Abstract
The olive tree, scientifically known as Olea europaea L., is an evergreen xerophytic tree that defines the natural flora, historical background, and cultural essence of the Mediterranean Basin. This study aimed to establish relationships using multivariate analysis methods between the nutrient content of soil conditions in which olive cultivars grown in Türkiye are cultivated and the nutrient element content, phytochemical contents, antioxidant activity, total chlorophyll amount, and leaf colors of these cultivars. All data sets used in the study were analyzed in 2022 and 2023, and average values were used in the research. According to Tukey's comparison result, it was determined that the soil structure of the garden was homogeneous in terms of nutrients it contained. In nutrient analysis conducted on leaves, in the ‘Çelebi’ cultivar, Al (59.25 mg kg−1), B (6.53 mg kg−1), Cu (48.36 mg kg−1), Fe (69.34 mg kg−1), K (1438.11 mg kg−1), Na (197.12 mg kg−1) nutrients are the highest; in the ‘Gemlik-21’ cultivar, Ca (5485.03 mg kg−1) nutrient is the highest; in the ‘Sarı Haşebi’ cultivar, Mg (928.11 mg kg−1), Mn (19.71 mg kg−1), S (632.77 mg kg−1) nutrients are the highest; and in the ‘Tavşan Yüreği’ cultivar, Ni (1.71 mg kg−1), Zn (9.76 mg kg−1) nutrients are the highest. The L* (49.19), b* (29.43) color values are highest in the ‘Sarı Yaprak’ cultivar, while the a* (28.84) value is highest in the ‘Girit Zeytini’ cultivar. The highest leaf chlorophyll content was determined in the ‘Girit Zeytini’ cultivar (95.57). Total phenolics, total flavonoids, antioxidant capacity were determined to be highest in the ‘Manzanilla’ (151.49 mg GAE/100 g), ‘Edincik Su’ (39.01 mg QE/100 g), ‘Nizip Yağlık’ (91.18%) cultivars, respectively. According to the principal component analysis, the first three principal components accounted for 82% of the total variation. The correlation matrix analysis revealed that high levels of certain minerals in the soil led to an increase in the leaves, resulting in positive correlation, while the opposite was true for negative correlation. According to heat map analysis, mineral elements in the leaf were in the same group, while other data sets were in different groups. The data obtained will shed light on future research on similar topics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Different ecological conditions resulting from the climate change in Türkiye from subtropical to continental cause many plant species to spread throughout the country. These plant species are grown by producers both naturally and economically. Olives are among the species that can be grown economically (Ercisli 2004; Ilhan 2024). The olive tree, belonging to the Oleaceae family, is an evergreen plant species. There are approximately 11 million hectares of olive groves in a total of 47 countries (Rallo et al. 2018). Mediterranean Basin countries alone account for over 97% of this area, and cultivation of olive cultivars, both for table olives and oil production, has been practiced since around 4000 BCE (Navajas-Porras et al. 2020).
Suggesting an alternative method to reduce waste disposal and minimize environmental impact, the cultivation of olive trees can be seen as an option driven by the increasing demand for natural products in various industrial sectors such as food and pharmaceuticals (Jabalbarezi Hukerdi et al. 2018). Among these, olive leaves (OL), traditionally used as a folk remedy, have gained prominence recently mainly due to their documented positive effects on human health (diuretic, antipyretic, bladder infections, hypotensive, headaches (Hutchings et al. 1996) and colon cancer (Zeriouh et al. 2017), attributed to their rich phytochemical content (total phenols, total flavonoids, antioxidant activity, etc.) (Pereira et al. 2007; Talhaoui et al. 2015). Consequently, while olives are primarily grown for the production of olive oil and table olives, OL has witnessed increasing demand in the global market, particularly for the extraction of valuable phytochemicals (Pasković et al. 2020).
Olive trees growth, productivity, fruit, and leaf nutrient content rely on essential nutrients such as Al (Aluminum), C (Carbon), H (Hydrogen), O (Oxygen), N (Nitrogen), P (Phosphorus), K (Potassium), Ca (Calcium), Mg (Magnesium), S (Sulfur), Fe (Iron), Zn (Zinc), Mn (Manganese), Mo (Molybdenum), Cu (Copper), B (Boro), Ni (Nickel), Si (Silicon), Co (Cobalt), Na (Sodium), and Cl (Chlorine) (Zipori et al. 2020). Additionally, all nutrients except for C and O are absorbed through the soil solution while being removed from olive orchards during fruit harvesting and pruning activities (Fernández-Escobar et al. 2015).
