Abstract
Asian ginseng (Panax ginseng) and American ginseng (Panax quinquefolium), are thought to be representative plant of Panax species, have important commercial value and are used in worldwide. Panax species produces triterpene saponins called ginsenosides, which are classified into two groups by the skeleton of aglycones, namely dammarane-type and oleanane-type. Dammarane-type ginsenosides dominate over oleanane-type not only in amount but also in structural varieties. Researches shows that the saponins content in American ginseng is higher than that in Asian ginseng, the higher part of ginsenosides is from dammarane-type biosynthesis. It has been proposed that protopanaxadiol derived from dammarenediol-II, is a key hydroxylation by cytochrome P450 for the biosynthesis of ginsenosides, and the gene number of protopanaxadiol synthase has been published independent in Asian ginseng (PgCYP716A47). However, little is known about genes involved in hydroxylation and glycosylation in American ginseng ginsenoside biosynthesis. Here, we first cloned and identified a P450 gene named PqD12H encoding enzymes catalyzed dammarenediol-II to protopanaxadiol by RT-PCR using degenerate primers designed based on sequence homology. In vitro, the ectopic expression of PqD12H in recombinant WAT21 yeast resulted in protopanaxadiol production after dammarenediol-II was added to the culture medium. In vivo, we established both PgCYP716A47 and PqD12H RNAi transgenic. The RT-PCR and HPLC analysis of the final products of protopanaxadiol and protopanaxatriol showed a result that declined level of protopanaxadiol-type and protopanaxatriol-type ginsenosides. It suggested that the P450 synthase content or expression in American ginseng exceed than in Asian ginseng. The result elucidated the evolution relationship of P450s and the reason of different saponins content among Panax species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Asian ginseng (Panax ginseng) and American ginseng (Panax quinquefolius) are the two most commonly used ginseng herbs and are thought to have exceptional curative properties, have an important commercial value and are used worldwide (Kitts et al. 2000). Although American ginseng is native to North American, it has spread to North Asian (Assinewe et al. 2003). Ginsenosides, which are glycosylated triterpenes, are considered to be the main compounds in American ginseng, showing many pharmacological activities including anti-cancer, anti-diabetic, neuroprotective, radioprotective, anti-amnestic and anti-aging effects (Chen et al. 2011). Both Asian and American ginseng contained a group of saponins generally referred to as ginsenosides. Up till now, saponins including ginsenosides Rg1, Re, Rb1, Rg2, Rb2, Rc and Rd, have been widely recognized as the main active ingredients of both the two plants (Devi et al. 2011; Barton et al. 2010). However, American ginseng has been used as a tonic for indications similar to those of Asian ginseng, but the two species differ in their respective quantities of the specific ginsenosides (Lee et al. 2008; Schlag and McIntosh 2006; Chen et al. 2009).
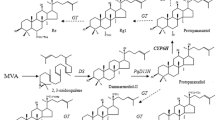
The current ideas of ginsenoside biosynthesis (Fig. 1), considered ginsenosides to be derived from the 2.3-oxidosqualene, which can be synthesized from the plastidial mevalonic acid pathway (MVA) (Augustin et al. 2011; Liang and Zhao 2008). From 2.3-oxidosqualene, the primary components of triterpenoid saponins are oleanane (β-amyrin), cycloartenol, lupeol or dammarene-type triterpenoid. Ginsenosides are classified into two groups by the structure of aglycones, dammarane-type and oleanane-type. Major ginsenosides are dammarane-type, including Rb1 and Rg1 whose genuine aglycones are protopanaxadiol and protopanaxatriol respectively (Punja 2012). Hydroxylation of dammarenediol-II by a P450 enzyme (D12H) at C-12 position generates protopanaxadiol then direct hydroxylation of protopanaxadiol to protopanaxatriol involve in another P450 enzyme (P6H) at C-6 position (Cai et al. 2007). Dammarenediol-II is thought to be converted to a ginsenoside after hydroxylation by P450 enzymes and subsequent glycosylation by glycosyltransferase (GT). Both P450 and GT are in plant genome supergene families (Inoue 2005; Qi et al. 2011). Currently, substantial progress has been obtained in dissecting the ginsenoside biosynthesis pathway; the genes encoding β-amyrin synthase, dammarenediol synthase have been cloned and identified from both the P. ginseng and P. quinquefolius (Han et al. 2011). However, only the protopanaxadiol P450 has been cloned from Asian ginseng (Han et al. 2012), for American ginseng there are few reports on cloning and identification of genes encoding enzymes involved in dammarene-type ginsenoside biosynthesis.
