Abstract
Fruits of Strychnos spinosa Lam. hold considerable food value within rural communities. However, no study has reported the nutritional profile of S. spinosa morphotypes. Therefore, this study is aimed to determine nutritional variation among the morphotypes. Proximate composition was analyzed using association of official analytical chemists’ methods, and minerals with inductively coupled plasma atomic emission spectroscopy. The following were the ranges of proximate and nutrient content determined: moisture content (10.29–60.50%); fat (0.95–2.67%); crude protein (2.85–9.19%); ash (4.78–18.05%); carbohydrates (37.39–42.24%); acid detergent fibre (7.94–21.75%), neutral detergent fibre (16.46–42.55%); calcium (0.30–35 mg/100 g); potassium (810–2510 mg/100 g); phosphorus (9–69 mg/100 g); sodium (7–54 mg/100 g); magnesium (9–55 mg/100 g); copper (0.10–2.70 mg/100 g); iron (0.10–5.50 mg/100 g); manganese (0.30–2.43 mg/100 g) and zinc (0.10–0.80 mg/100 g). Calcium, phosphorus, magnesium, copper, iron, manganese, fat, acid detergent fibre, neutral detergent fibre, sodium, crude protein showed positive association with principal components. Biplot and dendrogram grouped morphotypes with high and low nutrient content independently. Carbohydrates, protein, calcium, potassium, copper, iron, manganese, and zinc content of morphotypes were higher than those in commercialized fruits. This species is promising for domestication and commercialization, and thereby contributes significantly towards food security.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Strychnos species hold considerable value within rural communities, being highly esteemed fruits (Ngadze et al. 2017). Particularly, Strychnos spinosa find as prominent and versatile fruit tree species in KwaZulu-Natal (Nkosi et al. 2020). The fruit's popularity is rooted in the appealing nutritional and sensory attributes of its succulent pulp (Ngadze et al. 2018). Strychnos spinosa shows promising prospects for industrial utilization, particularly in the realm of producing fruit juice-based products (Rodrigues et al. 2018). Several rural communities have already embraced the creation of homemade fruit-based products using S. spinosa (Ngadze et al. 2017; Mbhele et al. 2022a).
Wild edible fruits that have been previously overlooked are now being recognized as a crucial element in human nutrition, carrying substantial implications for food security, well-being, and income generation (Achaglinkame et al. 2019). Despite the notable yield of fruit biomass that exceeds 40 kg per tree in its natural habitat (Ngemakwe et al. 2017; Akweni et al. 2020), due to their limited availability, these native fruits remain underutilized and many communities continue to face challenges related to inadequate food access (Bvenura and Sivakumar 2017). Hence, fruits of this species remain confined to local consumption and has not ventured into commercial markets (Rodrigues et al. 2018).
The limited diversity in our food sources has implications for nutrition in the face of many challenges we confront in the twenty-first century, the imperative of providing nutritious sustenance to the world's expanding population through sustainable and alternative food systems has become a pressing societal challenge (Fanzo et al. 2020). This challenge has been underscored by the COVID-19 pandemic, which has shed light on the necessity for transformative shifts in various aspects of modern life, including food sustainability and the preservation of underappreciated plants for future resource utilization (Fukalova et al. 2022).
Many households in South Africa are challenged with food insecurity (Mudzielwana et al. 2022). Food insecurity remains a significant contributor to malnutrition, particularly in low and middle-income countries (Siddiqui et al. 2020). This issue is delineated through a hierarchical framework that comprises of four levels, namely, availability, accessibility, utilization, and stability (Militao et al. 2022a). In the case of S. spinosa, a fruit-bearing tree known for its ability to thrive under drought conditions, hence it’s availability and accessibility for consumption stand out (Ntshidi et al. 2022). Rural communities utilize these fruits to develop a cultivar of food products (Ngadze et al. 2017). The concept of "stability" acknowledges the dynamic nature of food insecurity, which can be either transient and cyclic or chronic due to factors such as climate change that disrupt any of the preceding three dimensions (Militao et al. 2022b). Remarkably, S. spinosa remains abundant even in the face of severe climate changes, including prolonged drought conditions (Ntshidi et al. 2022). To truly achieve food security, all four dimensions of availability, accessibility, utilization, and stability must be addressed concurrently (Mudzielwana et al. 2022).
