway is a key component that activates Na+ exclusion and compartmentation from the cytosol through SOS1 (PM Na+/H+ antiporter), and NHX1 (tonoplast Na+/H+ antiporter). Cytosolic Na+ content determines ABA and ROS accumulation in cells which inhibits normal cellular functions such as promoting K+ exclusion via GORK (K+ outward rectified channel), inhibiting PM and tonoplast-based proton pump- VHA (vacuolar H+ ATPase), and AHA (PM H+ ATPase). H+ ATPase provides a proton source to drive K+ inward transporters-HAK1 (High-affinity K+ transporter), NHX2-4 (NHX type2 antiporter), and Na+ antiporters- NHX1, SOS1 located at PM and tonoplast. ANN (Ca2+ permeable channel annexin), MCA (Ca+ permeable mechanosensitive channels), SCABP8 (SOS3-like Ca2+ binding protein 8), RBOH (NADPH/respiratory burst oxidase protein), NSCC (non-selective cation channel)
In contrast to vegetables, tissue compartmentalization and exclusion of toxic ions plays an important role in redistribution of toxic ions in older or mature leaves in grasses (Liphschitz and Waisel 1974) and exclusion is carried out by salt glands or bladders. In many grasses, salt glands and bladders play a vital role in building tolerance of salinity (Ramadan and Flowers 2004; Yong et al. 2022). Salt glands mostly appear in epidermal cells, but they are usually found in mesophyll tissues in C4 grasses (Marcum 2006). The exclusion of salt toxic ions from salt glands in grasses is highly selective (Worku and Chapman 1998). The movement of toxic ions to salt glands is energy-dependent (Naidoo and Naidoo 1999). Other ions are also excreted from salt glands but in small quantities (Marcum and Murdoch 1994). There are many types of salt gland cells and epidermal bladder cells (EBCs) are an example (Shabala et al. 2014). EBCs take part in several roles, including acting as an external water reserve, depository of metabolites, a reservoir for ROS and osmolytes, and restricting sites for excessive toxic ions (Steudle et al. 1975; Agarie et al. 2007; Oh et al. 2015). Therefore, genetic engineering of salt glands in existing salt-sensitive vegetables and selection of new vegetable crops with salt glands will be among the promising molecular strategies for improving the salinity tolerance of vegetables.
Accumulation of toxic ions induces imbalances in other ions, i.e., K+ and Ca2+ (Munns and Tester 2008). Calcium ions play a vital role in transmitting external stimulus signals. These Ca2+ signals are communicated downstream by Ca2+-binding proteins (Hashimoto and Kudla 2011), ultimately transferring information to the systems that regulate the physiological and biochemical processes or gene expression (Kurusu et al. 2015). TPC1 cation channel is involved in the production of salinity stress-triggered systemic Ca2+ signal in roots and may contribute to whole-plant resistance to salinity stress (Choi et al. 2014; Gilroy et al. 2014). The development of high-resolution calcium biosensors and the identification of the downstream CBL-CIPK pathway have helped the establishment of Ca2+ waves as early signals of the sodium response and led to the identification of a novel cation sensing mechanism (Van Zelm et al. 2020). Tolerant plants seem to have certain genes that are not in sensitive plants. According to the literature, the genes involved in salt tolerance can be categorized into three groups: (i) genes that regulate salt absorption and distribution; (ii) genes involved in osmotic control; (iii) genes associated with plant growth. Analysis of sensitive Arabidopsis mutations in high external Na+ concentrations enabled the identification of three SOS genes involved in salt tolerance (i): SOS1 encapsulates the Na+/H+ code of the plasma transporter membrane involved in the exclusion of Na+ to the apoplast; SOS2 incorporates protein kinase, which activates SOS1; SOS3 incorporates calcium-binding protein and activates SOS2. In addition, the fourth gene (SOS4), appears to regulate SOS1, as it binds the cofactor, pyridoxal-5-phosphate, which binds SOS1. In addition to activating SOS2, there is also the protein SCaBP8 regulated by SOS2 (Parihar et al. 2015). In Arabidopsis plants where excessive exposure to SOS genes has been observed, salt tolerance and low Na+ concentration, and high K+ concentration have been reported (Yang et al. 2009). The relationship between genetic interactions SOS1, SOS2, and SOS3genes, salt stress tolerance, and high Na+/K+ levels have also been demonstrated in brassica (Kumar et al. 2009) and potato (Jaarsma and de Boer 2018) other genes include osmolytes or osmoprotectants or related solutes. These osmolytes are divided into four classes: N-containing solutes, such as proline and glycine betaine; sugars such as sucrose and raffinose; straight-chain polyhydric alcohols (polyols), such as mannitol and sorbitol; and cyclic polyhydric alcohols (cyclic polyols). The genes involved in plant growth are associated with signal molecules, hormones, and transcription factors, and are more common in other stress conditions. Depression molecules acting as protective molecules can be metabolites that change their concentrations or proteins that change their structures in response to drought, salt, and cold from roots to shoots to promote salt stress tolerance (Munns 2015).
Strategies to enhance vegetable production
Molecular breeding toward drought and salinity tolerance vegetables
To understand the complex mechanisms of drought and salinity and to augment their production, the focus of research is entering the era of omics. The implementation of multi-omics and improved breeding strategies is a dynamic step towards drought and salt tolerance in vegetables. The identification of drought and salt-responsive genes, proteins, metabolites, and miRNAs has become possible through studies of genomics, transcriptomics, metabolomics, proteomics, micromics, and phenomics. Many omics methods, tools, and resources have been developed for vegetable yield and quality improvement (Chaudhary et al. 2019). However, further investigation on the latest omics technologies will need to explore the myriad of pathways involved in drought and salt tolerance. Additionally, genome-wide association studies (GWAS) and quantitative trait loci (QTL) mapping techniques have made an impressive contribution to improving plant responses to drought and salt stresses. Regarding the drought stress condition, the SiDHN gene induced in tomato plants showed drought tolerance by maintaining their photosynthetic machinery and antioxidative defence mechanism (Guo et al. 2019). The overexpression of gene CsATAF1 enhanced the drought tolerance in cucumber seedlings by regulating ABA-dependent pathways and more efficient coping of ROS load (Wang et al. 2018). The tomato plants produced by crossing homozygous lines have showed upregulation of salt tolerance related genes LeNHX2 and SlSOS2 which are involved in improving the plant growth, water uptake, and yield under salt stress conditions as compared to their parental plants (Baghour et al. 2019). Similarly, the HAL1 gene responsible for salt tolerance in yeast was introduced in tomato plants. The overexpression of HAL1 significantly increases the crop tolerance under salt stress by improving the K+/Na+ ratio which leads to sustainable growth (Gisbert et al. 2000). The Arabidopsis gene LOS5 increases the salt tolerance in cucumber seedlings by enhancing germination, plant biomass, ABA, sugars, and antioxidative enzymes (Liu et al. 2013).
Priming
Seed priming is a major strategy to sustain or increase vegetable production in the current climate change scenario. Priming not only increases germination, but also helps the plant to tolerate different biotic and abiotic stress factors. It also enhances seedling establishment under harsh environmental conditions (Chen et al. 2012). Abiotic stresses such as drought, extreme temperatures, salinity, and heavy metals are major factors limiting global crop productivity and sustainability. Among them, drought has become a serious environmental constraint for horticultural production, especially in arid and semi-arid regions, and under rapidly changing climate scenarios (Khalid et al. 2019). Seed priming may help cells respond to drought stress through multiple mechanisms, including modulation of antioxidant defense systems, and upregulation of osmoprotectants and phenolic compounds (Savvides et al. 2016). The seed priming technique appears to be very much effective in water deficit conditions. Chakma et al. (2021a) observed that tomato plants primed with silicon showed higher fruit yield and quality as compared to controls under 75% and 100% field capacity. Cucumber plants when primed with ascorbic acid and pyridoxine improved the plant physiological and biochemical attributes under 65 and 80% field capacity. Pea primed with Bacillus thuringiensis, silicon, potassium silicate, and carrot extract, and onion primed with polyethylene glycol (PEG) and gibberellic acid showed improved germination, higher biomass, and better biochemical attributes at 50% deficit irrigation conditions (Arafa et al. 2021; Arvin and Kazemi 2003). Arvin and Kazemi (2003) also observed that seed priming of onion with PEG and gibberellic acid increased the tolerance against 85 mM salt stress. Di Stasio et al. (2020) showed that priming the tomato seeds with sea-weed extract enhances tomato production by up to 50% under salt-affected soils (6.3 dS m−1). In terms of priming techniques, osmopriming is regarded as the most efficient for sweet pepper against 60 and 80 mM NaCl (Shumaila and Ullah 2020). Similarly, various studies have been conducted to understand the priming compounds and techniques on vegetable seeds to mitigate the negative effect of drought and salt stress conditions (Table 1).
