Abstract
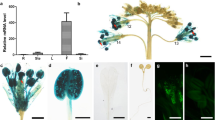
Arabinogalactan proteins (AGPs) are essential members of hyperglycosylated glycoproteins and are critical for the sexual reproduction of plants. To our knowledge, currently Arabodopsis FLA3 and Brassica BcMF8 are the only two members of AGPs reported concerning pollen intine formation. Previously, the orthologue of AtAGP6 in B. campestris, BcMF18, was isolated and proved to affect microspore development. In this study, western blot assay and subcellular localization showed that BcMF18 encoded an approximately 19.5 kDa secretory AGP protein which anchored to extracellular matrices. Ectopic overexpression of BcMF18 (OE) in Arabodopsis caused reduced male fertility and resulted in short siliques with low seed set. Approximately 46% of OE pollen grains were aborted and lacked viability, without all cytoplasmic materials and nuclei. Pollen abortion which led to defective microspore production with no cellulose was observed to start at the bicellular stage. At this point, intine layer displayed abnormalities and degraded from the bicellular stage, which led to the failure in keeping the internal environment stability and resulted in hollow remnants of microspores. However, the basic structure and patterning of the exine layer of collapsed pollen grains seemed unaffected, completed with intact nexine, baculum, and tectum to constitute a pronounced reticulate structure filled with typhine. Based on these results presented here, it is proposed that BcMF18 functions in Arabodopsis and is associated with pollen intine formation, possibly through participating in the formation of proteoglycan structures, which provide strong evidence for subfunctionalization from AtAGP6 to BcMF18.









Similar content being viewed by others
References
Agyare-Tabbi A, Zhang J, Xiong A-S et al (2011) A protein coding for a pollen-specific gene in alfalfa (Medicago sativa L.) is localized mainly in the intine of the pollen wall. Plant Cell Tissue Organ Cult 104:277–280
Alexander MP (1969) Differential staining of aborted and non-aborted pollen. Stain Technol 44:117–122
Ariizumi T, Toriyama K (2011) Genetic regulation of sporopollenin synthesis and pollen exine development. Annu Rev Plant Biol 62:1–24
Basu D, Tian L, Debrosse T et al (2016) Glycosylation of a fasciclin-like arabinogalactan-protein (SOS5) mediates root growth and seed mucilage adherence via a cell wall receptor-like kinase (FEI1/FEI2) pathway in Arabidopsis. PLoS ONE 11:e0145092
Blackmore S, Wortley AH, Skvarla JJ, Rowley JR (2007) Pollen wall development in flowering plants. New Phytol 174:483–498
Borner GHH, Lilley KS, Stevens TJ, Dupree P (2003) Identification of glycosylphosphatidylinositol-anchored proteins in Arabidopsis. A proteomic and genomic analysis. Plant Physiol 132:568–577
Cecchetti V, Altamura MM, Falasca G et al (2008) Auxin regulates Arabidopsis anther dehiscence, pollen maturation, and filament elongation. Plant Cell 20:1760–1774
Coimbra S, Costa M, Jones B et al (2009) Pollen grain development is compromised in Arabidopsis agp6 agp11 null mutants. J Exp Bot 60:3133–3142
Coimbra S, Costa M, Mendes MA et al (2010) Early germination of Arabidopsis pollen in a double null mutant for the arabinogalactan protein genes AGP6 and AGP11. Sex Plant Reprod 23:199–205
Costa M, Nobre MS, Becker JD et al (2013) Expression-based and co-localization detection of arabinogalactan protein 6 and arabinogalactan protein 11 interactors in Arabidopsis pollen and pollen tubes. BMC Plant Biol 13:7
Costa ML, Sobral R, Costa MMR et al (2015) Evaluation of the presence of arabinogalactan proteins and pectins during Quercus suber male gametogenesis. Ann Bot 115:81–92
De Azevedo Souza C, Kim SS, Koch S et al (2009) A novel fatty acyl-CoA synthetase is required for pollen development and sporopollenin biosynthesis in Arabidopsis. Plant Cell 21:507–525
Drakakaki G, Zabotina O, Delgado I et al (2006) Arabidopsis reversibly glycosylated polypeptides 1 and 2 are essential for pollen development. Plant Physiol 142:1480–1492
Driouich A, Zhang GF, Staehelin LA (1993) Effect of brefeldin A on the structure of the Golgi apparatus and on the synthesis and secretion of proteins and polysaccharides in sycamore maple (Acer pseudoplatanus) suspension-cultured cells. Plant Physiol 101:1363–1373
El-Tantawy AA, Solís MT, Costa ML et al (2013) Arabinogalactan protein profiles and distribution patterns during microspore embryogenesis and pollen development in Brassica napus. Plant Reprod 26:231–243