Proximate, micro, and macronutrients are integral components of a balanced diet, that provide various health benefits (Shahid et al. 2023). Macronutrients constitute the nutrients that the human body requires in substantial quantities which include fat, carbohydrate, and protein (Venn 2020). Unlike macronutrients, micronutrients are essential in smaller amounts to regulate diverse physiological functions that contribute to overall well-being (Awuchi et al. 2020). Deficiencies in proximate, micro, and macronutrients can result in various health problems, including malnutrition, chronic disease, compromised immune response, and cognitive deficits (Kiani et al. 2022; Savarino et al. 2021; Thaxton et al. 2018). This study marks the first documentation of the nutritional profiles of S. spinosa morphotypes. The insights gained from this research have the potential to serve as a basis for promoting the inclusion of S. spinosa into healthy diets, stimulating agricultural endeavours, and aiding in breeding programs. To advocate for the utilization of wild fruits as valuable dietary components, a comprehensive understanding of their nutritional value is essential (Sibiya et al. 2021). Therefore, this study aimed to document the nutritional attributes of S. spinosa morphotypes.
Materials and methods
Naming of morphotypes
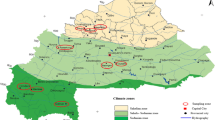
A representative set of Strychnos spinosa plant material was collected across the study area at Bonamanzi Game Reserve in KwaZulu-Natal to assess the nutritional variation among 32 morphotypes identified in the area (Table 1 and Fig. 1). Morphotype names were coined based on colour, texture, and shape of the immature fruits, colour of recently sprouted open leaves, as well as colour, shape, and form of fully developed leaves.
Differences in fruit colour, texture, and shape as well as leaf colour, shape and form among Strychnos spinosa morphotypes. Fruit colour: green (a), purple (b); Texture: smooth (c), smooth and corrugated (d), rough (e), rough and corrugated (f), very rough and corrugated (g); Shape: roundish (h), pyriform (i); Mature leaf colour: dark green (j) and green (k); and variation in leaf shape and form (l) (Mbhele et al. 2022b). Proximate composition
Moisture determination
Moisture was determined using the procedure described in American Association of Cereal Chemists (AACC) Method 44–15A (1999). Moisture crucibles were dried in a forced draft oven at 103°C for 1 h. The crucibles were then cooled in a desiccator for about 10 min. The crucibles were weighed, and 2 g of sample was weighed into the crucibles and dried in a forced draught oven for 4 h at 103°C. The samples were cooled for 10 min and weighed. The moisture content was determined by the difference between fresh matter and dry matter and calculated as follows:
Fat determination
Crude fat was determined by extracting the sample using petroleum ether, according to the AACC method 30–25 of the Soxhlet test apparatus (AACC 1983). Percentage of fat was calculated using the following formula:
Fat percentage of the fruit was categorized as low (≤ 1.30), moderate (> 1.30–1.70) and high (> 1.70).
Ash determination
To determine the ash content, which is the substance remaining after oxidative combustion of all the organic matter in food, the AACC method 08–01 was used (AACC 1999). Percentage ash was calculated as follows:
Ash percentage of the fruit was categorized as low (≤ 9.00), moderate (> 9.00–14.00) and high (> 14.00).
Crude proteins determination
To determine crude protein the micro Kjeldahl method described by the Association of Official Analytical Chemists was used (AOAC 2005). The nitrogen content was calculated and converted to percentage protein by using a protein conversion factor of 6.25. This was given as:
Protein percentage of the fruit was categorized as low (≤ 4.00), moderate (> 4.00–6.00) and high (> 6.00).
Acid detergent fibre and neutral detergent fibre
Both acid detergent fibre (ADF) and neutral detergent fibre (NDF) were determined as described by Nansikombi et al. (2019)
The neutral detergent fibre was calculated using the following equation:
The Acid detergent fibre was calculated using the following equation:
ADF percentage of the fruit was categorized as low (≤ 9.00), moderate (> 9.00–14.00) and high (> 14.00).
NDF percentage of the fruit was categorized as low (≤ 20.00), moderate (> 20.00–30.00) and high (> 30.00).