One of the other significant components found in olive leaves is chlorophylls (Chls) (Bahloul et al. 2014). Chls are green pigments that play a vital role in photosynthesis. They serve as catalysts during the process, converting solar energy into chemical energy (Kacar et al. 2006). Hence, understanding the chlorophyll content of the leaves is of utmost importance in terms of the region’s cultivation.
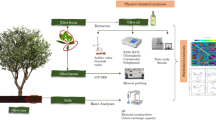
This study aims to establish relationships using multivariate analysis methods between the nutrient content of the soil conditions in which olive cultivars are grown in Türkiye and the nutrient element content, phytochemical contents, antioxidant activity, total chlorophyll amount, and leaf colors of these cultivars leaves.
Materials and methods
Plant material and research region
In the scope of the research, a total of 23 olive cultivars were used, including 20 local (‘As Topakaşı’, ‘Ayvalık’, ‘Çelebi’, ‘Domat’, 'Edincik Su’, ‘Elmacık’, ‘Gemlik’, ‘Gemlik-21’, ‘Girit Zeytini’, ‘Halhalı’, ‘Karamani’, ‘Kilis Yağlık’, ‘Memecik’, ‘Nizip Yağlık’, ‘Sarı Haşebi’, ‘Sarı Ulak’, ‘Sarı Yaprak’, ‘Saurani’, ‘Tavşan Yüreği’, ‘Uslu’) and 3 foreign (‘Arbequina’, ‘Frantoio’, ‘Manzanilla’) cultivars. The cultivars used were grown on their own roots without the use of rootstocks.
The olive grove located in the producer garden in Hatay province covers an area of 50 acres (36°42′44"N 36°30′30"E). The cultivars, planted at 4 × 4 spacing, are 7 years old, and cultural practices such as irrigation, fertilization, pruning, and spraying are regularly carried out. Hatay province is located in the Mediterranean region, therefore, summers are hot and dry, while winters are mild and rainy.
Soil analysis
Before starting the study, soil analysis was performed to ensure appropriate plant fertilization (Table 1), and the fertilization plan was adjusted accordingly.
Table 2 displays the optimal conditions for plant growth. Fertilization procedures persisted using compound fertilizer (20 + 20 + 20) supplemented with sulfur and micronutrients until the conclusion of April. Additionally, an extra 100 g of ammonium sulfate was administered according to the age of the trees. Fertilizer was incorporated into the soil under the tree canopy prior to irrigation.
Soil samples were collected from different regions of the field for use in the study (Gagour et al. 2024). Samples taken from five different regions of the field were dried at 40 °C and ground into powder to pass through a 2 mm sieve. The prepared samples were analyzed for minerals (Cu, K, Mg, Zn, Mn, Fe, and Na). The mineral analysis was conducted utilizing inductively coupled plasma optical emission spectrometry (ICP-OES), following the NF X31-121 and NF X31-108 standards (Afron 2002), and the results were expressed in mg kg−1. Soil analyses were conducted in five different regions of the area in 2022 and 2023, and average values were utilized.
Leaf nutrient analysis
Leaf nutrient analysis were conducted with 3 replicates, each consisting of 150 leaves. Leaf samples were collected when the fruits began to ripen, which occurred between late September, early October in 2022 and 2023 (Yıldız et al. 2023). Samples of leaves were selected from various sections of the tree in a randomized manner. In olive leaves, the total contents of Al, B, Ca, Cu, Fe, K, Mg, Mn, Na, Ni, S and Zn were determined. Microwave-assisted digestion process using nitric acid (HNO3) and hydrogen peroxide (H2O2) was employed for the decomposition of leaf samples. Dried and ground samples of olive leaves underwent decomposition with a mixture of HNO3-H2O2 acid (2:3 v/v). Following this, the samples were treated in a microwave oven (Refsan, RK55, Kütahya, Türkiye) through three stages: 145 °C with 75% relative humidity for 5 min; then 180 °C with 90% relative humidity for 10 min; and finally 100 °C with 40% relative humidity for 10 min (Mertens 2005a). The concentrations of nutrients in the samples were determined in mg kg−1 using inductively coupled plasma optical emission spectrometry (ICP-OES) (Agilent) (Mertens 2005b).