The proposed biosynthetic pathway of ginsenosides. MVA mevalonate, IPP isopentenyl diphosphate, GPP geranyl diphosphate, FPP farnesyl diphosphate, GPS geranyl diphosphate synthase, FPS farnesyl diphosphate synthase, SS squalene synthase, SE squalene epoxidase, CAS cycloartenol synthase, LS lupeol synthase, BAS beta-amyrin synthase, DS dammarenediol synthase, GT glucosyltransferase, dotted lines uncharacterized region of pathway
The genes coding for enzymes of biochemical pathways involved in triterpene biosynthesis are of considerable interest in the area of ginseng biotechnology. Up regulation or down regulation of phytosterol and triterpene production by genetic engineering of Panax species is an attractive strategy to achieve a higher medicinal value (Yue and Zhong 2005). A more detailed understanding of genes involved in saponin biosynthesis could facilitate the genetic modification of plants with altered or novel saponin content (Nelson 2006). Plant functional genomics involves generation of transgenic and mutant plants in association with multiparallel analysis of gene products such as mRNA and protein. In the last few years, significant technological progress has been made that will facilitate the generation and characterization of genetic diversity in plant systems (Li et al. 2009).
In this study, we first cloned a P450 gene (PqD12H) encoding enzymes catalyzing the reaction from dammarenediol-II to protopanaxadiol from 4 years American ginseng, based on homologous sequence analysis and amplified by RT-PCR. We constructed both PgCYP716A47 and PqD12H RNAi destination vector based on recombinant PCR from Asian and American ginseng 4 year roots. Functionally analyzed by ectopic expression of PqD12H in yeast yielded protopanaxadiol and measured of protopanaxadiol and protopanaxatriol products activity from RNAi transgenic, indicating that PqD12H is protopanaxadiol synthase, which is a critically important step in ginsenoside biosynthesis. RNAi in transgenic hairy roots also showed that even though the protopanaxadiol synthase was inhibited, P450 enzyme content or expression level in American ginseng is exceed than in Asian ginseng. The objective of the work was to explain the reason of different to American and Asian ginseng in their respective quantities of the specific ginsenosides and support the role of P450 in triterpenes saponins biosynthesis.
Results
Isolation and analysis of PqD12H
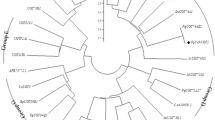
RT-PCR was performed with the designed degenerate primers, and several sequences were amplified. PCR products were inserted into plasmid vector and sequenced. An online BLAST P search revealed that one deduced amino acid sequence of these PCR products is most possibly involved in ginsenoside biosynthesis due to its high sequence similarity (99 and 98 %) with the cytochrome P450 (PgCYP716A47 and PnCYP450) catalyzing the formation of protopanaxadiol from dammarenediol-II in P. ginseng and P. notoginseng respectively (Fig. 2). Thus this sequence was selected for further studies and named as PqD12H. The cDNA of PqD12H (GeneBank accession number JX569336) was 1,459-bp long, with a 5′-untranslated region of 10 nucleotides, a predicted open reading frame (ORF) of 1,449 nucleotides encoding a protein of 482 amino acids with a calculated molecular mass of 60 kDa. According to the P450 nomenclature and the results of BLAST P, PqD12H should belong to CYP716A subfamily involved in terpenoid metabolism (Devi et al. 2011; Han et al. 2011). To further evaluate the homology and evolution relationship of PqD12H with other P450 genes from Panax species and other various species, phylogenetic tree was constructed (Fig. 3) at amino acid level. As expected, it could be observed that PqD12H is most closely related to PgCYP716A47 and PnCYP450 from P. ginseng and P. notoginseng. This indicated that PqD12H is most probably a gene encoding enzyme catalyzing the reaction from dammarenediol-II to protopanaxadiol.