Fei H, Sawhney VK (2001) Ultrastructural characterization of male sterile33 (ms33) mutant in Arabidopsis affected in pollen desiccation and maturation. Can J Bot 79:118–129
Feng X, Ni WM, Elge S et al (2006) Auxin flow in anther filaments is critical for pollen grain development through regulating pollen mitosis. Plant Mol Biol 61:215–226
Gan C (1989) Gene gun accelerates DNA-coated particles to transform intact cells. Scientist 3:25
Hafidh S, Fíla J, Honys D (2016) Male gametophyte development and function in angiosperms: a general concept. Plant Reprod 29:31–51
Hesse M (2000) Pollen wall stratification and pollination. Plant Syst Evol 222:1–17
Hijazi M, Velasquez SM, Jamet E et al (2014) An update on post-translational modifications of hydroxyproline-rich glycoproteins: toward a model highlighting their contribution to plant cell wall architecture. Front Plant Sci 5:395
Huang L, Cao J, Ye W et al (2008a) Transcriptional differences between the male-sterile mutant bcms and wild-type Brassica campestris ssp. chinensis reveal genes related to pollen development. Plant Biol 10:342–355
Huang L, Cao JS, Zhang AH, Ye YQ (2008b) Characterization of a putative pollen-specific arabinogalactan protein gene, BcMF8, from Brassica campestris ssp. chinensis. Mol Biol Rep 35:631–639
Huang L, Cao J, Zhang A et al (2009a) The polygalacturonase gene BcMF2 from Brassica campestris is associated with intine development. J Exp Bot 60:301–313
Huang L, Ye Y, Zhang Y et al (2009b) BcMF9, a novel polygalacturonase gene, is required for both Brassica campestris intine and exine formation. Ann Bot 104:1339–1351
Jiang J, Zhang Z, Cao J (2013) Pollen wall development: the associated enzymes and metabolic pathways. Plant Biol 15:249–263
Jiang J, Yao L, Yu Y et al (2014a) PECTATE LYASE-LIKE 10 is associated with pollen wall development in Brassica campestris. J Integr Plant Biol 56:1095–1105
Jiang J, Yao L, Yu Y et al (2014b) PECTATE LYASE-LIKE 9 from Brassica campestris is associated with intine formation. Plant Sci 229:66–75
Karimi M, Inzé D, Depicker A (2002) GATEWAY™ vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7:193–195
Lavallie ER, Diblasio EA, Kovacic S et al (1993) A thioredoxin gene fusion expression system that circumvents inclusion body formation in the E. coli cytoplasm. Biotechnology 11:187–193
Lee JY, Lee DH (2003) Use of serial analysis of gene expression technology to reveal changes in gene expression in Arabidopsis pollen undergoing cold stress. Plant Physiol 132:517–529
Levitin B, Richter D, Markovich I, Zik M (2008) Arabinogalactan proteins 6 and 11 are required for stamen and pollen function in Arabidopsis. Plant J 56:351–363
Li J, Yu M, Geng LL, Zhao J (2010) The fasciclin-like arabinogalactan protein gene, FLA3, is involved in microspore development of Arabidopsis. Plant J 64:482–497
Lin S, Dong H, Zhang F et al (2014) BcMF8, a putative arabinogalactan protein-encoding gene, contributes to pollen wall development, aperture formation and pollen tube growth in Brassica campestris. Ann Bot 113:777–788
Lin S, Yue X, Miao Y et al (2018) The distinct functions of two classical arabinogalactan proteins BcMF8 and BcMF18 during pollen wall development in Brassica campestris. Plant J 94:60–76
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Lou Y, Xu XF, Zhu J et al (2014) The tapetal AHL family protein TEK determines nexine formation in the pollen wall. Nat Commun 5:3855
Ma HL, Yu L, Liang RH, Zhao J (2015) Functional studies of arabinogalactan proteins in higher plants. Sci Sin Vitae 45:113–123
MacMillan CP, Mansfield SD, Stachurski ZH et al (2010) Fasciclin-like arabinogalactan proteins: specialization for stem biomechanics and cell wall architecture in Arabidopsis and Eucalyptus. Plant J 62:689–703
Marquez J, Seoane-Camba JA, Suarez-Cervera M (1997) The role of the intine and cytoplasm in the activation and germination processes of Poaceae pollen grains. Grana 36:328–342
Mcdonald BA, Martinez JP (1990) Restriction fragment length polymorphisms in Septoria tritici occur at a high frequency. Curr Genet 17:133–138
Mohebali M, Mirbakhsh M, Keshavarz H (2002) Rapid detection of Pneumocystis Carini in spiratory specimens of rats by calcofluor white staining. Iran J Public Health 31:108–110
Moon S, Kim SR, Zhao G et al (2013) Rice GLYCOSYLTRANSFERASE1 encodes a glycosyltransferase essential for pollen wall formation. Plant Physiol 161:663–675
Pereira AM, Masiero S, Nobre MS et al (2014) Differential expression patterns of arabinogalactan proteins in Arabidopsis thaliana reproductive tissues. J Exp Bot 65:5459–5471
