Abstract
Phytocystatins (PCs) are protein inhibitors of endogenous plant endopeptidases and exogenous pathogen proteinases. We have previously described the protein inhibitor TrcC-4, which is probably involved in the control of protein degradation during triticale seeds germination. The occurrence of the LARFAVXEHN motif supports the TrcC-4 designation as a PC. In this paper TrcC-4 was expressed in Escherichia coli using the pET28 expression vector. TrcC-4(6×His) was purified by affinity chromatography with a single step of purification. Western blot analysis showed the presence of TrcC-4 in both developing and germinating triticale seeds. TrcC-4 protein level was higher both in scutellum of germinating seeds and in developing grains of the triticale cultivar more resistant to pre-harvest sprouting (Zorro) than in a less resistant one (Disco). Furthermore it was demonstrated that the activity of EP8, cysteine endopeptidase responsible for the mass hydrolysis of prolamin during germination, is inhibited by TrcC-4(6×His), as confirmed by native PAGE with gliadin as a substrate. These results suggest that phytocystatin TrcC-4 controls the activity of cysteine endopeptidases involved in germination and, thus, is potentially involved in pre-harvest sprouting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of numerous metabolic functions of plant cysteine endopeptidases (CEPs), which belong to papain family (C1A), is degradation of storage proteins accumulated during seeds development (Grudkowska and Zagdanska 2004; Martinez et al. 2012). The activity of these enzymes supplies growing plants with amino acids, necessary for the synthesis of new proteins, however uncontrolled (or premature) could result in adverse effects. The natural protectors of storage proteins against such degradation, both during germination and seed development, appear to be the endogenous protein inhibitors of cysteine proteases called phytocystatins (PCs) (Abe et al. 1987; Arai et al. 2002).
Phytocystatins, together with the animal stefins, cystatins and kininogens, belong to the cystatin superfamily. All cystatins contain a conserved inhibitory domain, which consists of a conserved glycine residue (G) in the N terminus, a QXVXG motif located in the central region and a tryptophan residue (W) in the C terminus of the polypeptide chain (Turk and Bode 1991; Turk et al. 1997). These three elements form a hydrophobic tripartite wedge complementary to the active site cleft of target enzyme (Bode et al. 1988) while the first of them determines the specificity towards CPs (Machleidt et al. 1989; Björk et al.1995). PCs have an additional LARFAVXEHN sequence, which distinguishes them from animal cystatins (Margis et al. 1998). Similar to animal cystatins, PCs do not have either disulphide bonds nor predicted glycosylation sites. Based on their molecular weights (MW), phytocystatins are divided into three groups. Most of the phytocystatins are assigned to the first group with a MW range from 12 to 16 kDa. They inhibit the activity of CPs from the C1A family (Margis et al. 1998). The members of the second group have MW of approximately 23 kDa, an additional domain at the C-terminus and the ability to inhibit endopeptidases from the legumain (C13) family (Misaka et al. 1996; Martinez et al. 2005, 2007). The third group, called “multicystatins” (~80 kDa), include multi-domain inhibitors (Girard et al. 2007; Nissen et al. 2009). Proposed distinct evolutionary pathway for the mentioned three groups of PCs was presented by Benchabane et al. (2010). All three groups of PCs were identified both in generative and vegetative organs of monocotyledons and dicotyledons. Up to date PCs were obtained and characterised e.g. in rice (Kondo et al. 1990), maize (Abe et al. 1992; Massonneau et al. 2005), barley (Gaddour et al. 2001; Abraham et al. 2006; Martinez et al. 2009), wheat (Kuroda et al. 2001; Corre-Menguy et al. 2002), soya (Misaka et al. 1996), sunflower (Kouzuma et al. 1996), multicystatins from potato tubers (Walsh and Strickland 1993) and tomato leaves (Wu and Haard 2000).
Phytocystatins arouse interest mostly due to their ability to regulate the peptidase activities from pests to pathogens and to control the endopeptidases, whose expressions are induced by various abiotic stresses, such as drought, cold or hypoxia (reviewed by: Koiwa et al. 1997; Benchabane et al. 2010; Martinez et al. 2012). However, the role of PCs in the tissues of the developing and germinating seeds has not been clearly defined. They can either protect seeds against various pests or control the activity of endogenous CEPs responsible for the breakdown of storage proteins (Arai et al. 2002). These inhibitors, synthesised before storage proteins, probably protect the proteins against premature degradation by CEPs (Kuroda et al. 2001).