Phylogenic tree constructed with the deduced amino acid sequences of PqD12H (JX569336) and other genes of plant CYP family. PgCYP716A47, P. ginseng (AEY75212); PnCYP450, P. notoginseng (AED99867); VvCYP450716B2-like, V. vinifera (XP_002264643); VvCYP450716B2, V. vinifera (XP_002280969); PgCYP716A52v2, P. ginseng (AFO63032); MtCYP716A12, Medicago truncatula (ABC59076); PtCYP450, Populus trichocarpa (XP_002325964); BcCYP716A41, Bupleurum chinense (AFK79029); VvCYP716B1, V. vinifera (XP_002279492); GmCYP716B2-like, Glycine max (XP_003531849); StCYP450, Solanum tuberosum (BAC23044); SrCYP450, Stevia rebaudiana (ACL10147); PgCYP716A53v2, Panax ginseng (AFO63031); VvCYP716B1-like, V. vinifera (XP_003634587); AtCYP716A1, Arabidopsis thaliana (NP_198460)
Ectopic expression of PqD12H cDNA in WAT21 yeast
In order to determine the functional of PqD12H in ginsenosides biosynthesis, full-length cDNA of PqD12H was introduced in the yeast expression vector pAUR-123 and expressed in WAT21 yeast. The exogenously dammarenediol-II and protopanaxadiol synthesis in yeast extracts were analyzed and examined using HPLC. The retention time of the standard protopanaxadiol peaks was 50.14 min (Fig. 4a). Both dammarenediol-II and protopanaxadiol were identified in PqD12H-expressing yeast, the dammarenediol-II produces a peak at a retention time of 60.93. A protopanaxadiol signal was not detected in the control yeast extract with the empty vector, although a peak from the exogenously added dammarenediol-II was detected (Fig. 4b). The chromatogram from HPLC revealed that ectopic expression of dammarenediol-II and PqD12H in yeast clearly produces a peak at a retention time of 50.14 min, which is the same retention time as for the protopanaxadiol standard (Fig. 4c).
High performance liquid chromatography (HPLC) analysis of the PqD12H product in yeast. a The HPLC chromatogram of a protopanaxadiol standard. b The HPLC chromatogram of the yeast cell extract with an empty vector as a control. The arrow indicates exogenously dammarenediol-II peak at a retention time of 60.93 min. c The HPLC chromatogram of the yeast cell extract with pAUR-PqD12H. The arrow indicates a protopanaxadiol peak at a retention time of 50.14 min
Functional analysis of PqD12H by RNAi in hairy roots
To functionally analyze the role of protopanaxadiol synthase P450 gene in transgenic P. ginseng and P. quinquefolius plants, constitutively expressed P450-RNAi components were constructed by using recombinant PCR. Then plenty of P450 RNAi components were successfully constructed. It contained a sense fragment and an antisense fragment bind with the four restriction sites: EcoR1, Sac1, Apa1, Sal1 (Fig. 5a). P450-RNAi component was recombined into a plant expression vector pBI121 using double restriction enzyme digestion with Pst1 and HindIII which were the adaptors of RNAi component, and obtained P450-RNAi plant destination vector: pBI-PqD12H-RNAi and pBI-PgCYP716A47-RNAi.
Transgenic P. quinquefolius and P. ginseng roots segment excised from the whole plant and cultured on MS medium with 50 mg/l kanamycing. After about 5 weeks grown, transgenic plants were positively regenerated the hairy root at the side of the root segments, no signal was observed in the wild-type plants (Fig. 5b). The hairy root derived from independent transgenic P. quinquefolius and P. ginseng were regarded as independent lines. When the germination branch grows about 1 cm length, we cut the branch as a new hairy root and put them into one MS medium with 50 mg/l kanamycing to measured the dry weight level of hairy roots growth, meanwhile, the wild-type hairy root was used for control (Fig. 5c). The result both the P. quinquefolius and P. ginseng growth were inhibited compare with control, but the P. quinquefolius showed the stronger inhibition of transgenic growth. It suggested that the P. quinquefolius culture is more difficult than P. ginseng in same condition.
In order to determine the ginsenoside yield differ from American and Asian ginseng in their respective quantities of the specific ginsenosides. We measured the Dammarenediol-II content in Asian and American ginseng by HPLC. The results show that the Dammarenediol-II contents both in transgenic P. ginseng and quinquefolius were the same. This would be indicated silencing of P450 gene in ginsenosides biosynthesis could not caused the content of Dammarenediol-II (Fig. 6a, b, c).
The accumulation of P450 and DS expression of Asian and American ginseng was analyzed in P450 RNAi-transgenic plants by RT-PCR. Both the two plants DS showed no changed transcriptional activity in the wild-type and transgenic. RNAi interference caused the obvious decreased of P450 expression in transgenic P. ginseng and quinquefolius hairy roots compared with the wild-type. Densitometric analysis revealed that it was successful silenced the expression of P450 in ginsenosides biosynthesis (Fig. 7a). The result of analysis the Asian and American ginseng quantity of P450 indicated either the WT or transgenic Asian ginseng, protopanaxadiol synthase P450 quantity is lower than that in American ginseng (Fig. 7b).