Pereira AM, Pereira LG, Coimbra S (2015) Arabinogalactan proteins: rising attention from plant biologists. Plant Reprod 28:1–15
Preston J, Wheeler J, Heazlewood J et al (2004) AtMYB32 is required for normal pollen development in Arabidopsis thaliana. Plant J 40:979–995
Qin Y, Chen D, Zhao J (2007) Localization of arabinogalactan proteins in anther, pollen, and pollen tube of Nicotiana tabacum L. Protoplasma 231:43–53
Regan SM, Moffatt BA (1990) Cytochemical analysis of pollen development in wild-type Arabidopsis and a male-sterile mutant. Plant Cell 2:877–889
Schnurr JA, Storey KK, Jung HJG et al (2006) UDP-sugar pyrophosphorylase is essential for pollen development in Arabidopsis. Planta 224:520–532
Scott RJ, Spielman M, Dickinson HG (2004) Stamen structure and function. Plant Cell 16:S46–S60
Seifert GJ, Roberts K (2007) The biology of arabinogalactan proteins. Annu Rev Plant Biol 58:137–161
Seifert GJ, Xue H, Acet T (2014) The Arabidopsis thaliana FASCICLIN LIKE ARABINOGALACTAN PROTEIN 4 gene acts synergistically with abscisic acid signalling to control root growth. Ann Bot 114:1125–1133
Shi J, Cui M, Yang L et al (2015) Genetic and biochemical mechanisms of pollen wall development. Trends Plant Sci 20:741–753
Showalter AM (2001) Arabinogalactan-proteins: structure, expression and function. Cell Mol Life Sci 58:1399–1417
Song S, Qi T, Huang H, Xie D (2013) Regulation of stamen development by coordinated actions of jasmonate, auxin, and gibberellin in Arabidopsis. Mol Plant 6:1065–1073
Sun W, Kieliszewski MJ, Showalter AM (2004) Overexpression of tomato LeAGP-1 arabinogalactan-protein promotes lateral branching and hampers reproductive development. Plant J 40:870–881
Takano E, Maki M, Mori H et al (1988) Pig heart calpastatin: identification of repetitive domain structures and anomalous behavior in polyacrylamide gel electrophoresis. Biochemistry 27:1964–1972
Tan L, Eberhard S, Pattathil S et al (2013) An Arabidopsis cell wall proteoglycan consists of pectin and arabinoxylan covalently linked to an arabinogalactan protein. Plant Cell 25:270–287
Twell D (2010) Male gametophyte development. In: Pua EC, Davey MR (eds) Plant developmental biology-biotechnological perspectives. Springer, Berlin, pp 225–244
Ueda K, Yoshimura F, Miyao A et al (2013) COLLAPSED ABNORMAL POLLEN1 gene encoding the arabinokinase-like protein is involved in pollen development in rice. Plant Physiol 162:858–871
Wilson ZA, Zhang DB (2009) From Arabidopsis to rice: pathways in pollen development. J Exp Bot 60:1479–1492
Xu SL, Rahman A, Baskin TI, Kieber JJ (2008) Two leucine-rich repeat receptor kinases mediate signaling, linking cell wall biosynthesis and ACC synthase in Arabidopsis. Plant Cell 20:3065–3079
Xu T, Zhang C, Zhou Q, Yang ZN (2016) Pollen wall pattern in Arabidopsis. Sci Bull 61:832–837
Xu D, Shi J, Rautengarten C et al (2017) Defective Pollen Wall 2 (DPW2) encodes an acyl transferase required for rice pollen development. Plant Physiol 173:240–255
Zhang X, Henriques R, Lin SS et al (2006) Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat Protoc 1:641–646
Zhang D, Shi J, Yang X (2016) Role of lipid metabolism in plant pollen exine development. Subcell Biochem 86:315–337
Acknowledgements
We thank Prof. Dr Fengming Song for providing pET32a(+) vector and Escherichia coli strain DE3, and Prof. Dr Ying Miao for providing pB7YWG2,0 vector. This research received the support of grants from the National Natural Science Foundation of China [Grant Nos. 31501764, 31471877, and 31572126], the Grand Science and Technology Special Project of Zhejiang Province [Grant No. 2016C02051-6].
Author information
Authors and Affiliations
Contributions
SL, LH and JC participated in the design of the experimental plan. SL took charge of plant transformation and phenotype comparative observation. LH performed recombinant vector construction and gene expression analysis. YM took part in western blots and transient expression assays. YY performed bioinformatic analysis and statistical analysis. SL and LH wrote the manuscript, while RP provided commentes on it.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lin, S., Huang, L., Miao, Y. et al. Constitutive overexpression of the classical arabinogalactan protein gene BcMF18 in Arabidopsis causes defects in pollen intine morphogenesis. Plant Growth Regul 88, 159–171 (2019). https://doi.org/10.1007/s10725-019-00496-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-019-00496-0