Abstract
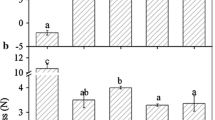
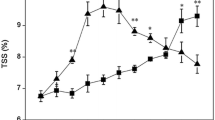
In contrast to climacteric fruits, the ripening regulation of non-climacteric fruits is not well understood. Strawberry is a representative example of this kind of fruit, so it has been used as a model system for this category. In this study, the effect of exogenous brassinosteroid (BR) on the expression of the receptor (FaBRI1) and two components of the signaling pathway (FaBIN2 and FaBRZ1) was analyzed in Fragaria × ananassa cultivar Camino Real by quantitative real-time polymerase chain reaction (RT-qPCR). The physicochemical and phytochemical characteristics of fruits were evaluated in the field and postharvest trials. Perception and signal transduction pathway show little gene action mainly when elicited by epibrassinolide, having treatment differences due mainly the pink stage. This leads us to suggest that BR is involved in strawberry fruit ripening, where the threshold to action seems to be very low and act in the pink stage according to perception and transduction signals. However, owing to the physicochemical and phytochemical characteristics, the BR influence mainly starts in the white stage for total sugar and soluble solid in field assay and for total sugar in the postharvest assays. In addition, there is a positive effect on vitamin C content and total anthocyanins for the treated red fruits in the postharvest assay. All results show that BR is involved in strawberry fruit ripening, in different stages, mainly in a phenylpropanoid pathway. However, new assays to confirm the real BR importance on strawberry maturation and fruit quality.




Similar content being viewed by others
References
Asghari M, Zahedipour P (2016) 24-Epibrassinolide acts as a growth-promoting and resistance-mediating factor in strawberry plants. J Plant Growth Regul 35:722–729
Ayub RA, Bosetto L, Galvão CW, Etto RM et al (2016) Abscisic acid involvement on expression of related gene and phytochemicals during ripening in strawberry fruit Fragaria × ananassa cv. Camino Real. Sci Hort 203:178–184
Belkhadir Y, Jaillais Y (2015) The molecular circuitry of brassinosteroid signaling. New Phytol 206:522–540
Bombarely A, Merchante C, Csukasi F, Cruz-Rus E et al (2010) Generation and analysis of ESTs from strawberry (Fragaria × ananassa) fruits and evaluation of their utility in genetic and molecular studies. BMC Genomics 11:503
Chai YM, Jia HF, Li CL, Dong QH et al (2011) FaPYR1 is involved in strawberry fruit ripening. J Exp Bot 62:5079–5089
Chai Y, Zhang Q, Tian L, Li C-L et al (2013) Brassinosteroid is involved in strawberry fruit ripening. Plant Growth Regul 69:63–69
Champa WAH, Gill MIS, Mahajan BVC, Arora NK (2014) Pre-harvest treatments of brassinosteroids on improving quality of table grapes (Vitis vinifera L.) cv. Flame Seedles. Int J Agric Sc Vet Med 2:96–104
Clouse SD (2011) Brassinosteroid signal transduction: from receptor kinase activation to transcriptional networks regulating plant development. Plant Cell 23:1219–1230
Cumplido-Laso G, Medina-Puche L, Moyano E, Hoffmann T et al (2012) The fruit ripening-related gene FaAAT2 encodes an acyl transferase involved in strawberry aroma biogenesis. J Exp Bot 63:4275–4290
Giovannoni JJ (2004) Genetic regulation of fruit development and ripening. Plant Cell 16(Suppl):S170-S180
Guo H, Li L, Aluru M, Aluru S et al (2013) Mechanisms and networks for brassinosteroid-regulated gene expression. Plant Biol 16:545–553
He J, Gendron JM, Yang Y, Li J et al (2002) The GSK3-like kinase BIN2 phosphorylates and destabilizes BZR1, a positive regulator of the brassinosteroid signaling pathway in Arabidopsis. PNAS USA 99:10185–10190
He J, Gendron JM, Sun Y, Gampala SSL et al (2005) BZR1 Is a transcriptional repressor with dual roles in brassinosteroid homeostasis and growth responses. Science 307:1634–1638
Kang C, Darwish O, Geretza A, Shahan R et al (2013) Genome-scale transcriptomic insights into early-stage fruit development in woodland strawberry Fragaria vesca. Plant Cell 25:1960–1978
Karlidag H, Yildirim E, Turan M (2012) Role of 24-epibrassinolide in mitigating the adverse effects of salt stress on stomatal conductance, membrane permeability, and leaf water content, ionic composition in salt stressed strawberry (Fragaria × ananassa). Sci Hort 130:133–140
Lee SK, Kader AA (2000) Pre-harvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol Technol 20:207–220
Li J, Nam KH, Vafeados D, Chory J (2001) BIN2, a new brassinosteroid-insensitive locus in Arabidopsis. Plant Phys 127:14–22
Li J, Wen J, Lease KA, Doke JT et al (2002) BAK1, an Arabidopsis LRR-Receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell 110:213–222
