Abstract
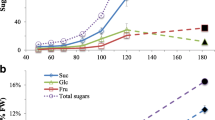
Carbohydrates are an important regulator of canola seed oil content. However, understanding the physiological regulation by carbohydrates governing seed oil accumulation is fragmented. In the present study, activities of sucrose and starch catalytic enzymes, including neutral and alkaline invertase, sucrose synthase (SUS), and starch phosphorylase, and biosynthetic enzymes, including sucrose phosphate synthase and ADP-glucose pyrophosphorylase, were compared in developing silique and seed of high oil content and low oil content lines (HOCL and LOCL, respectively). The results showed that the HOCL had significantly higher total soluble sugar concentration in the developing silique wall and seed during the seed lipid accumulation stage. Strikingly, all the enzymes showed very strong activities at 20 days after anthesis in the silique wall of the HOCL, which was in agreement with the higher amounts of corresponding gene expression. The result indicated that the homeostasis of the high efficiency of cleavage and biosynthetic of carbohydrates in the HOCL was beneficial for the rapid volume expansion of silique and transportation of carbohydrates, i.e., sucrose and starch, to the seed for utilization. At seed deposition stage, all the enzymes exhibited significantly higher activities in the HOCL than in the LOCL, which was helpful for increased production of carbohydrates. The results of gene expression at seed oil deposition stages revealed that one of the SUS genes, SUS3, showed significantly higher transcript amount in the HOCL than in the LOCL at all stages while most of other genes showed no significant differences between two lines at developing stages. Therefore, SUS3 might be played vital roles in the sucrose cleavage.





Similar content being viewed by others
Abbreviations
- AGPase:
-
ADP-glucose pyrophosphorylase
- DAA:
-
Days after anthesis
- HOCL:
-
High oil content line
- LOCL:
-
Low oil content line
- SPS:
-
Sucrose phosphate synthase
- SS:
-
Starch phosphorylase
- SUS:
-
Sucrose synthase
References
Agrawal GK, Hajduch M, Graham K, Thelen JJ (2008) In-depth investigation of soybean seed-filling proteome and comparison with a parallel study of rapeseed. Plant Physiol 148:504–518
Andriotis VME, Pike MJ, Kular B, Rawsthorne S, Smith AM (2010) Starch turnover in developing oilseed embryos. New Phytol 187:791–804
Angeles-Núñez JG, Tiessen A (2011) Mutation of the transcription factor LEAF COTYLEDON 2 alters to the chemical composition of Arabidopsis seeds, decreasing oil and protein content, while maintaining high levels of starch and sucrose in mature seeds. J Plant Physiol 168:1891–1900
Barratt DHP, Barber L, Kruger NJ, Smith AM, Wang TL, Martin C (2001) Multiple, distinct isoforms of sucrose synthase in pea. Plant Physiol 127:655–664
Bieniawska Z, Barratt DHP, Garlick AP, Thole V, Kruger NJ, Martin C, Zrenner R, Smith AM (2007) Analysis of the sucrose synthase gene family in Arabidopsis. Plant J 49:810–828
Chandra A, Verma PK, Islam MN, Grisham MP, Jain R, Sharma A, Roopendra K, Singh K, Singh P, Verma I, Solomon S (2015) Expression analysis of genes associated with sucrose accumulation in sugarcane (Saccharum spp. hybrids) varieties differing in content and time of peak sucrose storage. Plant Biol. doi:10.1111/plb.12276
Cuellar-Ortiz S, De La Arrieta-Montiel M, Acosta-Gallegos J, Covarrubias AA (2008) Relationship between carbohydrate partitioning and drought resistance in common bean. Plant Cell Environ 31:1399–1409
da Silva PMFR, Eastmond PJ, Hill LM, Smith AM, Rawsthorne S (1997) Starch metabolism in developing embryos of oilseed rape. Planta 203:480–487
Déjardin A, Rochat C, Wuillème S, Boutin JP (1997) Contribution of sucrose synthase, ADP-glucose pyrophosphorylase and starch synthase to starch synthesis in developing pea seeds. Plant Cell Environ 20:1421–1430