Phytochemical analysis
Sample preparation: Phytochemical analysis were conducted with 3 replicates, each consisting of 40 leaves. Preparation of the samples was carried out according to Hannachi et al. (2020); phytochemical analysis were extracted through a maceration process with agitation for 24 h at 25 °C, utilizing methanol as the extraction solvent. The resulting solution underwent filtration followed by centrifugation (OHAUS FC5718; Parsippany, New Jersey, USA) at 11, 200 × g for 15 min. The methanolic extracts obtained from olive leafs were employed to determine the contents of phytochemical analysis.
Total phenolics: TPs were assessed using the Folin-Ciocalteu method. A total of 500 μL of fresh leaf extract was mixed with 4.1 mL of distilled water, followed by the addition of 100 μL of Folin-Ciocalteu reagent and 2% sodium carbonate (Na2CO3). After incubating the solution in darkness for 2 h, resulting in a blue coloration, it was analyzed at a wavelength of 760 nm using a spectrophotometer (SOIF UV-5100H Spektrofotometre-UV/VIS 200–1000 nm). The obtained outcomes were presented as milligrams of gallic acid equivalent (GAE) per 100 g (fresh weight) (Eyduran et al. 2015). The total phenolic content (TPs) was determined using the equation provided in Eq. 1 (Tunç et al. 2024).
Total flavonoids: TFs were quantified following the procedure outlined by Yaman et al. (2024). Absorbance readings were obtained using a spectrophotometer (SOIF UV-5100H UV/VIS Spectrophotometer, 200–1000 nm) with measurements taken at a wavelength of 415 nm. The outcomes were expressed as mg/100 g in terms of quercetin equivalents (QE).
Total antioxidant activity
TAA was assessed utilizing the DPPH (2,2-diphenyl-1-picryl-hydrazyl) method as described by Liguori et al. (2016). The reduction in DPPH absorbance was measured at 517 nm using a spectrophotometer (SOIF UV-5100H UV/VIS Spectrophotometer, 200–1000 nm) at 25 °C (Asample). A solution devoid of olive lead served as the blank (Ablank), and its absorbance was recorded. TAA was calculated using the equation presented in Eq. 2 (Adiletta et al. 2018).
Color measurements and total chlorophyll content
Color measurements and leaf chlorophyll content were performed with 3 replications and 30 leaf in each replication. Color measurements (L*, a*, b*) of olive leafs were made with a colorimeter (3nh ST50) (Yaman 2022). To determine changes in leaf chlorophyll content, readings of chlorophyll amount were taken using a SPAD meter (SPAD 502). Measurements were conducted between 10:00–14:00 on two fully developed leaf pairs at the middle part of the shoot (Yaman 2024).
Statistical analysis
Since the study was conducted in 2022 and 2023, statistical analysis was performed by taking the average of 2 years. Soil and leaf nutrient content, biochemical properties, antioxidant activity, leaf color measurement and leaf chlorophyll content were analyzed using the JMP® Pro 17 (JMP® 2024) package program. The TUKEY multiple comparison test was used to evaluate the results, and the findings were presented at the 5% significance level. Principal component analysis (PCA), heatmap analysis and correlation matrix analysis in comparing soil nutrient content with other data sets were performed with the Origin Pro® 2024 (OriginLab® 2024) statistical program.
Results and discussion
Soil analysis
The mineral content of soil samples taken from five different regions of the research area is presented in detail in Table 3. As a result of the analysis, seven nutrients were identified in five regions. The nutrients Cu (0.77–0.91 mg kg−1), K (223.45–256.74 mg kg−1), Mg (154.13–172.83 mg kg−1), and Zn (1.64–1.74 mg kg−1) were found to be statistically insignificant, while minor differences were observed in the content of Fe (3.14–3.62 mg kg−1), Mn (1.25–1.67 mg kg−1), and Na (33.71–39.17 mg kg−1). The samples taken from five different regions demonstrate the homogeneity of the research area in terms of nutrients (Damak et al. 2021). Our findings are parallel to those obtained by Gagour et al. (2024) in their study conducted in Morocco.