Agronomic practices
The abiotic stresses cannot be addressed without management practices in the field. For instance, many agronomic practices have been developed by the Asian vegetable research and development center (AVRDC) now known as the World vegetable center to enhance vegetable yield under stress. To overcome water scarcity, the method of irrigation plays a pivotal role. It was reported by AVRDC (2005) that the use of drip irrigation enhances water use efficiency of capsicum by approximately 50–80% and the production was also increased as compared to furrow or flooding. Fewer chances of disease (fusarium wilt) were also reported in watermelon. The use of mulching is also very much important to maintain soil moisture and enhance nutrient conservation. Crop rotation, intercropping, crop diversification, use of organic mulches are important agronomic traits to conquer the stresses (Naik et al. 2017). These practices enhance the soil organic matter and nutrients in the soil which help the vegetables to tolerate abiotic stress conditions. The efficient use of fertilizers also helps the vegetables to tolerate abiotic stresses. The use of nutrients can also help under salt stress conditions. For example, potassium has been reported to increase tuber yield (Elkhatib et al. 2004). Phosphorous can promote radish plant health (De Oliveira et al. 2010). Sulphur seems to activate defense mechanisms in brassica and legumes (Rausch and Wachter 2005), and zinc application can reduce the uptake of sodium in pepper plants (Aktas et al. 2006).
Grafting
To counter the negative effects caused by climate change and to increase production, grafting is an environmentally friendly technique. Similar to perennial fruit crops, the rootstock and scion compatibility and their tolerance support each other. Tomato drought-tolerant rootstocks (cv. Fraidah, Zarina, Beaufort) grafted on drought-sensitive scions (cv. Unifort, Josefina, M28) has demonstrated maintenance of efficient growth, proper nutrients uptake, enhanced osmotic adjustment and improved fruit yield and quality (Ibrahim et al. 2014; Sanchez-Rodrigues et al. 2014; Altunlu and Gul 2012). Pepper cv. Verset used as rootstock and grafted with sensitive scion cv. Atlante showed better osmotic adjustment and strong photosynthetic machinery under water deficit conditions (Penella et al. 2014). Similarly, salt-tolerant Cucurbita hybrids rootstock cv. P360, PS1313 grafted with salt-sensitive cucumber scion cv. Akito and melon cv. Cyrano showed great tolerance against salt stress conditions by less decline in photosynthetic attributes and strong defence mechanisms (Rouphael et al. 2012; Colla et al. 2012).
Plant growth-promoting rhizobacteria
Salt and drought stresses are serious environmental challenges that greatly reduce the yield of vegetables. The application of plant growth-promoting microorganisms in vegetable crop production has yet to attract research attention. Enhanced use of plant growth-promoting rhizobacteria (PGPR) is a new option to address agricultural challenges posed by soil environmental stress. The few reports published underline that PGPR can enhance plant productivity by counteracting the negative effects of salt and water deficit stresses on plant growth, even in stressful environments. PGPR promotes plant growth through a variety of mechanisms, such as triggering osmotic responses, providing growth hormones and nutrients, acting as a biological control agent, and modifying plant root shoot signaling. The development of salt-tolerant crops is still being planned. Thus, the only viable alternative is the use of PGPR to increase vegetable yields in stressful environments. Under abiotic stress conditions, the complex and dynamic interactions between microorganisms and plant roots influence not only the plants themselves, but also the physical, chemical, and structural properties of soils. Selecting microorganisms from stressed ecosystems and their applications under stress conditions to alleviate the effects of abiotic stress on soils may increase the yield of soil vegetables under drought and salt stress conditions (Table 2). Similarly, Arbuscular mycorrhizal fungi (AMF) are also involved to mitigate the negative effects of drought and salinity on vegetable production (He and Huang 2013). Mycorrhizae have been reported to increase the absorptive surface area of plants. In salt-stressed and water-deficient soils, nutrients absorbed by hyphae of mycorrhizae can promote plant growth and reproduction and reduce abiotic environmental stress (Baum et al. 2015). The tolerance of salt stress in tomatoes was increased by using arbuscular mycorrhizal fungi (Latef and Chaoxing 2011), grafting (He et al. 2009), and application of phytohormones (Szepesi 2008). Salinity imposes negative effect on AMF but, still some studies showed that AMF could help the plant to tolerate more stress by enhancing host-plant nutrition, maintaining K+/Na+ ratio and better osmotic adjustment with improved photosynthesis, which together increase the plant tolerance against salinity (Baum et al. 2015). The inoculation of AMF in tomato (He and Huang 2013), pepper (Beltrano et al. 2013), and lettuce (Aroca et al. 2013) showed higher tolerance against salinity stress. Regarding the water deficit condition, the vegetables inoculated with AMF showed better tolerance by altering their physiology and gene expressions (Baum et al. 2015). Lettuce plants with AMF exposed to drought stress condition showed tolerance by increasing abscisic acid concentration which helps maintain balance in water movement through roots to leaf transpiration (Aroca et al. 2008). AMF also enhance the tolerance of pepper plants (Davies et al. 1992) by maintaining turgor pressure, leaf water potential and water content in leaves.
Plant growth regulators
Plant growth regulators (PGRs) are used in vegetables to improve plant health and yield in stress conditions. It was reported by Grand View Research that by 2025 the PGR market is expected to grow about 4.14 billion USD (GVR 2018). They can enhance plant growth and productivity, interact with several plant processes in response to stress, and increase the accumulation of antioxidant compounds that reduce plant susceptibility to stress. The application of gibberellic acid increases the relative water content and antioxidant defence mechanism of basil plants to tolerate drought stress conditions (Kiapour et al. 2015). Synthetic PGRs, i.e., melatonin (Ibrahim et al. 2020), salicylic acid (Chakma et al. 2021b) and strignolactones (Visentin et al. 2016) also showed improved tolerance in tomato plants and enhanced the fruit quality under water deficit condition. Similarly, strignolactones in tomato (Liu et al. 2022a, b), lettuce (Aroca et al. 2013), cucumber (Zhang et al. 2022a, b) and melatonin in cucumber (Zhang et al. 2020) and eggplant (Sofy et al. 2021) induced tolerance against salt stress environments. Natural or plant based PGRs also play vital role in tolerance mechanisms of vegetables against abiotic stresses. Foliar application of moringa leaf extract on pumpkin showed sustainable growth, maintained photosynthetic pigment, increase in proline and sugar content under water deficit conditions (Abd El-Mageed et al. 2017). Similarly, application of liquorice root extract increases the nutrient uptakes, vegetative growth, biochemical attributes, and yield in peppers (Desoky et al. 2019) and beans (Rady et al. 2019).
Nanoparticles
Nanotechnology is now widely used in many fields, such as pharmaceutical, engineering, agriculture, etc. It has an enormous potential in the agriculture sector and provides a green and important alternative for crop management. Many studies showed that the use of nanoparticles as foliar, soil, and priming enhances the crop performance in biotic and abiotic stress conditions (Aqeel et al. 2021; Alabdallah and Alzahrani 2020) observed that foliar application of zinc oxide nanoparticles enhanced crop growth and production of okra seedlings under saline conditions. Similarly, zinc, boron, silicon, and zeolite nanoparticles enhanced potato plant growth in salt-affected soils (Mahmoud et al. 2019). Cucumbers also showed resistance in a saline environment by inducing early stimulation of defence responses (antioxidative enzymes) when treated with cerium oxide nanoparticles (Chen et al. 2022) and manganese oxide nanoparticles (Lu et al. 2020). Nanoparticles also mitigate the drought stress in different vegetables. The cucumber (Ghani et al. 2022), tomato (El-Zohri et al. 2021) and eggplant (Semida et al. 2021) plants showed tolerance against drought stress when treated with zin oxide nanoparticles by enhancing their antioxidative enzymes and osmolytes accumulation. Alabdallah et al. (2021) also reported that silver nanoparticles enhanced the proline accumulation and upregulated the antioxidant enzymes in eggplant under water deficit conditions.