Our previous studies indicated that mRNA levels of TrcC-4 were higher and lasted longer in the triticale cultivar more resistant to pre-harvest sprouting (Zorro) than in a less resistant cultivar (Disco) (Szewińska et al. 2012). In this paper we analysed the protein level of TrcC-4 during development and germination of mentioned cultivars. Moreover, we checked the ability of recombinant TrcC-4 to complex with CEPs of the germinating endosperm, able to hydrolysis of storage prolamins, and thus, its possible participation in the protection of storage proteins against premature hydrolysis during germination/pre-harvest sprouting.
Materials and methods
Plant material
Seeds of two winter triticale cultivars (Zorro and Disco) with different resistance to pre-harvest sprouting were obtained from Danko Plant Breeders Ltd. in Laski, Poland. The developing seeds were collected 7, 10, 13, 17, 20, 27, 34, 41 and 48 days after pollination (dap). Mature seeds were allowed imbibition and then germinated at 22 °C. The seeds were collected after 2, 6, 12, 24, 48, 72 and 96 h, and the scutellum was separated from the endosperm. The prepared material was frozen in liquid nitrogen and stored at −80 °C until further use.
The total RNA extraction and cDNA synthesis
The total RNA was isolated according to the method of Chomczynski and Sacchi (1987) with additional initial extraction in a buffer containing 50 mM Tris–HCl pH 9.0, 200 mM NaCl, 1 % sarkosyl, 20 mM EDTA and 5 mM DTT. The samples were further extracted in a mixture of phenol/chloroform/isoamyl alcohol. Based on the RNA, the cDNA templates were synthesised using oligo(dT) 12–18 primers according to manufacturer’s protocol (Promega Corp., Madison, USA).
Expression and purification of the recombinant phytocystatin
The coding sequence of phytocystatin TrcC-4 (444 bp) was amplified using the specific forward primer: 5′-ATGCTAGCCGGAGATTCCACCGAGC-3′, which incorporated an NheI restriction site (underlined) and the reverse primer: 5′-TGAAGCTTTTAAACGTTGGGAGAGG-3′ which added a tail with a HindIII (underlined) restriction site at the 3′ end of the amplified fragment. The following PCR conditions were used: 2 min at 94 °C; 25 cycles: 30 s at 94 °C, 40 s at 56 °C and 30 s at 72 °C; and a final elongation step for 5 min at 72 °C. The final PCR product was inserted in-frame with N-terminal histidine tag (His6) into the fusion expression vector pET28a (Merck), previously restricted with NheI and HindIII endonucleases. The resulting expression plasmid was transformed into the Escherichia coli strain BL21(DE3) (Merck). Bacteria were cultivated in LB medium supplemented with kanamycin (50 μg/ml) at 37 °C until the OD600 reached ~0.8. Expression was induced by addition of 1 mM IPTG for 2 h. The bacteria were lysed by sonication in lysis buffer 1 [native conditions; containing 1 M NaCl, 20 mM Tris–HCl pH (7.5), 10 mM imidazole and 1 % Triton X-100] or lysis buffer 2 [denaturing conditions; containing 100 mM NaH2PO4, 10 mM Tris–HCl (pH 7.5) and 6 M GuHCl (guanidine hydrochloride)]. The recombinant protein was purified by affinity chromatography on Ni–NTA resin (300 μl) according to the manufacturer’s instruction (Invitrogen) and then eluted in elution buffer 1 [native conditions; containing 0.5 M NaCl, 20 mM Tris–HCl (pH 7.5) and 300 mM imidazole] or elution buffer 2 [denaturing conditions; containing 100 mM NaH2PO4, 10 mM Tris–HCl (pH 4.5) and 8 M urea], and dialysed against a solution of 20 mM Tris–HCl (pH 7.5) and 150 mM NaCl. Elution fractions (250 μl) were collected both under native and denaturing conditions. The quality of the eluted proteins was analysed by SDS-PAGE.