Leaf nutrient analysis
Mineral contents of olive leaves across the examined cultivars are given in detail in Table 4. Accordingly, Al ranged from 1.11 (‘Nizip Yağlık’) to 59.25 mg kg−1 (‘Çelebi’); B changed between 1.79 (‘Nizip Yağlık’) and 6.53 mg kg−1 (‘Çelebi’); Ca varied between 65.26 (‘Nizip Yağlık’) and 5485.03 mg kg−1 (‘Gemlik-21’); Cu changed between 0.08 (‘Nizip Yağlık’) and 48.36 mg kg−1 (‘Çelebi’); Fe varied between 6.95 (‘Nizip Yağlık’) and 69.34 mg kg−1 (‘Çelebi’); K ranged from 0.64 (‘Nizip Yağlık’) to 1438.11 mg kg−1 (‘Çelebi’); Mg changed between 17.00 (‘Nizip Yağlık’) and 928.11 mg kg−1 (‘Sarı Haşebi’); Mn varied between 0.02 (‘Nizip Yağlık’) and 19.71 mg kg−1 (‘Sarı Haşebi’); Na ranged from 25.74 (‘Edincik Su’) to 197.12 mg kg−1 (‘Çelebi’); Ni changed between 0.03 (‘Elmacık’) and 1.71 mg kg−1 (‘Tavşan Yüreği’); S varied between 24.42 (‘Nizip Yağlık’) and 525.14 mg kg−1 (‘Gemlik-21’), and Zn ranged from 0.12 (‘Nizip Yağlık’) to 9.76 mg kg−1 (‘Tavşan Yüreği’). Our findings differ from previous research conducted on similar topics in Greece (Dimassi et al. 1999), Portugal (Jordao et al. 1999), and Croatia (Pasković et al. 2013). Toplu et al. (2009) reported that leaf nutrient content varies according to nutrient uptake, ecological conditions, plant species, and cultivars.
Leaf color and chlorophyll content analysis
Color and chlorophyll values of olive cultivars are given in detail in Table 5. The color properties of fruits and leafs significantly influence various parameters, including phytochemical structures (Šamec et al. 2016; Yaman 2022). The L* value ranged from 31.79 (‘Domat’) to 49.19 (‘Sarı Yaprak’). The a* value varied between 6.58 (‘As Topakaşı’) and 28.84 (‘Girit Zeytini’). The b* value ranged from 9.94 (‘Frantoio’) to 29.43 (‘Sarı Yaprak’). Tunç and Nikpeyma (2023) reported L*, a*, b* values in olive cultivars varied between 17.66 and 42.74; -0.44 and 7.47; -2.88 and 20.62, respectively. Leaf chlorophyll content varied between 64.67 (‘As Topakaşı’) and 95.57 (‘Girit Zeytini’). Pouyafard et al. (2016) found that this value ranged between 71.21 and 97.08. Although the L* color value and chlorophyll content we obtained in the study are similar to the findings of researchers, the a* and b* color values do not show similarity. These differences are thought to stem from ecological, nutritional, and cultivar differences (Yaman 2022).
Phytochemical and antioxidant activity analysis
The phytochemical and antioxidant activity contents of olive cultivars given in detail in Table 6. Total phenolics, total flavonoids, antioxidant activity values varied between 51.43 (‘Nizip Yağlık) and 151.49 mg GAE/100 g (‘Manzanilla’), 14.49 (‘Sarı Ulak’) and 39.01 mg QE/100 g (‘Edincik Su’), 52.94 (‘Saurani’) and 91.18% (‘Nizip Yağlık’), respectively. In a similar study conducted in Iran, they found that total phenolics varied between 42.35 and 190.98 mg GAE/100 g, antioxidant activity ranged from 20.66 to 95.39% (Ghasemi et al. 2018). Our findings are in agreement with the findings of the researchers. In a study conducted in Tunisia, the total amount of flavonoids in olive leaves was found to be between 14.02–24.39 mg QE/100 g (Hannachi et al. 2020). Our total flavonoid findings are higher than the researchers findings. It is estimated that this difference is due to the nutritional status of the tree, the use of different cultivars and the different ecological conditions.