Doidy J, Grace E, Kühn C, Simon-Plas F, Casieri L, Wipf D (2012) Sugar transporters in plants and their interactions with fungi. Trends Plant Sci 17:413–422
Dubios M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for the determination sugars and related substances. Anal Chem 28:350–356
Fallahi H, Scofield GN, Badger MR, Chow WS, Furbank RT, Ruan Y (2008) Localization of sucrose synthase in developing seed and siliques of Arabidopsis thaliana reveals diverse roles for SUS during development. J Exp Bot 59:3282–3295
Gammelvind LH, Schjoerring JK, Mogensen VO, Jensen CR, Bock JGH (1996) Photosynthesis in leaves and siliques of winter oilseed rape (Brassica napus L.). Plant Soil 186:227–236
Geigenberger P, Reimholz R, Deiting U, Sonnewald U, Stitt M (1999) Decreased expression of sucrose phosphate synthase strongly inhibits the water stress induced synthesis of sucrose in growing potato tubers. Plant J 19:119–129
Gong X, Liu M, Zhang L, Ruan Y, Ding R, Ji Y, Zhang N, Zhang S, Farmer J, Wang C (2015) Arabidopsis AtSUC2 and AtSUC4, encoding sucrose transporters, are required for abiotic stress tolerance in an ABA-dependent pathway. Physiol Plant 153:119–136
Gutiérrez-Miceli FA, Rodríguez-Mendiola MA, Ochoa-Alejo N, Méndez-Salas R, Dendooven L, Arias-Castro C (2002) Relationship between sucrose accumulation and activities of sucrose phosphatase, sucrose synthase, neutral inverase and soluble acid invertase in micropropagated sugar plants. Acta Physiol Plant 24:441–446
Hädrich N, Hendriks JHM, Kötting O, Arrivault S, Feil R, Zeeman SC, Gibon Y, Schulze W, Stitt M, Lunn JE (2012) Mutagenesis of cysteine 81 prevents dimerization of the APS1 subunit of ADP-glucose pyrophosphorylase and alters diurnal turnover in Arabidopsis thaliana leaves. Plant J 70:231–242
Hajduch M, Casteel JE, Hurremeyer KE, Song Z, Agrawal GK, Thelen JJ (2006) Proteomic analysis of seed filling in Brassica napus. Developmental characterization of metabolic isoenzymes using high-resolution two-dimensional gel electrophoresis. Plant Physiol 141:32–46
Halford NG, Curtis TY, Muttucumaru N, Postles J, Mottram DS (2011) Sugar in crop plants. Ann Appl Biol 158:1–25
Hill LM, Morley-Smith ER, Rawsthorne S (2003) Metabolism of sugars in the endosperm of developing seeds of oilseed rape. Plant Physiol 131:228–236
Hirose T, Scofield GN, Terao T (2008) An expression analysis profile for the entire sucrose synthase gene family in rice. Plant Sci 174:534–543
Horst I, Welham T, Kelly S, Kaneko T, Sato S, Tabata S, Parniske M, Wang TL (2007) TILLING mutants of Lotus japonicus reveal that nitrogen assimilation and fixation can occur in the absence of nodule-enhanced sucrose synthase. Plant Physiol 144:806–820
Houston NL, Hajduch M, Thelen JJ (2009) Quantitative proteomics of seed filling in castor: comparison with soybean and rapeseed reveals differences between photosynthetic and nonphotosynthetic seed metabolism. Plant Physiol 151:857–868
Howard TP, Fahy B, Craggs A, Mumford R, Leigh F, Howell P, Greenland A, Smith AM (2012) Barley mutants with low rates of endosperm starch synthesis have low grain dormancy and high susceptibility to preharvest sprouting. New Phytol 194:158–167
Hua W, Li RJ, Zhan GM, Liu J, Li J, Wang XF, Liu GH, Wang HZ (2012) Maternal control of seed oil content in Brassica napus: the role of silique wall photosynthesis. Plant J 69:432–444
Hua S, Chen Z, Zhang Y, Yu H, Lin B, Zhang D (2014) Chlorophyll and carbohydrate metabolism in developing silique and seed are prerequisite to seed oil content of Brassica napus L. Bot Stud 55:34
Jiang Q, Deyholos MK (2010) Transcriptome analysis of secondary-wall-enriched seed coat tissues of canola (Brassica napus L.). Plant Cell Rep 29:327–342
Kimura S, Kondo T (2002) Recent progress in cellulose biosynthesis. J Plant Res 115:297–302
King SP, Lunn JE, Furbank RT (1997) Carbohydrate content and enzyme metabolism in developing canola siliques. Plant Physiol 114:153–160