Western blot analysis
Based on the purified fusion protein, TrcC-4(6×His), primary polyclonal anti-TrcC-4 antibodies were produced by Novazym (Poland).
Proteins were extracted from seed samples by grinding in 100 mM Tris–HCl (pH 7.5), 50 mM NaCl, 0.1 mM PMSF and 1 mM EDTA. Proteins were separated by SDS-PAGE (Laemmli 1970) and transferred onto PVDF membranes (Millipore). Immunological staining was performed using the primary polyclonal anti-TrcC-4 antibodies (1:100 v/v) overnight or anti-His antibodies for 1 h (1:10,000 v/v). After washing (5× 10 min) in TBST (0.8 % NaCl), the secondary antibodies (anti-rabbit or anti-mouse IgG, conjugated with alkaline phosphatase; Sigma) were added at a dilution of 1:20,000 v/v. The colour was developed by the reaction of nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate (Guerin and Carbonero 1997). Obtained antibodies recognized a protein exactly corresponding to the calculated molecular weight of TrcC-4.
Inhibitory activity assay—IC50 estimation
The inhibitory activity of the recombinant phytocystatin, TrcC-4(6×His), was tested against a purified cysteine endopeptidase, EP8, obtained according to Prabucka and Bielawski (2004) from 3 day endosperms of triticale using BANA (N-benzoyl-dl-arginine-β-naphthylamide) as a substrate. The inhibitor TrcC-4 (0.1–5.0 μM) was pre-incubated at 37 °C for 10 min with the enzyme EP8 (8 μg/ml) in 0.5 M sodium phosphate buffer, pH 6.0 containing 10 mM EDTA. Then the reaction was started by the addition of 1 mM BANA dissolved in DMSO and stopped with 2 % HCl in ethanol. Finally the colour was developed by adding 0.06 % w/v p-dimethyl-amino cinnamaldehyde in ethanol. Absorbance was measured at 540 nm after a 30 min incubation at room temperature. Inhibition was expressed as the percentage of remaining protease activity in treated samples compared to that in the controls without inhibitors. The effective cystatin concentration for 50 % activity of EP8 inhibition (IC50) was calculated.
Inhibitory activity assay after native PAGE with copolymerised gliadin
Crude extract of the endosperm, manually separated from 3-day-old seedlings, prepared according to Prabucka and Bielawski (2004) was incubated with or without (control) TrcC-4(6×His) or E-64 (diagnostic inhibitor of cysteine peptidases) at 4 °C for 15 min and then loaded onto the polyacrylamide gel with copolymerised gliadin. After electrophoresis, the gel slabs were incubated in a cold (4 °C) 0.2 M acetate buffer pH 3.6 with or without (control) inhibitor solution for 45 min; subsequently the gels were raised to 37 °C and incubated at this temperature for 16 h. After incubation, the gels were stained/destained with 0.1 % Naphthol Blue Black (Sigma)/7 % acetic acid.
Results
Purification of the recombinant protein TrcC-4(6×His) using affinity chromatography on Ni–NTA resin under native and denaturing conditions
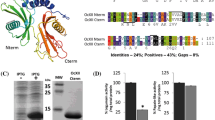
The fractions of TrcC-4(6×His), purified under native or denaturing conditions, are shown in Fig. 1. E. coli protein extract at the 2 h of IPTG induction was analysed in lane 2 (Fig. 1A) and a protein of the expected molecular weight 18.5 kDa was observed. In elution fractions (Fig. 1A, lanes 5–9), specific binding of a new protein gradually increased compared to other bacterial proteins. Specific binding of the protein purified under denaturing conditions was revealed in all elution fractions (Fig. 1B, lanes 2–6), and the protein was not observed in the wash fraction (Fig. 1B, lane 1).