Principal component analysis (PCA)
The 3D principal component analysis of the data sets consisting of three principal components is presented in detail in Fig. 1. PCA is a statistical method used in many branches of science to reduce the complexity in data sets (El Bakali et al. 2022). The 1st, 2nd and 3rd principal components account for 41.3%, 21.2% and 19.5% of the variation, respectively. Thus, the first three principal components account for 82% of the total variation. In the principal component analysis scatter plot, the values of AA, a*, L*, Fe (S), Zn (S), TFs, SPAD, Ca (L), Mg (L), S (L), Zn (L), region 1 and region 2 are located in the positive direction, while the other values are located in the negative direction. In the study conducted by Uslu Aksu et al. (2023), the first three principal components accounted for 62.05% of the total variation. Yaman et al. (2024) determined the variation accounted for by the first three principal components as 40.55%. Our findings regarding the variation captured by the first three principal components are higher than those reported by the researchers. The elevated variance values signify the effectiveness of the classification in the conducted study (Tunç et al. 2024).
Correlation matrix analysis
The correlation matrix of soil and leaf nutrient contents is presented in detail in Fig. 2. There is both a positive correlation and statistical significance between Al (L) and Fe (L) (r = 0.97**); Mn (L) (r = 0.96**); K (S) (r = 0.89*). There is a positive correlation between B (L) and Cu (L) (r = 0.89*); K (L) (r = 0.99***); S (L) (r = 0.93*). There is both a positive correlation and statistical significance between Cu (L) and S (L) (r = 0.89**); Zn (L) (r = 0.88*). There is a positive correlation between Fe (L) and K (L) (r = 0.88*); Mn (L) (r = 0.98**). There is both a positive correlation and statistical significance between K (L) and S (L) (r = 0.92*); Mg and Zn (L) (r = 0.94*); Mn (L) and K (S) (r = 0.88*); Na (L) and Ni (L) (r = 0.88*); Fe (S) and Zn (S) (r = 0.99**). Despite the negative correlation between Cu (S) and Fe (S) (r = -0.93*); Mn (S) and Zn (S) (r = -0.91*); Mg (S) and Na (S) (r = -0.91*), it is statistically significant.
The correlation matrix consisting of leaf colors, chlorophyll contents, phytochemicals and antioxidant activities is presented in detail in Fig. 3. There is both a positive correlation and statistical significance between L* and b* (r = 0.94*); SPAD and TPs (r = 0.78***); SPAD and TFs (r = 0.96**); TPs and TFs (r = 0.70***).
Correlation matrix consisting of leaf colors, chlorophyll contents, phytochemicals, antioxidant activities (The average of 2022 and 2023 years). L* a* b* Leaf colors, SPAD Leaf chlorophyll content, TPs Total phenolics (mg GAE/100 g), TFs Total flavonoids (mg QE/100 g), AA DPPH antioxidant activity (% inhibition). Pearson correlation type was used in the correlation matrix analysis
Our findings differ from the research results conducted in Morocco (Gagour et al. 2024) and Türkiye (Gurel et al. 2014). Elevated levels of certain minerals in the soil lead to corresponding increases in the leaves due to absorption, thus elucidating the positive associations among these minerals. Conversely, negative correlations between certain minerals in the soil and leaves may stem from their interchange during absorption or their limited uptake by plant roots (Gagour et al. 2024).
Heatmap analysis
Heatmap analysis of all data sets is presented in detail in Fig. 4. The soil and leaf nutrient contents, leaf color, chlorophyll, phytochemicals, and antioxidant activity were divided into two groups (A and B). Each group was further subdivided into two subgroups (A1, A2 and B1, B2). TPs, TFs, SPAD were included in subgroup A1, while a*, Na (S), Mn (S) were in subgroup A2. AA and Zn (S) were in subgroup B1, while all other datasets were in subgroup B2. The regions were divided into two groups (C and D). Only D was further subdivided into two subgroups (D1 and D2). Region 1 was in group C. While Region 5 was in subgroup D1, the other regions were in subgroup D2.