Ginsenoside content was determined in cultured hairy roots of wild-type and transgenic Asian and American RNAi lines by HPLC analysis after 2 month of culture. Accumulation of ginsenosides Rb1 of protopanaxadiol and Rg1 of protopanaxatriol were somewhat suppressed in both the two transgenic lines compared to these of wild-type. The results obviously revealed that P450 shown the strongest repression of ginsenoside content. The retention times of the standard Rb1 and Rg1 peaks were 46.7 and 83.5 min, respectively (Fig. 8a). The protopanaxadiol-type and protopanaxatriol-type ginsenoside (Rg1 and Rb1) levels were decreased by RNA interference of protopanaxadiol synthase (Fig. 8b, c, d, e). This further justified that PqD12H encodes an enzyme involved in formation of protopanaxadiol. Although saponins two transgenic plants have been inhibited, ginsenoside content of American ginseng was still higher than the content of Asian ginsenoside. This result indicates that P450 synthase content or expression in American ginseng is exceeded than in Asian ginseng.
Discussion
Ginsenosides have been regarded as the principal ingredients responsible for the pharmacological activities of Panax species. Both the Asian ginseng (P. ginseng) and American ginseng (P. quinquefolius) are saponin-rich plant because the content of triterpenoid saponin (ginsenosides), and triterpene saponins accumulated in P. ginseng and P. quinquefolius hairy roots have been reported to show various biological activities (Choi et al. 2001). There have been large-scale attempts to isolate the P450 s and GTs involved in ginsenoside biosynthesis in Panax species. The protopanaxadiol is synthesized from dammarenediol-II under the catalyzation of P450 enzymes (Shibuya et al. 2006). The P450 superfamily is a large and diverse group of enzymes. In A. thaliana, 246 P450 genes were reported (Haralampidis et al. 2002). In P. ginseng the CYP716A47 enzyme catalyzes this reaction has been identified (Han et al. 2012) by expressed sequence tags (EST), however the protopanaxadiol synthase has not been reported in P. quinquefolius. In this study, we cloned a P450 gene that involves in the formation of protopanaxadiol in P. quinquefolius using a reverse genetics approach. PqD12H shares strong similarity (99 and 98 %) with other cytochrome P450 s (PgCYP716A47 and PnCYP450). It is believed that PqD12H is protopanaxadiol synthase like several other P450 genes of Panax species. While our present work on PqD12H represents the first report on cloning and functional analysis of P450 gene involved in ginsenoside biosynthesis in P. quenquefolius. The high identity of PqD12H with PgCYP716A47 and PnCYP450 at amino acid level provide a piece of evidence for the close evolution relationship among P. quinquefolius, P. ginseng, and P. notoginseng.
RNA interference (RNAi) has been used for the identification or validation of biological function of the targeted transcripts of individual genes or small groups of genes in planta (Uchida et al. 2007) and heterologous identification in vitro is usually elucidate the specific gene expression of functional analysis, such as yeast expression or Escherichia coli analysis (Choi et al. 2005). Most recently, RNAi has been introduced as a potent naturally occurring biological strategy for gene silencing (He et al. 2008). In our study, we directly used a method of RNAi in vivo to analysised functional of PqD12H and PgCYP716A47. RNAi of PqD12H resulted in decreased protopanaxadiol-type ginsenoside, suggesting that PqD12H is involved in ginsenoside biosynthesis, most possibly participates in the hydroxylation of dammarenediol-II towards protopanaxadiol.
Diverse oxygenation of natural products generated by secondary metabolic synthetic pathways in plants was predominantly catalyzed by cytochrome P450 enzymes (Xu et al. 2009). In Panax species, two P450 genes are thought to be involved in dammarene-type ginsenoside biosynthesis (Takahashi et al. 2007). One of these genes might be involved in dammarenediol-II hydroxylation at the C-12 position for protopanaxadiol synthesis. Another gene is involved in protopanaxadiol hydroxylation at the C-6 position for protopanaxatriol synthesis, and these two compounds are used as the backbones for dammarene-type ginsenosides (Fig. 1). Using yeast expression analysis, PqD12H was characterized as a protopanaxadiol synthase with dammarenediol-II hydroxylase activity, as demonstrated by the construction of recombinant PqD12H-expressing yeast, which yielded protopanaxadiol from dammarenediol after the yeast were fed dammarenediol-II.