SDS-PAGE analysis of fusion protein TrcC-4 (6×His) purification under native conditions (A) 1 MW marker, 2 extract of BL21 (DE3) 2 h after IPTG induction, 3 flow-through fraction, 4 wash fraction, 5–9 the following fractions of protein elution, 9 purified recombinant TrcC-4(6xHis) after affinity chromatography on a nickel column; denaturing conditions (B) 1 wash fraction, 2–6 the following fractions of protein elution, 7 MW marker
To confirm that the purified protein (18.5 kDa) under native and denaturing conditions contains the histidine tail, western blot analysis was performed. Immunodetection was performed using primary anti-His antibodies and secondary antibodies conjugated with alkaline phosphatase. The reaction product of alkaline phosphatase with the substrates NBT and BCIP indicates the presence of a protein fused to the His-tag (Fig. 2). The recombinant protein TrcC-4(6×His), which was purified under native conditions, and TrcC-4(6×His), which was purified under denaturing conditions, were observed.
Western blotting analysis of purified triticale phytocystatin, TrcC-4(6xHis): 1 TrcC-4(6xHis) purified under native conditions, 2 TrcC-4(6xHis) purified under denaturing conditions, 3 MW marker. Protein was detected using primary anti-His antibodies and secondary antibodies conjugated with alkaline phosphatase
Immunodetection of endogenous triticale phytocystatin in developing and germinating seeds
To detect the phytocystatin protein in triticale seeds, primary polyclonal antibodies against the recombinant protein, TrcC-4(6×His) and secondary antibodies conjugated with alkaline phosphatase were used. Western blot analysis revealed the protein TrcC-4 at all stages of developing seeds of the two cultivars of winter triticale (Zorro and Disco), which differ greatly in their resistance to pre-harvest sprouting. In the first 2 weeks after pollination, high levels of the protein were observed (Fig. 3A). However, with the on-going process of seed development, the level of TrcC-4 significantly decreased. Changes of the protein levels were similar in both cultivars, but higher levels of TrcC-4 were observed in Zorro than in Disco.
Immunodetection of changes in the protein levels of TrcC-4 in developing (7, 10, 13, 17, 20, 27, 34, 41 and 48 days after pollination) (A) and germinating seeds (2, 6, 12, 24, 48, 72 and 96 h after imbibition): (B) scutellum (C) endosperm. In total, 40 μg of extracted protein was separated by 15 % SDS-PAGE, transferred to PVDF membrane and probed with primary polyclonal antibodies generated against purified recombinant TrcC-4. Z–Zorro cv. and D–Disco cv
The changes of the protein levels were also shown in the scutellum and endosperm of germinating triticale seeds of both Zorro and Disco cultivars (Fig. 3B, C). In the scutellum, the level of TrcC-4 was high and remained stable at all stages of seed germination (Fig. 3B). In the endosperm, high levels of TrcC-4 were observed in the first hours after imbibition, and then decreased slowly. The highest level of TrcC-4 in the endosperm was observed 2 h after imbibition (Fig. 3C). Strong expression signals were observed even after 6 h of germination, while 12 and 24 h after imbibition, the protein levels were already significantly lower than in the initial phase of the process. In the next stages of germination (after 48, 72 and 96 h), TrcC-4 levels were almost undetectable (Fig. 3C).
Inhibitory activity of TrcC-4(6×His) against endogenous cysteine endopeptidases of triticale
Electrophoretic analysis of crude extracts from the endosperm of 3-d germinating triticale seeds revealed six bands of endopeptidase activity: EP1, EP2, EP3, EP4, EP5 and EP8 (Fig. 4). Our previous studies indicated that endopeptidases EP2 to EP8 are cysteine endopeptidases from the papain family, while EP1 belongs to an aspartic endopeptidase (Prabucka and Bielawski 2004). Recombinant TrcC-4(6×His) (0.015 μM) especially decreased the activity of EP8 (Fig. 4, lane 2). At the lowest concentration of E-64 (0.015 μM) (Fig. 4, lane 5), the activity of the endopeptidases from EP2 to EP8 declined. At tenfold higher concentrations of TrcC-4(6×His) (0.15 μM) almost complete inhibition of EP2 to EP8 activity was observed (Fig. 4, lane 3) while at 0.15 μM of E-64 the level of EP8 activity (Fig. 4, lane 6) did not change compared with the tenfold lower concentration of the inhibitor. At the highest concentration of TrcC-4(6xHis) (1.5 μM) a complete inhibition of CEPs EP2-EP8 but not complete reduction of EP8 activity by E-64 were shown. The activity of the aspartic endopeptidase EP1 was not inhibited either by TrcC-4(6×His) or E-64. The ability of the recombinant protein TrcC-4(6×His) to inhibit the purified triticale cysteine endopeptidase EP8 was also tested by calculating IC50. The results showed that TrcC-4(6×His) obtained in native and denaturing conditions inhibited EP8 with IC50 values of 0.344 and 0.558 μM, respectively.