Conclusion and future perspectives
Climate change causes different biotic and abiotic stress factors which affect crop production. Among various abiotic factors, drought and salinity are the major factors that hinder vegetable production around the world. Droughts and salinity stresses affect the vegetable plant health which ultimately leads to the reduced yield. When vegetables are exposed to drought and salt stress conditions, they respond by activating specific genes and particular mechanisms (e.g. antioxidant defence mechanism) which enable tolerance against these stresses. To enhance their tolerance, different strategies can be adopted, including proper cultural practices, priming, grafting, and the use of PGPR, nanotechnology, and omics. Omics alone or together with other cutting-edge biotechnological technologies have revolutionized vegetable breeding by accelerating the identification of candidate genes, and non-coding RNAs, such as lncRNAs, miRNAs, and circRNAs for high yield, quality and stress response. However, limited research work has been conducted on key genes and some non-coding RNAs in regulating to drought and salt stress tolerance in vegetables. More research on key genes and some non-coding RNAs action under drought and salt stress in vegetables can therefore provide additional resources and tools for developing drought- and salt-tolerant vegetables. Genetic transformation has successfully improved vegetable varieties; however, public approval of GMOs using recombinant DNA hamper the genetically engineered vegetable crops in many countries. This issue may be solved in the near future with the development and application of CRISPR/Cas systems in vegetable breeding programs. To date there are very few drought and salt tolerance vegetable cultivars. The expansion of such cultivars should not focus on only yield attributes of vegetables but also those attributes which are directly affected by drought and salt stresses during plant growth and development. To manage the salt and drought stress is a complex matter that involves approaches of breeding, genetic engineering of salt and drought resistance cultivars, smart cultural practices and the use of mitigators for sustainable agriculture.
References
Abd El-Mageed TA, Semida WM, Rady MM (2017) Moringa leaf extract as biostimulant improves water use efficiency, physio-biochemical attributes of squash plants under deficit irrigation. Agric Water Manag 193:46–54
Abdelaal KA, EL-Maghraby LM, Elansary H, Hafez YM, Ibrahim EI, El-Banna M, El-Esawi M, Elkelish A (2020a) Treatment of sweet pepper with stress tolerance-inducing compounds alleviates salinity stress oxidative damage by mediating the physio-biochemical activities and antioxidant systems. Agronomy 10:26
Abdelaal KA, Mazrou YS, Hafez YM (2020b) Silicon foliar application mitigates salt stress in sweet pepper plants by enhancing water status, photosynthesis, antioxidant enzyme activity and fruit yield. Plants 9:733
Abdelaziz ME, Abdelsattar M, Abdeldaym EA, Atia MA, Mahmoud AW, Saad MM, Hirt H (2019) Piriformospora indica alters Na+/K + homeostasis, antioxidant enzymes and LeNHX1 expression of greenhouse tomato grown under salt stress. Sci Hortic 256:108532
Abebe T, Guenzi AC, Martin B, Cushman JC (2003) Tolerance of mannitol-accumulating transgenic wheat to water stress and salinity. Plant Physiol 131:1748–1755
Agarie S, Shimoda T, Shimizu Y, Baumann K, Sunagawa H, Kondo A, Cushman JC (2007) Salt tolerance, salt accumulation, and ionic homeostasis in an epidermal bladder-cell-less mutant of the common ice plant Mesembryanthemum crystallinum. J Exp Bot 58:1957–1967
Aghaei K, Ehsanpour AA, Komatsu S (2009) Potato responds to salt stress by increased activity of antioxidant enzymes. J Integr Plant Biol 51:1095–1103
Ahanger MA, Qi M, Huang Z, Xu X, Begum N, Qin C, Zhang C, Ahmad N, Mustafa NS, Ashraf M, Zhang L (2021) Improving growth and photosynthetic performance of drought stressed tomato by application of nano-organic fertilizer involves up-regulation of nitrogen, antioxidant and osmolyte metabolism. Ecotoxicol Environ Saf 216:112195
Ahmed S, Kouser S, Asgher M, Gandhi SG (2021) Plant aquaporins: a frontward to make crop plants drought resistant. Physiol Plant 172:1089–1105
Aktas H, Abak K, Ozturk L, Cakmak I (2006) The effect of zinc on growth and shoot concentration of sodium and potassium in pepper plants under salinity stress. Turk J Agric For 30:407–412
Alabdallah NM, Alzahrani HS (2020) The potential mitigation effect of ZnO nanoparticles on [Abelmoschus esculentus L. Moench] metabolism under salt stress conditions. Saudi J Biol Sci 27:3132–3137
Alabdallah NM, Hasan MM, Salih AM, Roushdy SS, Al-Shammari AS, Alsanie SI, El-Zaidy M (2021) Silver nanoparticles improve growth and protect against oxidative damage in eggplant seedlings under drought stress. Plant Soil Environ 67:617–624
Al-Abdallat AM, Al-Debei HS, Ayad JY, Hasan S (2014) Over-expression of SlSHN1 gene improves drought tolerance by increasing cuticular wax accumulation in tomato. Int J Mol Sci 15:19499–19515
Almeida P, de Boer GJ, de Boer AH (2014) Differences in shoot Na+ accumulation between two tomato species are due to differences in ion affinity of HKT1; 2. J Plant Physiol 171:438–447
Altaf MA, Shahid R, Ren MX, Naz S, Altaf MM, Khan LU, Tiwari RK, Lal MK, Shahid MA, Kumar R, Nawaz MA (2022) Melatonin improves drought stress tolerance of tomato by modulating plant growth, root architecture, photosynthesis, and antioxidant defense system. Antioxidants 11:309
Altunlu H, Gul A (2012) Increasing drought tolerance of tomato plants by grafting. Acta Hort 960:183–190
Aqeel U, Aftab T, Khan MMA, Naeem M, Khan MN (2021) A comprehensive review of impacts of diverse nanoparticles on growth, development and physiological adjustments in plants under changing environment. Chemosphere. https://doi.org/10.1016/j.chemosphere.2021.132672
Arafa SA, Attia KA, Niedbała G, Piekutowska M, Alamery S, Abdelaal K, Alateeq TK, Ali AM, Elkelish M, Attallah A (2021) Seed priming boost adaptation in pea plants under drought stress. Plants 10:2201
Arkhipova TN, Prinsen E, Veselov SU, Martinenko EV, Melentiev AI, Kudoyarova GR (2007) Cytokinin producing bacteria enhance plant growth in drying soil. Plant Soil 292:305–315
Aroca R, Vernieri P, Ruiz-Lozano JM (2008) Mycorrhizal and non-mycorrhizal Lactuca sativa plants exhibit contrasting responses to exogenous ABA during drought stress and recovery. J Exp Bot 59:2029–2041
Aroca R, Ruiz-Lozano JM, Zamarreño ÁM, Paz JA, García-Mina JM, Pozo MJ, López-Ráez JA (2013) Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. J plant physiol 170:47–55
Arvin MJ, Kazemi Poor N. (2003) Response of onion cultivars to drought and salinity stresses at germination stage and seed priming by chemicals to improve germination. Iran J Hortic Sci Tech 4:95–104
Assaha DV, Mekawy AMM, Ueda A, Saneoka H (2015) Salinity-induced expression of HKT may be crucial for Na+ exclusion in the leaf blade of huckleberry (Solanum scabrum Mill.), but not of eggplant (Solanum melongena L.). Biochem Biophys Res Commun 460:416–421