Panax species is a saponin-rich plant because the content of triterpenoid saponin is more than 4–5 % in dry roots. Research shows that the content of total ginsenoside in Asian ginseng is about 4 % and in American ginseng is about 5 %. The reason of this result is not explain clearly enough (Wang et al. 2005). The total content of ginsenosides in American ginseng is higher than Asian ginseng about 1 %. Some studies indicated that the extra 1 % ginsenoside is from dammarane type ginsenoside biosynthesis (Sun et al. 2009). To confirm in vivo relationship of PgCYP716A47 and PqD12H in plants, RNA interference of the two P450 by constructed of recombinant RNAi plant destination vector. This would be useful to confirm the expression of protopanaxadiol synthase in Panax species. In transgenic hairy roots, the decreased protopanaxadiol and protopanaxatriol production might be attributed to the low flux of P450 toward secondary metabolites when dammarane-type biosynthesis is suppressed. The objective HPLC measure result show the level of the dammarenediol-II were the same, American ginseng was obvious higher than Asian ginseng in the expression of P450 (Table 1). It suggested that the P450 synthase content or expression in American ginseng is exceeded than in Asian ginseng. It indicated that the expression of protopanaxadiol synthase differ cause the higher ginsenosides content in natural American ginseng than in Asian ginseng.
In addition to the ginsenosides, panaxadiol (Rb1, Rb2, Rc and Rd) and panaxatriol (Rg1, Re, Rf and Rg2) showed different pharmacological effects, including anti-stress, anti-hyperglycemic, anti-inflammatory, anti-oxidant and anti-cancer effects (Shibata 2001). Because the production of ginsenosides is not practical through organic synthesis, these compounds must be isolated from natural Panax or by ginsenoside hydrolysis. Protopanaxdiol production through ectopic expression of DS and D12H in yeast might represent a promising way to produce useful dammarene-type ginsenosides using genetic engineering. The first step of this engineering theory is to known all the genes in this biosynthesis pathway.
There has been a great progress in elucidation of ginsenoside synthetic pathway in P. ginseng. However, as to P. quinquefolius, the progress seems slower in this respect. The researches on dissecting ginsenoside biosynthetic pathway in P. ginseng would be instructive for the attempts in P. quinquefolius. In this article, our data showed that inhibited the expression of protopanaxadiol synthase is able to depress the levels of protopanaxadiol and protopanaxatriol. The results revealed declined level of protopanaxadiol-type ginsenoside and protopanaxatriol-type ginsenoside suggest that the P450 synthase content or expression in American ginseng is exceeded than in Asian ginseng, this might caused the different ginsenosides content in Asian and American ginseng. Our work should be helpful for elucidation of ginsenoside biosynthesis in P. quinquefolius and for clarification of the P450 s evolution relationship of Panax species.
Materials and methods
Plant material and strain
Actively growing 4-year-old American ginseng was harvested from cultivated fields in Jing-Yu County, JiLin, China in September 2011. Four-year-old Asian ginseng was provided by Agricultural Engineering laboratory, Jilin University. The plants were cleaned by sterilized H2O, the fresh plants were packaged with handi-wrap and immediately stored at 4 °C until use. Agrobacterium rhizogenes A4, was stored in laboratory at −80 °C with 20 % glycerol.
Cloning of the PqD12H gene and bioinformatic analyses
RT-PCR was performed through a standard program using total RNA extracted from 4-year-old American ginseng roots with RNA Reagent kit (TaKaRa). The degenerate primers were designed as PqD12HF (RCAGYAGCAATNBTGTTGNT); and PqD12HR (TYHATTGTGGGGATVTAGA) based on conserved regions resulted from BLAST P search with Arabidopsis thaliana CYP85A1 and P. ginseng CYP716A47 as query sequence. The full length sequences were obtained by 5′ and 3′ Race. The amplified products were separated by electrophoresis, recovered from the agarose gels, inserted into the PMD19-T vector (TaKaRa), sequenced, and transformed into E. coli.
BLAST P searches with the deduced amino acid sequences from these amplified cDNAs as query sequences respectively were carried out to distinguish the one (named as PgP450) that is most possibly involved in ginsenoside biosynthesis. Sequence alignment and phylogenetic analysis were done by the softwares CLUSTAL -X and MEGA 5.1.
Expression of PqD12H in yeast
To construct an expression plasmid vector for yeast, primers for the amplification of the PqD12H coding sequence and introduction of Sac1 and Sma1 restriction sites were designed. The sequences were: 5′-GAGCTCATGGTGTTGTTTTTCTCCCT-3′, 5′-CCCGGGTTAATTGTGGGGATGTAGA-3′. PCR was performed total RNA isolated from the established hairy roots as a template, in the following cycling conditions: 3 min at 92 °C, than 35 cycles for 1 min at 92 °C, 45 s at 54 °C and 2 min at 72 °C. The amplification product was purified on a 0.8 % agarose gel. The cloned Saccharomyces cerevisiae pAUR-123 vector (Takara) with the promoter ADH1 (Alcohol dehydrogenase 1 gene) was transformed into E. coli. The coding sequence was then ligated to the pAUR-123 vector by digested with Sac1 and Sma1. Expression vectors for PqD12H and the empty expression vector was used to transform the Saccharomyces cerevisiae strain WAT21.