Native PAGE (with copolymerised gliadin) of endopeptidases from 3-d germinating triticale endosperm. Gels were incubated in the presence of inhibitor TrcC-4(6×His) or E-64 (cysteine proteinase inhibitor). 1 crude extract from 3-d germinating triticale seeds without inhibitors, 2 crude extract with the addition of TrcC-4 at 0.015 μM, 3 0.15 μM, 4 1.5 μM, 5 crude extract with the addition of E-64 at 0.015 μM, 6 0.15 μM, 7 1.5 μM
Discussion
In this paper, the first fusion protein of triticale phytocystatin, TrcC-4, was obtained using an E. coli expression system. As we previously demonstrated, TrcC-4 belongs to group A of the PCs from Poaceae family (Szewińska et al. 2012), the inhibitors that may regulate the activity of endogenous CEPs (Arai et al. 2002; Corre-Menguy et al. 2002). Western blot analysis, using anti-TrcC-4 antibodies, demonstrated the relatively high level of TrcC-4 protein in developing grains of triticale in the initial stage of the process, decreasing in later stages (Fig. 3A). These results correspond to the high mRNA level of TrcC-4 observed during the first 2 weeks of development, just before the accumulation of storage proteins (Szewińska et al. 2012). The high mRNA level of wheat phytocystatin, WC4 (84 % amino acid sequence identity with TrcC-4), was also observed during the first 2 weeks after pollination (Kuroda et al. 2001). It seems that the role of triticale inhibitor TrcC-4, as well as WC4 and PCs of barley (HvCPI-1-4), maize (CC1, CC2) and rice (OC-I, OC-II) in phylogenetic group A (Martinez et al. 2005; Abraham et al. 2006), is to protect accumulated storage material against premature hydrolysis. Furthermore, higher levels of TrcC-4 protein and mRNA observed in cultivar more resistant to sprouting (Zorro) than in cultivar more susceptible to sprouting (Disco), might confirm this hypothesis, however further investigations proving common subcellular location of both the inhibitor and the enzyme are necessary. The correlation between expression of phytocystatins and the the ability to germination may confirms the results obtained by Hong et al. (2007) and Hwang et al. (2009). They showed that over-expression of BrCYS1 and AtCYS6 encoding Chinese cabbage and Arabidopsis phytocystatins respectively retard seed germination and seedling growth inhibiting stored CP activity.
Cysteine endopeptidases, the potential target for PCs, are recognised as the main group of peptidases responsible for storage protein hydrolysis. These endopeptidases are synthesised de novo in the scutellum and aleurone layer in response to gibberellin signalling (Fincher 1989; Holwerda et al. 1990; Mikkonen et al. 1996; Zhang and Jones 1996). It was confirmed by evaluating their transcript and activity level in rice (Watanabe et al. 1991), wheat (Kiyosaki et al. 2007, 2009; Shi and Xu 2009) and barley (Mikkonen et al. 1996; Martinez et al. 2009). In our previous study the activity of seven CEPs, EP2–EP8, and one aspartyl proteinase, EP1, hydrolysing wheat gliadin, was observed in the endosperm of triticale grains (Prabucka and Bielawski 2004). EP3 and EP4 seem to be the major endopeptidases active during the first 2 days of germination, while EP8 (GeneBank ID: JN398461), induced by GA3 (Drzymała et al. 2008), is the major endopeptidase activity in the endosperm of triticale grains on the third day of germination (Prabucka and Bielawski 2004). Activity of any of the mentioned triticale CEPs was observed in the scutellum (Prabucka and Bielawski 2004), what correlates with high TrcC-4 protein levels (Fig. 3B). Furthermore, higher levels of TrcC-4 mRNA (Szewinska et al. 2012) and TrcC-4 protein in the cultivar Zorro (compared to those in Disco) (Fig. 3B) and TrcC-4 mRNA (Szewińska et al. 2012) may be related to increased resistance of Zorro to pre-harvest sprouting. Whereas high TrcC-4 levels in the endosperm of both triticale cultivars in the first 12 h of germination may protect storage proteins against too rapid hydrolysis (Fig. 3C). Rapid decreases in TrcC-4 levels from the second day of germination seem to be related to the increased activities of endopeptidases that hydrolyse grain proteins (Prabucka and Bielawski 2004), including inhibitory proteins with low molecular weight. Especially the high activity of aspartic endopeptidase EP1 (Fig. 4) increasing from the second day after imbibition (Prabucka and Bielawski 2004) may be responsible for the lack of correlation between the mRNA (Szewińska et al. 2012) and protein level of TrcC-4 observed after 48 h after imbibition in this tissue.