AVRDC (2005) Annual report. AVRDC—The World Vegetable Center, Shanhua
Baghbani A, Forghani AH, Kadkhodaie A (2013) Study of salinity stress on germination and seedling growth in greenhouse cucumber cultivars. J Basic Appl Sci Res 3:1137–1140
Baghour M, Gálvez FJ, Sánchez ME, Aranda MN, Venema K, Rodríguez-Rosales MP (2019) Overexpression of LeNHX2 and SlSOS2 increases salt tolerance and fruit production in double transgenic tomato plants. Plant Physiol Biochem 135:77–86
Bahadur A, Chatterjee A, Kumar R, Singh M, Naik PS (2011) Physiological and biochemical basis of drought tolerance in vegetables. Vege Sci 38:1–16
Bahmani K, Noori SA, Darbandi AI, Akbari A (2015) Molecular mechanisms of plant salinity tolerance: a review. Aust J Crop Sci 9:321–336
Bakhshi B, Mohseni FE, Nikpay N, Ebrahimi MA, Bihamta MR, Mardi M, Salekdeh GH (2016) MicroRNA signatures of drought signaling in rice root. PLoS ONE 11:e0156814
Baum C, El-Tohamy W, Gruda N (2015) Increasing the productivity and product quality of vegetable crops using arbuscular mycorrhizal fungi: a review. Sci Hortic 187:131–141
Behera TK, Krishna R, Ansari WA, Aamir M, Kumar P, Kashyap SP, Pandey S, Kole C (2022) Approaches involved in the vegetable crops salt stress tolerance improvement: present status and way ahead. Front Plant Sci. https://doi.org/10.3389/fpls.2021.787292
Beltrano J, Ruscitti M, Arango MC, Ronco M (2013) Effects of arbuscular mycorrhiza inoculation on plant growth, biological and physiological parameters and mineral nutrition in pepper grown under different salinity and p levels. J Soil Sci Plant Nutr 13:123–141
Blum A (2009) Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crops Res 112:119–123
Bolton A, Simon P (2019) Variation for salinity tolerance during seed germination in diverse carrot [Daucus carota (L.)] germplasm. Hort Sci 54:38–44
Cai S, Papanatsiou M, Blatt MR, Chen ZH (2017) Speedy grass stomata: emerging molecular and evolutionary features. Mol Plant 10:912–914
Cattivelli L, Rizza F, Badeck FW, Mazzucotelli E, Mastrangelo AM, Francia E, Marè C, Tondelli A, Stanca AM (2008) Drought tolerance improvement in crop plants: an integrated view from breeding to genomics. Field crops res 105:1–4
Chakma R, Biswas A, Saekong P, Ullah H, Datta A (2021b) Foliar application and seed priming of salicylic acid affect growth, fruit yield, and quality of grape tomato under drought stress. Sci Hort 280:109904
Chakma R, Saekong P, Biswas A, Ullah H, Datta A (2021a) Growth, fruit yield, quality, and water productivity of grape tomato as affected by seed priming and soil application of silicon under drought stress. Agric Water Manag 256:107055
Chartzoulakis K, Klapaki G (2000) Response of two greenhouse pepper hybrids to NaCl salinity during different growth stages. Sci Hort 86:247–260
Chatterjee A, Solankey SS (2015) Functional physiology in drought tolerance of vegetable crops-an approch to mitigate climate change impact. Clim Dy Hortic Sci 1:149–171
Chaudhary J, Khatri P, Singla P, Kumawat S, Kumari A, Vikram A, Jindal SK, Kardile H, Kumar R, Sonah H, Deshmukh R (2019) Advances in omics approaches for abiotic stress tolerance in tomato. Biology 8:90
Chen Z, Newman I, Zhou M, Mendham N, Zhang G, Shabala S (2005) Screening plants for salt tolerance by measuring K+ flux: a case study for barley. Plant Cell Environ 28:1230–1246
Chen J, Chang SX, Anyia AO (2011) Gene discovery in cereals through quantitative trait loci and expression analysis in water-use efficiency measured by carbon isotope discrimination. Plant Cell Environ 34:2009–2023
Chen K, Fessehaie A, Arora R (2012) Dehydrin metabolism is altered during seed osmopriming and subsequent germination under chilling and desiccation in Spinacia oleracea L. cv. Bloomsdale: possible role in stress tolerance. Plant Sci 183:27–36
Chen ZH, Chen G, Dai F, Wang Y, Hills A, Ruan YL, Zhang G, Franks PJ, Nevo E, Blatt MR (2017) Molecular evolution of grass stomata. Trends Plant Sci 22:124–139
Chen L, Meng J, Luan Y (2019) miR1916 plays a role as a negative regulator in drought stress resistance in tomato and tobacco. Biochem Biophys Res Commun 508:597–602
Chen T, Shabala S, Niu Y, Chen ZH, Shabala L, Meinke H, Venkataraman G, Pareek A, Xu J, Zhou M (2021) Molecular mechanisms of salinity tolerance in rice. Crop J 9:506–520
Chen L, Peng Y, Zhu L, Huang Y, Bie Z, Wu H (2022) CeO2 nanoparticles improved cucumber salt tolerance is associated with its induced early stimulation on antioxidant system. Chemosphere 299:134474
Choi WG, Toyota M, Kim SH, Hilleary R, Gilroy S (2014) Salt stress-induced Ca2 + waves are associated with rapid, long-distance root-to-shoot signaling in plants. Proc Natl Acad Sci USA 111:6497–6502
Colla G, Rouphael Y, Rea E, Cardarelli M (2012) Grafting cucumber plants enhance tolerance to sodium chloride and sulfate salinization. Sci Hort 135:177–185
Condon AG, Richards RA, Rebetzke GJ, Farquhar GD (2004) Breeding for high water-use efficiency. J Exp Bot 55:2447–2460
Davies FT Jr, Potter JR, Linderman RG (1992) Mycorrhiza and repeated drought exposure affect drought resistance and extraradical hyphae development of pepper plants independent of plant size and nutrient content. J Plant Physiol 139:289–294
De Oliveira Oliveira FRAFAD, Medeiros JFD, Sousa FLD, Freir AG (2010) Phosphorus-salinity interaction in radish. Rev Cienc Agron 41:519–526
Deng F, Zeng F, Shen Q, Abbas A, Cheng J, Jiang W, Chen G, Shah AN, Holford P, Tanveer M, Chen Zhang D, ZH, (2022) Molecular evolution and functional modification of plant miRNAs with CRISPR. Trends Plant Sci. https://doi.org/10.1016/j.tplants.2022.01.009
Desoky ES, Elrys AS, Rady MM (2019) Integrative moringa and licorice extracts application improves Capsicum annuum fruit yield and declines its contaminant contents on a heavy metals-contaminated saline soil. Ecotoxicol Environ Saf 169:50–60
Devi N, Arumugam T (2019) Salinity tolerance in vegetable crops: a review. J Pharm Phytochem 8:2717–2721
Dhankher OP, Foyer CH (2018) Climate resilient crops for improving global food security and safety. Plant Cell Environ 41:877–884
Di Stasio E, Cirillo V, Raimondi G, Giordano M, Esposito M, Maggio A (2020) Osmo-priming with seaweed extracts enhances yield of salt-stressed tomato plants. Agronomy 10:1559
Elkhatib HA, Elkhatib EA, Allah AMK, El-Sharkawy AM (2004) Yield response of salt-stressed potato to potassium fertilization: Apreliminary mathematical model. J Plant Nutr 27:111–122
El-Zohri M, Al-Wadaani NA, Bafeel SO (2021) Foliar sprayed green zinc oxide nanoparticles mitigate drought-induced oxidative stress in tomato. Plants 10:2400
Fan HF, Ding L, Xu YL, Du CX (2017) Antioxidant system and photosynthetic characteristics responses to short-term PEG-induced drought stress in cucumber seedling leaves. Russ J Plant Physiol 64:162–173
FAO (2021) World Food and Agriculture—statistical Yearbook 2021. FAO, Rome
Farzane A, Nemati H, Shoor M, Ansari H (2021) Foliar application of potassium on antioxidant enzyme activities of tomato plants under drought stress. Adv Hortic Sci 35:1