WAT21 yeast cells were transformed using a modified lithium acetate procedure, as described previously (Gietz et al. 1992). Transformed cells were selected by AbA (Aureobasidin A) and after 3 days of growth were subcultured on YPD medium (Kribii et al. 1997). The dammarenediol-II (50 mg/l) dissolved with ethanol was added to the medium as a substrate. After cultured for 1 day, the cells were collected by centrifugation at 500×g for 15 min and refluxed with 2 ml of 20 % KOH and 50 % Alcohol for 5 min. After extraction with the same volume of hexane, the extracts were analyzed by HPLC.
Silence of PqD12H and CYP85a47 in transgenic hairy roots by RNAi
For understanding relationship of P450 among Panax species, we selected recombinant PCR to constructed RNAi component. Sequence analysis of PqD12H and CYP716A47 gene was performed using CLUSTAL X software and we designed the primer in the homology conservation coding domain of Asian and American ginseng sequence. So the primers of Asian and American ginseng RNAi component are conformity. Total RNA was extracted from 4-year-old Asian and American ginseng roots independently.
Recombinant PCR contains three round PCR amplification. First PCR primers included: 5′-ATCGTCGTCCACGAAAGCTTTTTGGTTCCCGAGCAGTG-3′ and 5′-GAATTCGAGCTCGGGCCCGTCGACGGAGTCCGGCCTCTATGT-3′ for sence fragment. Second PCR primers included: 5′-GTCGACGGGCCCGAGCTCGAATTCGGAGTCCGGCCTCTATGT-3′ and 5′-GACGCCCTTATTTTACTGCAGTTTGGTTCCCGAGCAGTG for antisense fragment. Both the first and second PCR performed as follows: 94 °C for 5 min, followed by 30 cycles of 94 °C for 1 min, 57 °C for 50 s, and 72 °C for 50 s, with a final 8-min extension at 72 °C. For the third round PCR, we designed a modified sence primer with a Hind III adaptor 5′-ATCGTCGTCCACGAAAGCTT-3′ and a antisence primer with a Pst1 adaptor 5′-GACGCCCTTATTTTACTGCAG-3′, which performed as follows: 94 °C for 5 min, followed by 35 cycles of 94 °C for 1 min, 65 °C for 50 s, and 72 °C for 50 s, with a final 8 min extension at 72 °C.
Both the two P450 RNAi component was recombined into a plant expression vector pBI121 using HindIII and Pst1 double digestion. The obtained recombined plasmid was transformed into competent E.coli cells by heat. Recombined plasmid pBI-PqD12H-RNAi component and pBI-PgCYP716A47-RNAi were subcloned into competent Agrobacterium rhizogenes A4 cells to yield the P450 RNAi plant expression vector engineering bacteria.
The 4-year-old Asian and American ginseng were sterilized with mercury for 10 min respectively. The whole plant body was cutted into about 1 cm thick root segments. Root segments excised from both the two sterilized plants and maintained on MS medium with 3 % sucrose at 25 °C. After about 2 days grown, the root segments were immersed in Agrobacterium rhizogenes A4 strain for 8–10 min at room temperature, the root segments were placed on sterilized filter paper for 10 min and cultured on MS medium supplemented with 20 mg/l kanamycin, 3 % sucrose at 25 °C. After cultivation for 4–5 weeks, the hairy roots started to appear at the side of the root segments. In order to obtain the transgenic hairy root, single root segment with hairy root were picked off and placed onto new media. We cutted the hairy roots when it grows about 1 cm length and transferred to selection MS medium with no kanamycin, 3 % sucrose at 25 °C. With the growth of hairy root, it isolated some branches. We cut the branch as a new hairy root. The dry weight level was measured at 15, 30 and 45 days compare with control.
RT–PCR analysis and ginsenoside analyses
For analysed the expression of dammarenediol-II synthase (DS) and protopanaxadiol synthase. Total RNA was isolated from the transgenic hairy roots and control group of American ginseng. They were then converted into cDNA using the method mentioned above. Primers included: 5′-TTTGGTAGTCAACTATGGGA-3′ and 5′-CAACCACCTTCTTCATTTT-3′ for P. quinquefolius DS (GU997679), 5′-TTTGGTTCCCGAGCAGTG-3′ and 5′-GGAGTCCGGCCTCTATGT-3′ for PqD12H (JX569336). The Asian ginseng transgenic hairy roots were treated for the same way with American ginseng. Primers included: 5′-CTAAGCATACCGCCGTTGA-3′ and 5′-GTTGCACCCTTCCCACTC-3′ for P. ginseng DS (JN596111), 5′-AGTTTGGTTCCCGAGCAG-3′ and 5′-TGGCACGATTCATAGCAGTC-3′ for CYP716A47 (JN604536).