Li F, Ma C, Wang X, Gao C, Zhang J, Wang Y, Cong N, Li X, Wen J, Yi B, Shen J, Tu J, Fu T (2011) Characterization of Sucrose transporter alleles and their association with seed yield-related traits in Brassica napus L. BMC Plant Biol 11:168
Liepman AH, Wightman R, Geshi N, Turner SR, Scheller HV (2010) Arabidopsis—a powerful model system for plant cell wall research. Plant J 61:1107–1121
Livak JK, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 25:402–408
Miron D, Petreikov M, Carmi N, Shen S, Levin I, Granot D, Zamski E, Schaffer AA (2002) Sucrose uptake, invertase localization and gene expression in developing fruit of Lycopersicon esculentum and the sucrose-accumulating Lycopersicon hirsutum. Physiol Plant 115:35–47
Nägele T, Henkel S, Hörmiller I, Sauter T, Sawodny O, Ederer M, Heyer AG (2010) Mathematical modeling of the central carbohydrate metabolism in Arabidopsis reveals a substantial regulatory influence of vacuolar invertase on whole plant carbon metabolism. Plant Physiol 153:260–272
Ruuska SA, Thomas G, Benning C, Ohlrogge JB (2002) Contrapuntal networks of gene expression during Arabidopsis seed filling. Plant Cell 14:1191–1206
Sanjaya Durrett TP, Weise SE, Benning C (2011) Increasing the energy density of vegetative tissues by diverting carbon from starch to oil biosynthesis in transgenic Arabidopsis. Plant Biotech J 9:874–883
Sauer N, Tanner W (1993) Molecular biology of sugar transporters in plants. Bot Acta 106:277–286
Schmalstig JG, Hitz WD (1987) Contributions of sucrose synthase and invertase to the metabolism of sucrose in developing leaves: estimation by alternate substrate utilization. Plant Physiol 85:407–412
Smith AM (1990) Enzymes of starch synthesis. In: Lea PJ (ed) Methods in plant biochemistry, vol 3. Academic Press, London 99, pp 93–102
Smith AM, Bettey M, Bedford ID (1989) Evidence that the rb locus alters the starch content of developing pea embryos through an effect on ADP glucose pyrophosphorylase. Plant Physiol 89:1279–1284
Taylor NG (2008) Cellulose biosynthesis and deposition in higher plants. New Phytol 178:239–252
Taylor JG Jr, Owen TP, Koonce LT, Haigler CH (1992) Dispersed lignin in tracheary elements treated with cellulose synthesis inhibitors provides evidence that molecules of the secondary wall patterning. Plant J 2:959–970
Tuncel A, Cakir B, Hwang S, Okita TW (2014) The role of the large subunit in redox regulation of the rice endosperm ADP-glucose pyrophosphorylase. FEBS J 281:4951–4963
Wang Q, Zhang X, Li F, Hou Y, Liu X, Zhang X (2011) Identification of a UDP-glucose pyrophosphorylase from cotton (Gossypium hirsutum L.) involved in cellulose biosynthesis in Arabidopsis thaliana. Plant Cell Rep 30:1303–1312
White JA, Todd J, Newman T, Focks N, Girke T, de Iláduya OM, Jaworski JG, Ohlrogge JB, Benning C (2000) A new set of Arabidopsis expressed sequence tags from developing seeds. The metabolic pathway from carbohydrates to seed oil. Plant Physiol 124:1582–1594
Acknowledgments
Great appreciations were expressed to the anonymous reviewers for their critical review. This work was supported by the Major State Basic Research Development Program of China (973 program) (2015CB150205); Natural Science Foundation of Zhejiang Province (LY12C13006); Program for Zhejiang Leading Team of S & T Innovation (2011R50026-04 and 2012C12902-1); the earmarked fund for China Agriculture Research System (CARS-13); the key and integrated technology for canola high yield improvement.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Z., Hua, S., Zhang, D. et al. Comparison on the carbohydrate metabolic enzyme activities and their gene expression patterns in canola differing seed oil content. Plant Growth Regul 78, 357–369 (2016). https://doi.org/10.1007/s10725-015-0098-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-015-0098-y