Li L, Li D, Luo Z, Huang X, Li X (2016) Proteomic response and quality maintenance in postharvest fruit of strawberry (Fragaria × ananassa) to exogenous cytokinin. Sci Rep 6:27094
Lopes PZ, Fornazzari IM, Almeida AT, Galvão CW et al (2015) Effect of ethylene treatment on phytochemical and ethylene-related gene expression during ripening in strawberry fruit Fragaria × ananassa cv. Camino Real GMR 14:16113–16125
Martínez GA, Civello PM, Chaves AR et al (2001) Characterization of peroxidase-mediated chlorophyll bleaching in strawberry fruit. Phytochemistry 58:379–387
Merchante C, Vallarino JZ, Osorio S, Aragüez I et al (2013) Ethylene is involved in strawberry fruit ripening in an organ-specific manner. J Exp Bot 64:4421–4439
Muñoz C, Sánchez-Sevilla JF, Botella MA et al (2011) Polyphenol composition in the ripe fruits of Fragaria species and transcriptional analyses of key genes in the pathway. J Agric Food Chem 59:12598–12604
Oksanen J, Kindt R, Legendre P, O’Hara RB (2007) Vegan: community ecology package version 18-6. Available from: http://cran.r-project.org/
Russinova E, Borst J, Kwaaitaal M, Caño-Delgado A et al (2004) Heterodimerization and endocytosis of Arabidopsis brassinosteroid receptors BRI1 and AtSERK3 (BAK1). Plant Cell 16:3216–3229
Singh R, Rastogi S, Dwivedi UN (2010) Phenylpropanoid metabolism in ripening fruits. Compr Rev Food Sci Food Saf 9:398–416
Smirnoff N, Wheeler GL (2000) Ascorbic acid in plants: biosynthesis and function. Crit Rev Biochem Mol Biol 35:291–314
Sun JH, Luo JJ, Tian L, Li CL et al (2013) New evidence for the role of ethylene in strawberry fruit ripening. J Plant Growth Regul 32:461–470
Symons GM, Davies C, Shavrukov Y, Dry iB et al (2006) Grapes on steroids. Brassinosteroids are involved in grape berry ripening. Plant Phys 140:150–158
Symons GM, Ghua YJ, Ross JJ, Quittenden LJ et al (2012) Hormonal changes during non-climacteric ripening in strawberry. J Exp Bot 12:2–10
Team Development Core, R. (2007) A language and environment for statistical computing. R Foundation for statistical computing. Vienna. Available in http://www.R-project.org/
Valero D, Serrano M (2010) Fruit ripening. In: Valero D, Serrano M (eds) Postharvest biology and technology for preserving fruit quality, 1st edn. CRC press, New York, pp 7–42
Vidya Vardhini B, Rao SS (2002) Acceleration of ripening of tomato pericarp discs by brassinosteroids. Phytochemistry 61:843–847
Villarreal NM, Bustamante CA, Civello PM et al (2010) Effect of ethylene and 1-MCP treatments on strawberry fruit ripening. J Sci Food Agric 90:683–689
Wang X, Chory J (2006) Brassinosteroids regulate dissociation of BKI1, a negative regulator of BRI1 signaling, from the plasma membrane. Science 313:1118–1122
Wang Z, Nakano T, Gendron J, He J et al (2002) Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev Cell 2:505–513
Xi Z, Zhang Z, Huo S et al (2013) Regulating the secondary metabolism in grape berry using exogenous 24-epibrassinolide for enhanced phenolics content and antioxidant capacity. Food Chem 141:3056–3065
Xu F, Gao X, Xi Z, Zhang H et al (2015) Application of exogenous 24-epibrassinolide enhances proanthocyanidin biosynthesis in Vitis vinifera ‘Cabernet Sauvignon’ berry skin. Plant Growth Regul 75:741–750
Yang G, Komatsu S (2004) Microarray and proteomic analysis of brassinosteroid- and gibberellin-regulated gene and protein expression in rice. Genom Proteom Bioinform 2:77–83
Yin Y, Wang Z, Mora-Garcia S, Li J et al (2002) BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell 109:181–191
Yin Y, Vafeados D, Tao Y, Yoshida S et al (2005) A new class of transcription factors mediates brassinosteroid-regulated gene expression in Arabidopsis. Cell 120:249–259
Zhang Q, Wang HQ, Len P et al (2008) Mechanism of anthocyanins and flavonols in fruit development of strawberries. Acta Hortic 35:1735–1741
Zhu T, Tan W-R, Deng X-G et al (2015) Effects of brassinosteroids on quality attributes and ethylene synthesis in postharvest tomato fruit. Postharvest Biol Technol 100:196–204
Acknowledgements
Research support by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação Araucária, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and to Universidade Estadual de Ponta Grossa (UEPG).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ayub, R.A., Reis, L., Bosetto, L. et al. Brassinosteroid plays a role on pink stage for receptor and transcription factors involved in strawberry fruit ripening. Plant Growth Regul 84, 159–167 (2018). https://doi.org/10.1007/s10725-017-0329-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-017-0329-5