Dammarenediol-II and Ginsenosides were extracted by the method of Samukawa et al. (1995). Two ginsenosides Rb1 of protopanaxadiol and Rg1 of protopanaxatriol accurately weighed, were dissolved in methanol and diluted to volume with methanol, which was taken as the sample solution. Analysis of ginsenosides by HPLC was carried out as described in Han et al. (2006). Quantitative analysis was performed on a one-point curve method using external standards of authentic ginsenosides.
References
Assinewe VA, Baum BR, Gagnon D, Arnason JT (2003) Phytochemistry of wild populations of Panax quinquefolius L. (North American ginseng). J Agric Food Chem 51:4549–4553
Augustin JM, Kuzina V, Andersen SB, Bak S (2011) Molecular activities, biosynthesis and evolution of triterpenoid saponins. Phytochemistry 72:435–457
Barton DL, Soori GS, Bauer BA, Sloan JA, Johnson PA, Figueras C, Duane S, Mattar B, Liu HS, Atherton PJ, Christensen B, Loprinzi CL (2010) Pilot study of Panax quinquefolius (American ginseng) to improve cancer-related fatigue: a randomized, double-blind, dose-finding evaluation: NCCTG trial N03CA. Care Cancer 18:179–187
Cai J, Yue XZ, Zhong JJ (2007) Protopanaxadiol 6-hydroxylase and its role in regulating the Ginsenoside heterogeneity in Panax notoginseng cells. Biotechnol Bioeng 100:933–939
Chen J, Zhao R, Zeng YM, Meng H, Zuo WJ, Li X, Wang JH (2009) Three new triterpenoid saponins from the leaves and stems of Panax quinquefolium. J Asian Nat Prod Res 11:195–201
Chen S, Luo H, Li Y, Sun Y, Wu Q, Niu Y (2011) 454 EST analysis detects genes putatively involved in ginsenoside biosynthesis in Panax ginseng. Plant Cell Rep 30:1593–1601
Choi YE, Yang DC, Kusano T, Sano H (2001) Rapid and efficient Agrobacterium mediated genetic transformation by plasmolyzing pretreatment of cotyledons in Panax ginseng. Plant Cell Rep 20:616–621
Choi DW, Jung J, Ha YI, Park HW, In DS, Chung HJ, Liu JR (2005) Analysis of transcripts in methyl jasmonate-treated ginseng hairy roots to identify genes involved in the biosynthesis of ginsenosides and other secondary metabolites. Plant Cell Rep 23:557–566
Devi BS, Kim YJ, Sathiyamoorthy S, Khorolragchaa A, Gayathri S, Parvin S, Yang DU, Selvi SK, Lee OR, Lee S, Yang DC (2011) Classification and characterization of putative cytochrome P450 genes from Panax ginseng C. A Meyer Biochem (Mosc) 76:1347–1359
Gietz D, St Jean A, Woods RA, Schiestl RH (1992) Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res 20:1425
Han JY, Kwon YS, Yang DC, Jung YR, Choi YE (2006) Expression and RNA interference-induced silencing of the dammarenediol synthase gene in Panax ginseng. Plant Cell Physiol 47:1653–1662
Han JY, Kim HJ, Kwon YS, Choi YE (2011) The Cyt P450 enzyme CYP716A47 catalyzes the formation of protopanaxadiol from dammarenediol-II during ginsenoside biosynthesis in Panax ginseng. Plant Cell Physiol 52:2062–2073
Han JY, Kim HJ, Kwon YS, Choi YE (2012) The Cyt P450 Enzyme CYP716A47 Catalyzes the Formation f Protopanaxadiol from Dammarenediol-II During insenoside Biosynthesis in Panax ginseng. Plant Cell Physiol 52:2062–2073
Haralampidis K, Trojanowska M, Osbourn AE (2002) Biosynthesis of triterpenoid saponins in plants. Adv Biochem Eng Biotechnol 75:31–49
He F, Zhu Y, He M, Zhang Y (2008) Molecular cloning and characterization of the gene encoding squalene epoxidase in Panax notoginseng. Mitochond DNA 19:270–273
Inoue K (2005) Cytochrome P450 enzymes in biosyntheses of some plant secondary metabolites. Yakugaku Zasshi 125:31–49
Kitts DD, Wijewickreme AN, Hu C (2000) Antioxidant properties of a North American ginseng extract. Mol Cell Biochem 203(1–2):1–10