Conclusion
As a result of the research, very small differences were found in the nutrient contents of soil samples taken from five different regions. Therefore, it was determined that the field soil is generally homogeneous. Despite the general homogeneity of the field soil, significant differences were determined in the leaf nutrient content of the examined olive cultivars. In nutrient analysis conducted on leaves, in the ‘Çelebi’ cultivar, Al (59.25 mg kg−1), B (6.53 mg kg−1), Cu (48.36 mg kg−1), Fe (69.34 mg kg−1), K (1438.11 mg kg−1), Na (197.12 mg kg−1) nutrients are the highest; in the ‘Gemlik-21’ cultivar, Ca (5485.03 mg kg−1) nutrient is the highest; in the ‘Sarı Haşebi’ cultivar, Mg (928.11 mg kg−1), Mn (19.71 mg kg−1), S (632.77 mg kg−1) nutrients are the highest; and in the ‘Tavşan Yüreği’ cultivar, Ni (1.71 mg kg−1), Zn (9.76 mg kg−1) nutrients are the highest. Additionally, significant differences have been detected in leaf color values (L*, a*, b*), chlorophyll content, phytochemical properties, and antioxidant capacity. The L* (49.19), b* (29.43) color values are highest in the ‘Sarı Yaprak’ cultivar, while the a* (28.84) value is highest in the ‘Girit Zeytini’ cultivar. The highest leaf chlorophyll content was determined in the ‘Girit Zeytini’ cultivar (95.57). Total phenolics, total flavonoids, antioxidant capacity were determined to be highest in the ‘Manzanilla’ (151.49 mg GAE/100 g), ‘Edincik Su’ (39.01 mg QE/100 g), ‘Nizip Yağlık’ (91.18%) cultivars, respectively. These differences are directly associated with the nutritional status of the cultivars, as confirmed by principal component analysis, correlation matrix analysis, and heatmap analysis. This study is indicative of guiding future research and highlights the necessity of its application on different olive cultivars and different fruit types.
Data availability
No datasets were generated or analysed during the current study.
References
Adiletta G et al (2018) Study of pomological traits and physico-chemical quality of pomegranate (Punica granatum L.) genotypes grown in Italy. Eur Food Res Technol 244:1427–1438. https://doi.org/10.1007/s00217-018-3056-x
Afron N (2002) X 31–108; Soil quality–Determination of Ammonium Acetate Extractable Ca++, Mg++, K+ and Na+ cations–Agitation Method. AFNOR: Paris, France
Aksu Uslu N (2023) Evaluation of quality traits and phytochemical compounds of persimmon (Diospyros kaki L.) cultivars grown in Samsun, Turkey. Erwerbs-Obstbau 65(4):889–897. https://doi.org/10.1007/s10341-022-00753-z
Bahloul N, et al (2014) Comparative investigation of minerals, chlorophylls contents, fatty acid composition and thermal profiles of olive leaves (Olea europeae L.) as by-product. Grasas y Aceites 65(3):e035-e035. https://doi.org/10.3989/gya.0102141
Cheng HH, Kurtz LT (1963) Chemical distribution of added nitrogen in soils. Soil Sci Soc Am J 27(3):312–316. https://doi.org/10.2136/sssaj1963.03615995002700030029x
Damak F et al (2021) Soil geochemistry, edaphic and climatic characteristics as components of Tunisian olive terroirs: Relationship with the multielemental composition of olive oils for their geographical traceability. Euro-Mediterr J Environ Integr 6(37):1–23. https://doi.org/10.1007/s41207-021-00241-y
Dimassi K, et al (1999) Genotypic effect on leaf mineral levels of 17 olive cultivars grown in Greece. Acta Horticulturae 474:345–348. https://doi.org/10.17660/ActaHortic.1999.474.71
El Bakali I, et al (2022) A comparative phytochemical profiling of essential oils isolated from three hemp (Cannabis sativa L.) cultivars grown in central-northern Morocco. Biocatal Agric Biotechnol 42:102327. https://doi.org/10.1016/j.bcab.2022.102327
Epstein E, Bloom A (2005) Mineral Nutrition of Plants: Principles and Perspectives, 2nd edn. Sinauer Associates, USA, Sunderland, Mass
Ercisli S (2004) A short review of the fruit germplasm resources of Turkey. Genet Resour Crop Evol 51:419–435. https://doi.org/10.1023/B:GRES.0000023458.60138.79