To evaluate the ability of TrcC-4 to inhibit the activity of endogenous CEPs capable of hydrolysis of prolamins—the main triticale storage protein, the endopeptidases from endosperms of germinating triticale grains (72 h after imbibition) were incubated with recombinant TrcC-4(6×His). This ability has been confirmed in vitro with all of the examined CEPs, including EP8 (Fig. 4). The activity of EP1 was not inhibited by TrcC-4(6×His), which confirms the inability of TrcC-4(6×His) to inhibit aspartyl endopeptidases. The total inhibition of EP8 activity was observed with 1.5 μM TrcC-4(6×His), while the same concentration of E-64 did not completely suppress the activity of this enzyme (Fig. 4).
Due to the particularly important role of EP8 in triticale germination, the effectiveness of recombinant TrcC-4(6×His) (purified in both native and denaturing conditions) to inhibit purified EP8 was examined. The IC50 value for the native TrcC-4(6×His) was circa 1.6 times lower than that of the renatured TrcC-4(6×His). Observed differences in IC50 can be explained by incorrect refolding of a fraction of the inhibitor protein.
The presented results suggest that phytocystatin TrcC-4 may control the activity of CEPs involved in germination and, thus, is potentially involved in pre-harvest sprouting. However, verification of this hypothesis requires further investigation. Based on data detailing inhibitor-enzyme relationships, complete knowledge of a mechanism that controls CEPs involved in pre-harvest sprouting can be applied by breeders to obtain new triticale cultivars with increased phytocystatin levels and increased resistance to pre-harvest sprouting.
References
Abe K, Emori Y, Kondo H, Suzuki K, Arai S (1987) Molecular cloning of a cysteine proteinase inhibitor of rice (oryzacystatin). Homology with animal cystatins and transient expression in the ripening process of rice seeds. J Biol Chem 262:16793–16797
Abe M, Abe K, Kuroda M, Arai S (1992) Corn kernel cysteine proteinase inhibitor as a novel cystatin superfamily member of plant origin. Molecular cloning and expression studies. Euro J Biochem 209:933–937
Abraham Z, Martinez M, Carbonero P, Diaz I (2006) Structural and functional diversity within the cystatin gene family of Hordeum vulgare. J Exp Bot 57:4245–4255
Arai S, Matsumoto I, Emori Y, Abe K (2002) Plant seed cystatins and their target enzymes of endogenous and exogenous origin. J Agric Food Chem 50:6612–6617
Benchabane M, Schlüter U, Vorster J, Goulet MC, Michaud D (2010) Plant cystatins. Biochimie 92:1657–1666
Björk I, Brieditis I, Abrahamson M (1995) Probing the functional role of the N-terminal region of cystatins by equilibrium and kinetic studies of the binding of Gly-11 variants of recombinant human cystatin C to target proteinases. Biochem J 306:513–518
Bode W, Engh R, Musil D, Thiele U, Huber R, Karshikov A, Brzin J, Kos J, Turk V (1988) The 2.0 A X-ray crystal structures of chicken egg white cystatin and its possible mode of interaction with cysteine proteinases. EMBO J 7:2593–2599
Chomczynski P, Sacchi N (1987) Single-step method of total RNA isolation by a single extraction with an acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159
Corre-Menguy F, Cejudo FJ, Mazubert C, Vidal J, Lelandais-Briere C, Torres G, Rode A, Hartmann C (2002) Characterization of the expression of a wheat cystatin gene during caryopsis development. Plant Mol Biol 50:687–698
Drzymała A, Prabucka B, Gajo I, Bielawski W (2008) Endogenous action of cysteine endopeptidase and three carboxipeptidases on triticale prolamins. Cereal Chem 85:366–371