Fikret Y, Manar T, Şebnem E, Şebnem K, Özlem U (2013) SOD, CAT, GR and APX enzyme activities in callus tissues of susceptible and tolerant eggplant varieties under salt stress. Res J Biotech 8:45–51
Fu Q, Liu C, Ding N, Guo B (2010) Ameliorative effects of inoculation with the plant growth promoting rhizobacterium Pseudomonas sp. DW1 on growth of eggplant (Solanum melongena L.) seedlings under salt stress. Agric Water Manage 97:1994–2000
Furtana GB, Tipirdamaz R (2010) Physiological and antioxidant response of three cultivars of cucumber (Cucumis sativus L.) to salinity. Turk J Biol 34:287–296
Gamalero E, Berta G, Massa N, Glick BR, Lingua G (2010) Interactions between Pseudomonas putida UW4 and Gigaspora rosea BEG9 and their consequences for the growth of cucumber under salt-stress conditions. J Appl Microbiol 108:236–245
Gérard F, Blitz-Frayret C, Hinsinger P (2017) Modelling the interactions between root system architecture, root functions and reactive transport processes in soil. Plant Soil 413:161–180
Ghani MI, Saleem S, Rather SA, Rehmani MS, Alamri S, Rajput VD, Kalaji HM, Saleem N, Sial TA, Liu M (2022) Foliar application of zinc oxide nanoparticles: an effective strategy to mitigate drought stress in cucumber seedling by modulating antioxidant defense system and osmolytes accumulation. Chemosphere 289:133202
Gharsallah C, Fakhfakh H, Grubb D, Gorsane F (2016) Effect of salt stress on ion concentration, proline content, antioxidant enzyme activities and gene expression in tomato cultivars. AoB Plants. https://doi.org/10.1093/aobpla/plw055
Gilroy S, Suzuki N, Miller G, Choi WG, Toyota M, Devireddy AR, Mittler R (2014) A tidal wave of signals: calcium and ROS at the forefront of rapid systemic signaling. Trends Plant Sci 19:623–630
Gisbert C, Rus AM, Bolarín MC, López-Coronado JM, Arrillaga I, Montesinos C, Caro M, Serrano R, Moreno V (2000) The yeast HAL1 gene improves salt tolerance of transgenic tomato. Plant Physiol 123:393–402
Gruda N, Bisbis M, Tanny J (2019) Impacts of protected vegetable cultivation on climate change and adaptation strategies for cleaner production-a review. J Clean Prod 225:324e339
Guo X, Zhang L, Wang X, Zhang M, Xi Y, Wang A, Zhu J (2019) Overexpression of Saussurea involucrata dehydrin gene SiDHN promotes cold and drought tolerance in transgenic tomato plants. PLoS ONE 14:pe0225090
Gupta K, Sengupta A, Chakraborty M, Gupta B (2016) Hydrogen peroxide and polyamines act as double edged swords in plant abiotic stress responses. Front plant sci 7:1343
GVR (2018) Biostimulants market size worth $4.14 Billion By 2025|CAGR: 10.2%. https://www.grandviewresearch.com/press-release/global-biostimulants-market. Accessed 13 Mar 2022
Habib SH, Kausar H, Saud HM (2016) Plant growth-promoting rhizobacteria enhance salinity stress tolerance in okra through ROS-scavenging enzymes. Biomed Res Int. doi:https://doi.org/10.1155/2016/6284547
Hannachi S, Van Labeke M-C (2018) Salt stress affects germination, seedling growth and physiological responses differentially in eggplant cultivars (Solanum melongena L.). Sci Hort 228:56–65
Hashimoto K, Kudla J (2011) Calcium decoding mechanisms in plants. Biochimie 93:2054–2059
He Z, Huang Z (2013) Expression analysis of LeNHX1 gene in mycorrhizal tomato under salt stress. J Microbiol 51:100–104
He Y, Zhu Z, Yang J, Ni X, Zhu B (2009) Grafting increases the salt tolerance of tomato by improvement of photosynthesis and enhancement of antioxidant enzymes activity. Environ Exp Bot 66:270–278
He F, Wang HL, Li HG, Su Y, Li S, Yang Y, Feng CH, Yin W, Xia X (2018) Pe CHYR 1, a ubiquitin E3 ligase from Populus euphratica, enhances drought tolerance via ABA-induced stomatal closure by ROS production in Populus. Plant Biotechnol J 16:1514–1528
Hopmans JW, Qureshi AS, Kisekka I, Munns R, Grattan SR, Rengasamy P, Ben-Gal A, Assouline S, Javaux M, Minhas PS, Raats PA (2021) Critical knowledge gaps and research priorities in global soil salinity. Advan Agron 169:1–191
Horie T, Motoda J, Kubo M, Yang H, Yoda K, Horie R, Chan WY, Leung HY, Hattori K, Konomi M, Osumi M (2005) Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na + unloading from xylem vessels to xylem parenchyma cells. Plant J 44:928–938
Hussain S, Khalid MF, Hussain M, Ali MA, Nawaz A, Zakir I, Fatima Z, Ahmad S (2018) Role of micronutrients in salt stress tolerance to plants. In: Hasanuzzaman M, Fujita M, Oku H, Nahar K, Hawrylak-Nowak B (eds) Plant nutrients and abiotic stress tolerance. Springer, Singapore, pp 363–376
Ibrahim A, Wahb-Allah M, Abdel-Razzak H, Alsadon A (2014) Growth, yield, quality and water use efficiency of grafted tomato plants grown in greenhouse under different irrigation levels. Life Sci J 11:118–126
Ibrahim MF, Elbar OH, Farag R, Hikal M, El-Kelish A, El-Yazied AA, Alkahtani J, El-Gawad HG (2020) Melatonin counteracts drought induced oxidative damage and stimulates growth, productivity and fruit quality properties of tomato plants. Plants 9:1276
Jaarsma R, de Boer AH (2018) Salinity tolerance of two potato cultivars (Solanum tuberosum) correlates with differences in vacuolar transport activity. Front Plant Sci 9:737
Jiang W, Tong T, Chen X, Deng F, Zeng F, Pan R, Zhang W, Chen G, Chen ZH (2021) Molecular response and evolution of plant anion transport systems to abiotic stress. Plant Mol Biol 30:1–6
Jing LZ, Kui GY, Hang LS, Gang BJ (2009) Effects of exogenous hydrogen peroxide on ultrastructure of chloroplasts and activities of antioxidant enzymes in greenhouse-ecotype cucumber under drought stress. Acta Hort Sinica 36:1140–1146
Khalid MF, Hussain S, Ahmad S, Ejaz S, Zakir I, Ali MA, Ahmed N, Anjum MA (2019) Impacts of abiotic stresses on growth and development of plants. Plant tolerance to environmental stress: role of phytoprotectants. CRC-Press, USA, pp 1–8
Khalid MF, Hussain S, Anjum MA, Ahmad S, Ali MA, Ejaz S, Morillon R (2020) Better salinity tolerance in tetraploid vs. diploid volkamer lemon seedlings is associated with robust antioxidant and osmotic adjustment mechanisms. J Plant Physiol 244:153071
Khalid MF, Vincent C, Morillon R, Anjum MA, Ahmad S, Hussain S (2021) Different strategies lead to a common outcome: different water-deficit scenarios highlight physiological and biochemical strategies of water deficit tolerance in diploid vs tetraploid volkamer lemon. Tree Physiol 41:2359–2374
Kiapour H, Moaveni P, Habibi D (2015) Evaluation of the application of gibbrellic acid and titanium dioxide nanoparticles under drought stress on some traits of basil (Ocimum basilicum L.). Int J Agron Agric Res 6:138–150
Koevoets IT, Venema JH, Elzenga JTM, Testerink C (2016) Roots withstanding their environment: exploiting root system architecture responses to abiotic stress to improve crop tolerance. Front Plant Sci 7:1335
Kohler J, Hernandez JA, Caravaca F, Roldan A (2009) Induction of antioxidant enzymes is involved in the greater effectiveness of a PGPR versus AM fungi with respect to increasing the tolerance of lettuce to severe salt stress. Environ Exp Bot 65:245–252
Kopta T, Sekara A, Pokluda R, Ferby V, Caruso G (2020) Screening of chilli pepper genotypes as a source of capsaicinoids and antioxidants under conditions of simulated drought stress. Plants 9:364