Eyduran SP et al (2015) Organic acids, sugars, vitamin C, antioxidant capacity, and phenolic compounds in fruits of white (Morus alba L.) and black (Morus nigra L.) mulberry genotypes. J Appl Bot Food Qual 88:134–138. https://doi.org/10.5073/JABFQ.2015.088.019
FAO (1990) Micronutrient. Assessment at the Country Level: An International Study. FAO Soil Bulletin By Mikko Sillanpaa, Rome. Follet, R.H., 1969. Zn, Fe, Mn, and Cu in Colorado Soils. PhD Thesis. Colo. State Univ
Fernández-Escobar R, et al (2015) Nutrient removal from olive trees by fruit yield and pruning. HortScience 50(3):474–478. https://doi.org/10.21273/HORTSCI.50.3.474
Gagour J et al (2024) Leaf mineral profiling and its correlation with oil physicochemical traits from four olive (Olea europaea L.) cultivars grown in Morocco as affected by olive ripening stages. Eur Food Res Technol 250:1443–1456. https://doi.org/10.1007/s00217-024-04475-2
Ghasemi S et al (2018) Investigation of phenolic compounds and antioxidant activity of leaves extracts from seventeen cultivars of Iranian olive (Olea europaea L.). J Food Sci Technol 55:4600–4607. https://doi.org/10.1007/s13197-018-3398-1
Gurel S, et al (2014) Antioxidative properties of olive fruits (Olea europaea L.) from ‘Gemlik’variety and relationship with soil properties and mineral composition. Oxidation Communications 37(4):985–1004
Hannachi H, et al (2020) Chemical profiles and antioxidant activities of leaf, pulp, and stone of cultivated and wild olive trees (Olea europaea L.). Int J Fruit Sci 20(3):350–370. https://doi.org/10.1080/15538362.2019.1644574
Hutchings A et al (1996) Zulu medicinal plants: An inventory. University of Natal Press, Scottsville, South Africa, p 464p
Ilhan G, et al (2024) Morphological characterization of 96 wild-grown genotypes of sea buckthorn (Hippophae rhamnoides spp.) in eastern Türkiye. Genetic Resources and Crop Evolution 71(2):773–784. https://doi.org/10.1007/s10722-023-01656-y
Jabalbarezi Hukerdi Y, et al (2018) The study of physicochemical properties and nutrient composition of Mari olive leaf cultivated in Iran. Nutr Food Sci Res 5(2):39–46. https://doi.org/10.29252/nfsr.5.2.39
JMP® (2024) https://www.jmp.com/en_us/home.html. Accessed 24 March 2024
Jordao PV, et al (1999) Effect of cultivar on leaf-mineral composition of olive tree. Acta Horticulturae 474:349–352. https://doi.org/10.17660/ActaHortic.1999.474.72
Kacar B, et al (2006) Plant Physiology. Nobel Publishing, vol 848
Liguori L et al (2016) Quality attributes of low-alcohol top-fermented beers produced by membrane contactor. Food Bioprocess Tech 9:191–200. https://doi.org/10.1007/s11947-015-1612-y
Loue A (1968) Diagnostic petiolaire de prospection. Etudes Sur la Nutrition et la Fertilisation Potassiques de la Vigbe Societe Commerciale des Potasses d’Alsace Services Agroomiques 31–41
Mertens D (2005a) Plants Preparation of Laboratory Sample. In: Horwitz W, Latimer GW (eds) Official Methods of Analysis 18th Chapter 3. Gaitherburg, Maryland USA, pp 1–2
Mertens D (2005b) Metal in Plants and Pet Foods. In: Horwitz W, Latimer GW (eds) Official Methods of Analysis 18th Chapter 3. Gaitherburg, Maryland USA, pp 3–4
Navajas-Porras B et al (2020) Relationship of quality parameters, antioxidant capacity and total phenolic content of EVOO with ripening state and olive variety. Food Chem 325:126926. https://doi.org/10.1016/j.foodchem.2020.126926
OriginLab® (2024) https://www.originlab.com/. Accessed 24 March 2024
Pasković I et al (2013) Leaf mineral concentration of five olive cultivars grown on calcareous soil. J Cent Eur Agric 14(4):1471–1478. https://doi.org/10.5513/jcea.v14i4.2345
Pasković I et al (2020) Temporal variation of phenolic and mineral composition in olive leaves is cultivar dependent. Plants 9(9):1099. https://doi.org/10.3390/plants9091099
Pereira AP, et al (2007) Phenolic compounds and antimicrobial activity of olive (Olea europaea L. Cv. Cobrançosa) leaves. Molecules 12(5):1153–1162. https://doi.org/10.3390/12051153