Fincher GB (1989) Molecular and cellular biology associated with endosperm mobilization in germinating cereal grains. Annu Rev Plant Physiol Plant Mol Biol 40:305–346
Gaddour K, Vicente-Carbajosa J, Lara P, Isabel-Lamoneda I, Díaz I, Carbonero P (2001) A constitutive cystatin-encoding gene from barley (icy) responds differentially to abiotic stimuli. Plant Mol Biol 45:599–608
Girard C, Rivard D, Kiggundu A, Kunert K, Gleddie SC, Cloutier C, Michaud D (2007) A multicomponent, elicitor-inducible cystatin complex in tomato, Solanum lycopersicum. New Phytol 173:841–851
Grudkowska M, Zagdanska B (2004) Multifunctional role of plant cysteine proteinases. Acta Biochim Polon 51:609–624
Guerin J, Carbonero P (1997) The spatial distribution of sucrose synthase isozymes in barley. Plant Physiol 114:55–62
Holwerda BC, Galvin NJ, Baranski TJ, Rogers JC (1990) In vitro processing of aleurain, a barley vacuolar thiol protease. Plant Cell 2:1091–1106
Hong JK, Hwang JE, Lim ChJ, Yang KA, Jin Z-L, Kim ChY, Koo JCh, Chung WS, Lee KO, Lee SY, Cho MJ, Lim ChO (2007) Over-expression of Chinese cabbage Phytocystatin 1 retards seed germination in Arabidopsis. Plant Sci 172:556–563
Hwang JE, Hong JK, Je JH, Lee KO, Kim DY, Lee SY, Lim CO (2009) Regulation of seed germination and seedling growth by an Arabidopsis phytocystatin isoform, AtCYS6. Plant Cell Rep 28:1623–1632
Kiyosaki T, Matsumoto I, Asakura T, Funaki J, Kuroda M, Misaka T, Arai S, Abe K (2007) Gliadain, a gibberellin-inducible cysteine proteinase occurring in germinating seeds of wheat, Triticum aestivum L., specifically digests gliadin and is regulated by intrinsic cystatins. FEBS J 8:1908–1917
Kiyosaki T, Asakura T, Matsumoto I, Tamura T, Terauchi K, Funaki J, Kuroda M, Misaka T, Abe K (2009) Wheat cysteine proteases triticain alpha, beta and gamma exhibit mutually distinct responses to gibberellin in germinating seeds. J Plant Physiol 166:101–106
Koiwa H, Bressan RA, Hasegawa PM (1997) Regulation of protease inhibitors and plant defense. Trends Plant Sci 2:379–384
Kondo H, Abe K, Nishimura I, Watanabe H, Emori Y, Arai S (1990) Two distinct cystatin species in rice seeds with different specificities against cysteine proteinases. Molecular cloning, expression, and biochemical studies on oryzacystatin-II. J Biol Chem 265:15832–15837
Kouzuma Y, Kawano K, Kimura M, Yamasaki N, Kadowaki T, Yamamoto K (1996) Purification, characterization, and sequencing of two cysteine proteinase inhibitors, Sca and Scb, from sunflower (Helianthus annuus) seeds. J Biochem 119:1106–1113
Kuroda M, Kiyosaki T, Matsumoto I, Misaka T, Arai S, Abe K (2001) Molecular cloning, characterization and expression of wheat cystatins. Biosci Biotechnol Biochem 65:22–28
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Machleidt W, Thiele U, Laber B, Assfalg-Machleidt I, Esterl A, Wiegand G, Kos J, Turk V, Bode W (1989) Mechanizm of inhibition of papain by chicken egg white cystatin. Inhibition constants of N-terminally truncated forms and cyanogens bromide fragments of the inhibitor. FEBS Lett 243(2):234–238
Margis R, Reis EM, Villeret V (1998) Structural and phylogenetic relationships among plant and animal cystatins. Arch Biochem Biophys 359:24–30
Martinez M, Abraham Z, Carbonero P, Díaz I (2005) Comparative phylogenetic analysis of cystatin gene families from arabidopsis, rice and barley. Mol Genet Genomics 273:423–432