Kosma DK, Jenks MA (2007) Eco-physiological and molecular-genetic determinants of plant cuticle function in drought and salt stress tolerance. In: Jenks MA, Hasegawa PM, Jain SM (eds) Advances in molecular breeding toward drought and salt tolerant crops. Springer, Dordrecht, pp 91–120
Kulkarni M, Soolanayakanahally R, Ogawa S, Uga Y, Selvaraj MG, Kagale S (2017) Drought response in wheat: key genes and regulatory mechanisms controlling root system architecture and transpiration efficiency. Front Chem 5:106
Kumar G, Purty RS, Sharma MP, Singla-Pareek SL, Pareek A (2009) Physiological responses among Brassica species under salinity stress show strong correlation with transcript abundance for SOS pathway-related genes. J Plant Physiol 166:507–520
Kumar R, Solankey SS, Singh M (2012) Breeding for drought tolerance in vegetables. Veg Sci 39:1–15
Kurusu T, Kuchitsu K, Tada Y (2015) Plant signaling networks involving Ca2 + and Rboh/Nox-mediated ROS production under salinity stress. Front Plant Sci 6:427
Laporte MM, Shen B, Tarczynski MC (2002) Engineering for drought avoidance: expression of maize NADP-malic enzyme in tobacco results in altered stomatal function. J Exp Bot 53:699–705
Latef AA, Chaoxing H (2011) Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition, antioxidant enzymes activity and fruit yield of tomato grown under salinity stress. Sci Horti 127:228–233
Lim JH, Kim SD (2013) Induction of drought stress resistance by multi-functional PGPR Bacillus licheniformis K11 in pepper. Plant Pathol J 29:201–208
Liphschitz N, Waisel Y (1974) Existence of salt glands in various genera of Gramineae. New Phytol 73:507–513
Liu L, Duan L, Zhang J, Mi G, Zhang X, Zhang Z, Ren H (2013) Arabidopsis LOS5 gene enhances chilling and salt stress tolerance in cucumber. J Integr Agri 12:825–834
Liu X, Mak M, Babla M, Wang F, Chen G, Veljanoski F, Wang G, Shabala S, Zhou M, Chen ZH (2014) Linking stomatal traits and expression of slow anion channel genes HvSLAH1 and HvSLAC1 with grain yield for increasing salinity tolerance in barley. Front Plant Sci 5:634
Liu Y, Wen L, Shi Y, Su D, Lu W, Cheng Y, Li Z (2021) Stress-responsive tomato gene SlGRAS4 function in drought stress and abscisic acid signaling. Plant Sci 304:110804
Liu D, Guo W, Guo X, Yang L, Hu W, Kuang L, Huang Y, Xie J, Liu Y (2022a) Ectopic overexpression of CsECR from navel orange increases cuticular wax accumulation in tomato and enhances its tolerance to drought stress. Front Plant Sci 13:924552
Liu H, Li C, Yan M, Zhao Z, Huang P, Wei L, Wu X, Wang C, Liao W (2022b) Strigolactone is involved in nitric oxide-enhanced the salt resistance in tomato seedlings. J Plant Res. https://doi.org/10.1007/s10265-022-01371-2
Lu L, Huang M, Huang Y, Corvini PFX, Ji R, Zhao L (2020) Mn3O4 nanozymes boost endogenous antioxidant metabolites in cucumber (Cucumis sativus) plant and enhance resistance to salinity stress. Environ Sci Nano 7:1692–1703
Maham S, Muhammad S (2019) Mitigation of drought stress-induced adverse effects on antioxidant system of eggplant by exogenous application of alpha-tocopherol. Int J Agric Biol 21:971–978
Mahmood T, Rana RM, Ahmar S, Saeed S, Gulzar A, Khan MA, Wattoo FM, Wang X, Branca F, Mora-Poblete F, Mafra GS (2021) Effect of drought stress on capsaicin and antioxidant contents in pepper genotypes at reproductive stage. Plants 10:1286
Mahmoud AWM, Abdeldaym EA, Abdelaziz SM, El-Sawy MB, Mottaleb SA (2019) Synergetic effects of zinc, boron, silicon, and zeolite nanoparticles on confer tolerance in potato plants subjected to salinity. Agronomy 10:19
Malcheska F, Ahmad A, Batool S, Müller HM, Ludwig-Müller J, Kreuzwieser J, Randewig D, Hänsch R, Mendel RR, Hell R, Wirtz M (2017) Drought-enhanced xylem sap sulfate closes stomata by affecting ALMT12 and guard cell ABA synthesis. Plant physiol 174:798–814
Marcum KB (2006) Saline tolerance physiology in grasses. In: Khan MA, Weber DJ (eds) Ecophysiology of high salinity tolerant plants. Springer, Heidelberg, pp 157–172
Marcum KB, Murdoch CL (1994) Salinity tolerance mechanisms of six C4 turfgrasses. J Am Soc Hort Sci 119:779–784
Maser P, Eckelman B, Vaidyanathan R, Horie T, Fairbairn DJ, Kubo M, Yamagami M, Yamaguchi K, Nishimura M, Uozumi N, Robertson W, Sussman MR, Schroeder JI (2002) Altered shoot/root NaR distribution and bifurcating salt sensitivity in Arabidopsis by genetic disruption of the NaR transporter AtHKT1. FEBS Lett 531:157–161
Mittler R (2002) Oxidative stress, antioxidant and stress tolerance. Trends Plant Sci 7:405–410
Mittler R (2017) ROS are good. Trends Plant Sci 22:11–19
Moller IM, Sweetlove LJ (2010) ROS signalling-specificity is required. Trends Plant Sci 15:370–374
Møller IS, Gilliham M, Jha D, Mayo GM, Roy SJ, Coates JC, Haseloff J, Tester M (2009) Shoot Na+ exclusion and increased salinity tolerance engineered by cell type-specific alteration of Na+ transport in Arabidopsis. Plant Cell 21:2163–2178
Mombeini M, Alamzadeh Ansari N, Abdossi V, Naseri A (2021) Reducing destructive effects of drought stress on cucumber through seed priming with silicic acid, pyridoxine, and ascorbic acid along with foliar spraying with silicic acid. Agric Conspec Sci 86:35–49
Muñiz García MN, Cortelezzi JI, Fumagalli M, Capiati DA (2018) Expression of the Arabidopsis ABF4 gene in potato increases tuber yield, improves tuber quality and enhances salt and drought tolerance. Plant Mol Biol 98:137–152
Munns R (1993) Physiological processes limiting plant growth in saline soil:some dogmas and hypotheses. Plant Cell Environ 16:15–24
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Enviro 25:239–250
Munns R (2005) Genes and salt tolerance: bringing them together. New Phytol 167:645–663
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Munns R, Day DA, Fricke W, Watt M, Arsova B, Barkla BJ, Bose J, Byrt CS, Chen ZH, Foster KJ, Gilliham M (2020) Energy costs of salt tolerance in crop plants. New Phytol 225:1072–1090
Naidoo Y, Naidoo G (1999) Cytochemical localization of adenosine triphosphatase activity in salt glands of Sporobolus virginicus (L.) Kunth. S Afr J Bot 65:370–373
Naik PS, Singh M, Ranjan JK (2017) Impact of climate change on vegetable production and adaptation measures. Abiotic stress management for resilient agriculture. Springer, Singapore, pp 413–428
Niu S, Luo Y, Li D, Cao S, Xia J, Li J, Smith MD (2014) Plant growth and mortality under climatic extremes: an overview. Environ Exp Bot 98:13–19
Oh DH, Barkla BJ, Vera-Estrella R, Pantoja O, Lee SY, Bohnert HJ, Dassanayake M (2015) Cell type‐specific responses to salinity—the epidermal bladder cell transcriptome of Mesembryanthemum crystallinum. New Phytol 207:627–644
Osakabe Y, Yamaguchi-Shinozaki K, Shinozaki K, Tran LSP (2014) ABA control of plant macroelement membrane transport systems in response to water deficit and high salinity. New Phytol 202:35–49
Pagès L (2021) Simulating the diversity and plasticity of root systems using 3D models of the root system architecture. The root systems in sustainable agricultural intensification. Wiley, Hoboken, pp 355–373