Pouyafard N et al (2016) Determination of some physiologic and morphologic changes of young olive (Cv Ayvalik) trees under different water stress in coastal part of aegean region. J Tekirdag Agric Fac 13(1):88–98
Rallo L et al (2018) Quality of olives: A focus on agricultural preharvest factors. Sci Hortic 233:491–509. https://doi.org/10.1016/j.scienta.2017.12.034
Richards LA (1954) Diagnosis and improvement of saline and alkali soils (No. 60) (Ed.). US Government Printing Office
Šamec D et al (2016) Assessment of the differences in the physical, chemical and phytochemical properties of four strawberry cultivars using principal component analysis. Food Chem 194:828–834. https://doi.org/10.1016/j.foodchem.2015.08.095
Talhaoui N et al (2015) Phenolic compounds in olive leaves: Analytical determination, biotic and abiotic influence, and health benefits. Food Res Int 77:92–108. https://doi.org/10.1016/j.foodres.2015.09.011
Toplu C et al (2009) Leaf mineral composition of olive varieties and their relation to yield and adaptation ability. J Plant Nutr 32(9):1560–1573. https://doi.org/10.1080/01904160903094321
Tunç Y, et al (2024) Elucidating the genetic diversity in wild olive (Olea europaea L. subps. oleaster) genotypes from the Mesopotamia region with multivariate analyses. Genet Resour Crop Evol 1–12. https://doi.org/10.1007/s10722-024-01948-x
Tunç Y, Nikpeyma Y (2023) Effects of kaolin clay application on the Gemlik and Ayvalık (Edremit) varieties of olive (Olea europaea L.) trees against sunburn in dry and watery conditions. Osmaniye Korkut Ata Univ J Inst Sci Technol 6(2):1384–1394
Tüzüner A (1990) Soil and Water Analysis Laboratories Handbook. T.R. Ministry of Agriculture, Forestry and Rural Affairs, General Directorate of Rural Services. pp 21–27
Yaman I (2024) Investigation of the polissibilities of using Gemlik olive (Olea europaea L.) as a rootstock some local vareties. PhD thesis. Mustafa Kemal Univ Inst Sci Technol Dep Horticulture. Hatay, Türkiye
Yaman M (2022) Determination of genetic diversity in european cranberrybush (Viburnum opulus L.) genotypes based on morphological, phytochemical and ISSR markers. Genetic Resources and Crop Evolution 69(5):1889–1899. https://doi.org/10.1007/s10722-022-01351-4
Yaman M, et al (2024) Determination of fruit characteristics, nutrients and biochemical contents of Transvalia (Prunus persica L.) peach cultivar grafted on different clonal rootstocks obtained by selection and hybridization. Scientia Horticulturae 330:113093. https://doi.org/10.1016/j.scienta.2024.113093
Yıldız E, et al (2023) Assessing the genetic diversity in hawthorn (Crataegus spp.) genotypes using morphological, phytochemical and molecular markers. Genet Resour Crop Evol 70(1):135–146. https://doi.org/10.1007/s10722-022-01414-6
Zeriouh W, et al (2017) Phenolic extract from oleaster (Olea europaea var. sylvestris) leaves reduces colon cancer growth and induces caspase-dependent apoptosis in colon cancer cells via the mitochondrial apoptotic pathway. PLoS One. 12(2):e0170823. https://doi.org/10.1371/journal.pone.0170823
Zipori I et al (2020) Sustainable management of olive orchard nutrition: a review. Agriculture 10(1):11. https://doi.org/10.3390/agriculture10010011
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
Yazgan TUNÇ: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Mehmet YAMAN: Data curation, formal analysis, methodology, writing – original draft. Yusuf Murat KEÇE: Methodology. Kadir Uğurtan YILMAZ: Methodology, Conceptualization. Ercan YILDIZ: Project administration, Methodology. Adem GÜNEŞ: Formal analysis, Conceptualization.
Consent to Participate and Publish
Authors read the manuscript and showed their willingness to publish this study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tunç, Y., Yaman, M., Keçe, Y.M. et al. Characterization of olive (Olea europaea L.) cultivars; colour properties, biochemical contents, antioxidant activity and nutrient contents. Genet Resour Crop Evol (2024). https://doi.org/10.1007/s10722-024-01991-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10722-024-01991-8