Martinez M, Diaz-Mendoza M, Carrillo L, Diaz I (2007) Carboxy terminal extended phytocystatins are bifunctional inhibitors of papain and legumain cysteine proteinases. FEBS Lett 581:2914–2918
Martinez M, Cambra I, Carrillo L, Diaz-Mendoza M, Diaz I (2009) Characterization of the entire cystatin gene family in barley and their target cathepsin L-like cysteine proteases, partners in the hordein mobilization during seed germination. Plant Physiol 151:1531–1545
Martinez M, Cambra I, Gonzalez-Melendi P, Santamaria ME, Diaz I (2012) C1A cysteine-proteases and their inhibitors in plants. Physiol Plant 145:85–94
Massonneau A, Condamine P, Wisniewski JP, Zivy M, Rogowsky PM (2005) Maize cystatins respond to developmental cues, cold stress and drought. Biochim Biophys Acta 1729:186–199
Mikkonen A, Porali I, Cercos M, Ho TH (1996) A major cysteine proteinase, EPB, in germinating barley seeds: structure of two intronless genes and regulation of expression. Plant Mol Biol 31:239–254
Misaka T, Kuroda M, Iwabuchi K, Abe K, Arai S (1996) Soyacystatin, a novel cysteine proteinase inhibitor in soybean, is distinct in protein structure and gene organization from other cystatins of animal and plant origin. Euro J Biochem 240:609–614
Nissen MS, Kumar GN, Youn B, Knowles DB, Lam KS, Ballinger WJ, Knowles NR, Kang C (2009) Characterization of Solanum tuberosum multicystatin and its structural comparison with other cystatins. Plant Cell 21:861–875
Prabucka B, Bielawski W (2004) Purification and partial characteristic of a major gliadin-degrading cysteine endopeptidase from germinating triticale seeds. Acta Physiol Plant 26:383–392
Shi Ch, Xu L (2009) Characters of cysteine endopeptidases in wheat endosperm during seed germination and subsequent seedling growth. J Integr Plant Biol 51(1):52–57
Szewińska J, Zdunek-Zastocka E, Pojmaj M, Bielawski W (2012) Molecular cloning and expression analysis of triticale phytocystatins during development and germination of seeds. Plant Mol Biol Rep 30:867–877
Turk V, Bode W (1991) The cystatins: protein inhibitors of cysteine proteinases. FEBS Lett 285:213–219
Turk B, Turk V, Turk D (1997) Structural and functional aspects of papain-like cysteine proteinases and their protein inhibitors. Biol Chem 378:141–150
Walsh TA, Strickland JA (1993) Proteolysis of the 85-kilodalton crystalline cysteine proteinase inhibitor from potato releases functional cystatin domains. Plant Physiol 103:1227–1234
Watanabe H, Abe K, Emori Y, Hosoyama H, Arai S (1991) Molecular cloning and gibberellin-induced expression of multiple cysteine proteinases of rice seeds (oryzains). J Biol Chem 266:16897–16902
Wu J, Haard NF (2000) Purification and characterization of a cystatin from the leaves of methyl jasmonate treated tomato plants. Comp Biochem Physiol C: Toxicol Pharmacol 127:209–220
Zhang N, Jones BL (1996) Purification and partial characterization of a 31-kDa cysteine endopeptidase from germinated barley. Planta 199:565–572
Acknowledgments
The research was founded by a grant from the Ministry of Science and Higher Education, Warsaw, Poland (Project N N310 151335). We thank Dr. E. Zdunek-Zastocka (Department of Biochemistry, Warsaw University of Life Sciences) for critical reading of the manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Szewińska, J., Prabucka, B., Krawczyk, M. et al. The participation of phytocystatin TrcC-4 in the activity regulation of EP8, the main prolamin degrading cysteine endopeptidase in triticale seeds. Plant Growth Regul 69, 131–137 (2013). https://doi.org/10.1007/s10725-012-9756-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-012-9756-5