Palaniyandi SA, Damodharan K, Yang SH, Suh JW (2014) Streptomyces sp. strain PGPA39 alleviates salt stress and promotes growth of ‘Micro Tom’ tomato plants. J Appl Microbiol 117:766–773
Parihar P, Singh S, Singh R, Singh VP, Prasad SM (2015) Effect of salinity stress on plants and its tolerance strategies: a review. Environ Sci Pollut Res 22:4056–4075
Park S, Li J, Pittman JK, Berkowitz GA, Yang H, Undurraga S, Morris J, Hirschi KD, Gaxiola RA (2005) Up-regulation of a H+ - pyrophosphatase (H+ - PPase) as a strategy to engineer drought-resistant crop plants. Proc Natl Acad Sci USA 102:18830–18835
Parkash V, Singh S (2020) A review on potential plant-based water stress indicators for vegetable crops. Sustainability 12:3945
Passioura JB, Munns R (1984) Hydraulic resistance of plants. II. Effects of rooting medium, and time of day, in barley and lupin. Funct Plant Biol 11:341–350
Patel J, Mishra A (2021) Plant aquaporins alleviate drought tolerance in plants by modulating cellular biochemistry, root-architecture, and photosynthesis. Physiol Plant 172:1030–1044
Pathak TB, Maskey ML, Dahlberg JA, Kearns F, Bali KM, Zaccaria D (2018) Climate change trends and impacts on California agriculture: a detailed review. Agronomy 8:25
Penella C, Nebauer SG, San Bautista A, López-Galarza S, Calatayud Á (2014) Rootstock alleviates PEG-induced water stress in grafted pepper seedlings: physiological responses. J Plant Physiol 171:842–885
Petretto GL, Urgeghe PP, Massa D, Melito S (2019) Effect of salinity (NaCl) on plant growth, nutrient content, and glucosinolate hydrolysis products trends in rocket genotypes. Plant Physiol Biochem 141:30–39
Rady MM, Desoky ES, Elrys AS, Boghdady MS (2019) Can licorice root extract be used as an effective natural biostimulant for salt-stressed common bean plants? S Afr J Bot 121:294–305
Rahnama H, Ebrahimzadeh H (2005) The effect of NaCl on antioxidant enzyme activities in potato seedlings. Biol plant 49:93–97
Ramadan T, Flowers JJ (2004) Effects of salinity and benzyl adenine on development and function of microhairs of Zea mays L. Planta 219:639–648
Ranjan A, Sinha R, Lal SK, Bishi SK, Singh AK (2021) Phytohormone signalling and cross-talk to alleviate aluminium toxicity in plants. Plant Cell Rep 40:1331–1343
Rausch T, Wachter A (2005) Sulfur metabolism a versatile platform for launching defense operations. Trends Plant Sci 10:503–509
Razi K, Muneer S (2021) Drought stress-induced physiological mechanisms, signaling pathways and molecular response of chloroplasts in common vegetable crops. Crit Rev Biotechnol 41:1–40
Romero-Aranda MR, González-Fernández P, Pérez-Tienda JR, López-Diaz MR, Espinosa J, Granum E, Traverso J, Pineda B, Garcia-Sogo B, Moreno V, Asins MJ (2020) Na + transporter HKT1; 2 reduces flower Na + content and considerably mitigates the decline in tomato fruit yields under saline conditions. Plant Physiol Biochem 154:341–352
Rouphael Y, Cardarelli M, Rea E, Colla G (2012) Improving melon and cucumber photosynthetic activity, mineral composition, and growth performance under salinity stress by grafting onto Cucurbita hybrid rootstocks. Photosynthetica 50:180–188
Safdar H, Amin A, Shafiq Y, Ali A, Yasin R, Shoukat A, Hussan MU, Sarwar MI (2019) A review: impact of salinity on plant growth. Nat Sci 17:34–40
Samancioglu A, Yildirim E, Turan M, Kotan R, Sahin U, Kul R (2016) Amelioration of drought stress adverse effect and mediating biochemical content of cabbage seedlings by plant growth promoting rhizobacteria. Int J Agri Biol. https://doi.org/10.17957/IJAB/15.0195
Sánchez-Rodríguez E, del Rubio-Wilhelmi M, Blasco M, Leyva B, Romero R, Ruiz L JM (2012) Antioxidant response resides in the shoot in reciprocal grafts of drought-tolerant and drought-sensitive cultivars in tomato under water stress. Plant Sci 188:89–96
Sánchez-Rodríguez E, Leyva R, Constán-Aguilar C, Romero L, Ruiz JM (2014) How does grafting affect the ionome of cherry tomato plants under water stress? Soil Sci Plant Nutr 60:145–155
Sang-Mo K, Radhakrishnan R, Latif Khan A, Kim M-J, Jae-Man P, Bo-Ra K, Dong-Hyun S, In-Jung L (2014) Gibberellin secreting rhizobacterium, Pseudomonas putida H-2-3 modulates the hormonal and stress physiology of soybean to improve the plant growth under saline and drought conditions. Plant Physiol Biochem 84:115–124
Sarwar M, Amjad M, Ayyub CM (2017) Alleviation of salt stress in cucumber (Cucumis sativus) through seed priming with triacontanol. Int J Agri Biol 19(4):771–778
Savvides A, Ali S, Tester M, Fotopoulos V (2016) Chemical priming of plants against multiple abiotic stresses: mission possible? Trend Plant Sci 21:329–340
Semida WM, Abdelkhalik A, Mohamed G, El-Mageed A, Taia A, El-Mageed A, Shimaa A, Rady MM, Ali EF (2021) Foliar application of zinc oxide nanoparticles promotes drought stress tolerance in eggplant (Solanum melongena L.). Plants 10:421
Seymen M (2021) Comparative analysis of the relationship between morphological, physiological and biochemical properties in spinach (Spinacea oleracea L.) under deficit irrigation conditions. Turkish J Agric For 45:55–67
Shabala SN, Shabala SI, Martynenko AI, Babourina O, Newman IA (1998) Salinity effect on bioelectric activity, growth, Na+ accumulation and chlorophyll fluorescence of maize leaves: a comparative survey and prospects for screening. Funct Plant Biol 25:609–616
Shabala S, Bose J, Hedrich R (2014) Salt bladders: do they matter? Trends Plant Sci 19:687–691
Shabala S, Chen G, Chen ZH, Pottosin I (2020) The energy cost of the tonoplast futile sodium leak. New Phytol 225:1105–1110
Shahid SA, Zaman M, Heng L (2018) Soil salinity: historical perspectives and a world overview of the problem. Guideline for salinity assessment, mitigation and adaptation using nuclear and related techniques. Springer, Cham, pp 43–53
Shannon MC, Grieve CM (1998) Tolerance of vegetable crops to salinity. Sci Hortic 78:5–38
Shepherd T, Wynne Griffiths D (2006) The effects of stress on plant cuticular waxes. New Phytol 171:469–499
Shumaila S, Ullah S (2020) Mitigation of salinity-induced damages in Capsicum annum L. (sweet pepper) seedlings using priming techniques: a future perspective of climate change in the region. Commun Soil Sci Plant Anal 51:1602–1625
Siddikee MA, Glick BR, Chauhan PS, Yim WJ, Sa T (2011) Enhancement of growth and salt tolerance of red pepper seedlings (Capsicum annuum L.) by regulating stress ethylene synthesis with halotolerant bacteria containing 1-aminocyclopropane-1-carboxylic acid deaminase activity. Plant Physiol Biochem 49:427–434
Singh J, Sastry EV, Singh V (2012) Effect of salinity on tomato (Lycopersicon esculentum Mill.) during seed germination stage. Physiol Mol Biol Plants 18:45–50
Sivritepe N, Sivritepe HO, Eris A (2003) The effects of NaCl priming on salt tolerance in melon seedlings grown under saline conditions. Sci Hort 97:229–237
Slavin JL, Lloyd B (2012) Health benefits of fruits and vegetables. Adv Nutr 3:506e516
Sofy AR, Sofy MR, Hmed AA, Dawoud RA, Refaey EE, Mohamed HI, El-Dougdoug NK (2021) Molecular characterization of the Alfalfa mosaic virus infecting Solanum melongena in Egypt and the control of its deleterious effects with melatonin and salicylic acid. Plants 10:459