Kribii R, Arro′ M, Del Arco A, Gonza′lez V, Balcells L, Delourme D (1997) Cloning and characterization of the Arabidopsis thaliana SQS1 gene encoding squalene synthase–involvement of the C-terminal region of the enzyme in the channeling of squalene through the sterol pathway. Eur J Biochem 249:61–69
Lee LS, Wise SD, Chan C, Parsons TL, Flexner C, Lietman PS (2008) Possible differential induction of phase 2 enzyme and antioxidant pathways by American ginseng, Panax quinquefolius. J Clin Pharmacol 48:599–609
Li HF, Ye M, Guo HZ, Tian Y, Zhang J, Zhou JP, Hu YC, Guo DA (2009) Biotransformation of 20(S)-protopanaxadiol by Mucor spinosus. Phytochemistry 70:1416–1420
Liang Y, Zhao S (2008) Progress in understanding of ginsenoside biosynthesis. Plant Biol (Stuttg) 10:415–421
Nelson DR (2006) Plant cytochrome P450 s from moss to poplar. Phytochem 5:193–204
Punja ZK (2012) American ginseng research developments, opportunities, and challenges. J Ginseng Res 35:368–374
Qi LW, Wang CZ, Yuan CS (2011) Ginsenosides from American ginseng: chemical and pharmacological diversity. Phytochemistry 72:689–699
Samukawa K, Yamashita H, Matsuda H, Kubo M (1995) Simultaneous analysis of saponins in ginseng radix by high performance liquid chromatography. Chem Pharm Bull 43:137–141
Schlag EM, McIntosh MS (2006) Ginsenoside content and variation among and within American ginseng (Panax quinquefolius L.) populations. Phytochemistry 67:1510–1519
Shibata S (2001) Chemistry and cancer preventing activities of ginseng saponins and some related triterpenoid compounds. J Korean Med Sci 16:S28–S37
Shibuya M, Hoshino M, Katsube Y, Hayashi H, Kushiro T, Ebizuka Y (2006) Identification of beta-amyrin and sophoradiol 24-hydroxylase by expressed sequence tag mining and functional expression assay. FEBS J 273:948–959
Sun BS, Gu LJ, Fang ZM, Wang CY, Wang Z, Lee MR et al (2009) Simultaneous quantification of 19 ginsenosides in black ginseng developed from Panax ginseng by HPLC-ELSD. J Pharm Biomed Anal 50(1):15–22
Takahashi S, Yeo YS, Zhao Y, O’Maille PE, Greenhagen BT, Noel JP (2007) Functional characterization of premnaspirodiene oxygenase, a cytochrome P450 catalyzing region- and stereo-specific hydroxylations of diverse sesquiterpene substrates. J Biol Chem 282:31744–31754
Uchida H, Sugiyama R, Nakayachi O, Takemura M, Ohyama K (2007) Expression of the gene for sterol-biosynthesis enzyme squalene epoxidase in parenchyma cells of the oil plant, Euphorbia tirucalli. Planta 226:1109–1115
Wang AB, Wang CZ, Wu JA, Osinski J, Yuan CS (2005) Determination of major ginsenosides in Panax quinquefolius (American ginseng) using, highperformance liquid chromatography. Phytochem Anal 16:272–277
Xu L, Chen WF, Wong MS (2009) Ginsenoside Rg1 protects dopaminergic neurons in a rat model of Parkinson’s disease through the IGF-I receptor signalling pathway. Br J Pharmacol 158:738–748
Yue CJ, Zhong JJ (2005) Purification and characterization of UDPG: ginsenoside Rd glucosyltransferase from suspended cells of Panax notoginseng. Process Biochem 40:3742–3748
Acknowledgments
This work was supported by National High Technology Research and Development Program of China (863), NO. 2013AA102604. Projects of National Science Foundation of China NO. 30970259. 31270337 and Research Fund for the Doctoral Program of Higher Education of China NO. 20120061110038.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yan-Long Liang, Wang le, Hao-Jie Cao assisted to this work.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Sun, Y., Zhao, SJ., Liang, YL. et al. Regulation and differential expression of protopanaxadiol synthase in Asian and American ginseng ginsenoside biosynthesis by RNA interferences. Plant Growth Regul 71, 207–217 (2013). https://doi.org/10.1007/s10725-013-9821-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-013-9821-8