Steudle E, Lüttge U, Zimmermann U (1975) Water relations of the epidermal bladder cells of the halophytic species Mesembryanthemum crystallinum: direct measurements of hydrostatic pressure and hydraulic conductivity. Planta 126:229–246
Sun J, Cao H, Cheng J, He X, Sohail H, Niu M, Huang Y, Bie Z (2018) Pumpkin CmHKT1; 1 controls shoot Na+ accumulation via limiting Na+ transport from rootstock to scion in grafted cucumber. Intl J Mol Sci 19:2648
Szepesi Á (2008) Influence of exogenous salicylic acid on antioxidant enzyme activities in the roots of salt stressed tomato plants. Acta Biol Szeg 52:199–200
Tavakkoli E, Fatehi F, Coventry S, Rengasamy P, McDonald GK (2011) Additive effects of Na+ and Cl–ions on barley growth under salinity stress. J Exp Bot 62:2189–2203
Tyerman SD, Munns R, Fricke W, Arsova B, Barkla BJ, Bose J, Bramley H, Byrt C, Chen Z, Colmer TD, Cuin T (2019) Energy costs of salinity tolerance in crop plants. New Phytol. https://doi.org/10.1111/nph.15555
Tyerman SD, McGaughey SA, Qiu J, Yool AJ, Byrt CS (2021) Adaptable and multifunctional ion-conducting aquaporins. Annu Rev Plant Biol 72:703–36
Ullah U, Ashraf M, Shahzad SM, Siddiqui AR, Piracha MA, Suleman M (2016) Growth behavior of tomato (Solanum lycopersicum L.) under drought stress in the presence of silicon and plant growth promoting rhizobacteria. Soil Environ 35(1):65–75
Van Zelm E, Zhang Y, Testerink C (2020) Salt tolerance mechanisms of plants. Ann Rev Plant Biol 71:403–433
Visentin I, Vitali M, Ferrero M, Zhang Y, Ruyter-Spira C, Novák O, Strnad M, Lovisolo C, Schubert A, Cardinale F (2016) Low levels of strigolactones in roots as a component of the systemic signal of drought stress in tomato. New Phyto 212:954–963
Walter J, Jentsch A, Beierkuhnlein C, Kreyling J (2013) Ecological stress memory and cross stress tolerance in plants in the face of climate extremes. Environ Exp Bot 94:3–8
Wang JW, Wang LJ, Mao YB, Cai WJ, Xue HW, Chen XY (2005a) Control of root cap formation by microRNA-targeted auxin response factors in Arabidopsis. Plant Cell 17:2204–2216
Wang Y, Ying J, Kuzma M, Chalifoux M, Sample A, McArthur C, Uchacz T, Sarvas C, Wan J, Tennis DT, McCourt P, Huang Y (2005b) Molecular tailoring of farnesylation for plant drought tolerance and yield protection. Plant J 43:413–424
Wang W, Zhang Y, Xu C, Ren J, Liu X, Black K, Gai X, Wang Q, Ren H (2015) Cucumber ECERIFERUM1 (CsCER1), which influences the cuticle properties and drought tolerance of cucumber, plays a key role in VLC alkanes biosynthesis. Plant Mol Biol 87:219–233
Wang L, Li QT, Lei Q, Feng C, Zheng X, Zhou F, Li L, Liu X, Wang Z, Kong J (2017) Ectopically expressing MdPIP1; 3, an aquaporin gene, increased fruit size and enhanced drought tolerance of transgenic tomatoes. BMC Plant Biol 17:1–3
Wang J, Zhang L, Cao Y, Qi C, Li S, Liu L, Wang G, Mao A, Ren S, Guo YD (2018) CsATAF1 positively regulates drought stress tolerance by an ABA-dependent pathway and by promoting ROS scavenging in cucumber. Plant Cell Physiol 59:930–945
Worku W, Chapman GP (1998) The salt secretion physiology of a Chloridoid grass, Cynodon dactylon (L.) Pers., and its implications. Sinet 21:1–16
Wu X, Zhu Z, Li X, Zha D (2012) Effects of cytokinin on photosynthetic gas exchange, chlorophyll fluorescence parameters and antioxidative system in seedlings of eggplant (Solanum melongena L.) under salinity stress. Acta Physiol Planta 34:2105–2114
Wu H, Liu L, Chen Y, Liu T, Jiang Q, Wei Z, Li C, Wang Z (2022) Tomato SlCER1–1 catalyzes the synthesis of wax alkanes, increasing drought tolerance and fruit storability. Horti Res. https://doi.org/10.1093/hr/uhac004
Xue D, Zhang X, Lu X, Chen G, Chen ZH (2017) Molecular and evolutionary mechanisms of cuticular wax for plant drought tolerance. Front Plant Sci 8:621
Yan M (2015) Seed priming stimulate germination and early seedling growth of Chinese cabbage under drought stress. S Afr J Bot 99:88–92
Yan M (2016) Hydro-priming increases seed germination and early seedling growth in two cultivars of Napa cabbage (Brassica rapa subsp. pekinensis) grown under salt stress. J Hortic Sci Biotechnol 91:421–426
Yang Q, Chen ZZ, Zhou XF, Yin HB, Li X, Xin XF, Hong XH, Zhu JK, Gong Z (2009) Over-expression of SOS (salt overly sensitive) genes increases salt tolerance in transgenic Arabidopsis. Mol Plant 2:22–31
Yong MT, Solis CA, Amatoury S, Sellamuthu G, Rajakani R, Mak M, Venkataraman G, Shabala L, Zhou M, Ghannoum O, Holford P (2022) Proto Kranz-like leaf traits and cellular ionic regulation are associated with salinity tolerance in a halophytic wild rice. Stress Biol 2:1–9
Zhang X, Zou Z, Gong P, Zhang J, Ziaf K, Li H, Xiao F, Ye Z (2011) Over-expression of microRNA169 confers enhanced drought tolerance to tomato. Biotechnol lett 33:403–409
Zhang FP, Sussmilch F, Nichols DS, Cardoso AA, Brodribb TJ, McAdam SA (2018) Leaves, not roots or floral tissue, are the main site of rapid, external pressure-induced ABA biosynthesis in angiosperms. J Exp Bot 69:1261–1267
Zhang T, Shi Z, Zhang X, Zheng S, Wang J, Mo J (2020) Alleviating effects of exogenous melatonin on salt stress in cucumber. Sci Hort 262:109070
Zhang H, Zhu J, Gong Z, Zhu JK (2022a) Abiotic stress responses in plants. Nat Rev Genet 23:104–119
Zhang X, Zhang L, Ma C, Su M, Wang J, Zheng S, Zhang T (2022b) Exogenous strigolactones alleviate the photosynthetic inhibition and oxidative damage of cucumber seedlings under salt stress. Sci Hort 297:110962
Zhou R, Kong L, Yu X, Ottosen CO, Zhao T, Jiang F, Wu Z (2019) Oxidative damage and antioxidant mechanism in tomatoes responding to drought and heat stress. Acta Physiol Planta 41:20
Zhu JK (2001) Plant salt tolerance. Trends Plant Sci 6:66–71
Zhu Z, Wei G, Li J, Qian Q, Yu J (2004) Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci 167:527–533
Funding
Open Access funding provided by the Qatar National Library. This report was made possible by the NPRP award [MME01-0826-190018] from the Qatar National Research Fund, a member of The Qatar Foundation. The statements made herein are solely the responsibility of the authors.
Author information
Authors and Affiliations
Contributions
MFK: collected the data and wrote the first draft. SH, Z-HC, and TA: contributed in the conceptualization and critically revised of the article. MY: prepared the salinity aspect and illustration of figures. LihL: revised in the agronomic aspect of the article. LiL: conducted in the English language editing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors have no conflict of interest to declare.
Ethical approval
Not applicable.
Research involving human and animal participants
Not applicable.
Informed consent
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Communicated by Fei Dai.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khalid, M.F., Huda, S., Yong, M. et al. Alleviation of drought and salt stress in vegetables: crop responses and mitigation strategies. Plant Growth Regul 99, 177–194 (2023). https://doi.org/10.1007/s10725-022-00905-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-022-00905-x