Carbohydrate determination
The available carbohydrate content in the samples was calculated by difference as follows: % carbohydrate = 100 – (% moisture + % ash + % crude protein + % crude fat + % crude fibre)
Mineral composition
Calcium (Ca), copper (Cu), iron (Fe), potassium (K), magnesium (Mg), manganese (Mn), phosphorus (P), sodium (Na), and zinc (Zn) content of the fruits were measured in triplicates using ICP-OES (Manson and Roberts 2000). Plant samples were analyzed using Manson and Roberts' batch-handling technique (Manson and Roberts 2000). The materials were dried at 750C and sieved through a 0.84 mm sieve. After sieving, the product was ash dried overnight at 4500C before being exposed to 1 M of HCl. ICP-OES was used to determine the elements Calcium (Ca), potassium (K), phosphorus (P), magnesium (Mg), sodium (Na), copper (Cu), iron (Fe), manganese (Mn) and zinc (Zn).
The fruit’s calcium levels (mg/100 g) were classified as low (≤ 18.0), moderate (> 18.0–25.0), and high (> 25.0). Its potassium content was categorized as low (≤ 1230), moderate (> 1230–2000), and high (> 2000). While phosphorus content was low (≤ 30), moderate (30–50), high (> 50). The magnesium content in the fruit was sorted into three groups: low (≤ 30.0), moderate (> 30–46), and high (> 46). Similarly, the sodium content was divided into low (≤ 15), moderate (> 15–28), and high (> 28) categories. The copper content of the fruit fell into the low (≤ 1.30), moderate (> 1.30–1.80), and high (> 1.80) ranges. Regarding iron content, it was classified as low (≤ 2.25), moderate (> 2.25–4.00), and high (> 4.00). Whereas manganese was low (≤ 1.50), moderate (> 1.50–2.00) and high (> 2.00). Finally, the fruit’s zinc content was grouped as low (≤ 0.40), moderate (> 0.40–0.70), and high (> 0.70).
Data analysis
All samples were prepared and analyzed in triplicate. Data were subjected to analysis of variance (ANOVA) using the GenStat 15th edition. Analysis was combined for both growing seasons. Means were separated using Tukey’s Honest Significant Difference (HSD) at 5% significant level. Correlations and principal component analysis (PCA) were implemented to determine multi-character variation. Cluster analysis through biplot and dendrogram was conducted to study the similarities and dissimilarities of closely related morphotypes. The dendrogram was obtained using Ward’s method of linking based on Euclidean distance in the XLSTAT version 2022.1.2.
Results and discussion
Proximate composition
Almost all proximate traits varied significantly among S. spinosa morphotypes, except for the dry matter (Table 2). The lowest moisture content (10.29%) recorded in morphotype GRR-GRO and a range from 10.29 to 60.53% among almost all morphotypes, differed significantly to 7.12% that was determined in S. spinosa fruits from Nigeria (Jacob et al. 2016). The low fruit moisture content found in almost all S. spinosa morphotypes from KwaZulu-Natal, can be attributed to a longer shelf life for these fruits (Hassan et al. 2014). The highest moisture content (60.53%) in GSR-GEO (Table 2) was comparable to 78.8% found in the same species from Zimbabwe (Ngadze et al. 2017). However, higher moisture content can lead to limited shelf life during storage (Hassan et al. 2014), as ripe fruits can only be stored for up to two weeks under ambient conditions, primarily in shaded environments, before they deteriorate (Ngadze et al. 2017). Generally, fresh fruits with elevated moisture content, like S. spinosa, tend to experience direct or indirect losses in nutrients and quality during storage (Ngadze et al. 2017). As a result, there is a necessity to process S. spinosa into more stable products to address this challenge (Jiru et al. 2023).
Morphotype GRxCP-GEF produced the highest fat content (2.63%), whereas morphotype GSR-GEF had the lowest (0.95%). The majority (53%) of morphotypes that bore fruits characterized by the high fat levels in a range from 1.97 to 2.67% (Table 2) related to 1.98% (Lockett et al. 2000) and 2.04% (Jacob et al. 2016) recorded in S. spinosa fruits from Nigeria. In contrast, fat content determined in S. spinosa fruits from Malawi was very high (31.2%) (Saka and Msonthi 1994) and was comparable to a range from 26.3 to 27.8% in S. madagascarensis (Chemane et al. 2022), which is recognized as a source of vegetable oil (Zharare et al. 2022). However, opting for low-fat fruits such as S. spinosa can confer numerous health benefits, encompassing weight management, cardiovascular well-being, blood sugar regulation, and enhanced nutrient intake (Sharma et al. 2016; Ngadze et al. 2017).
The diversity in ash content among S. spinosa morphotypes from 4.78% (GSR-GEO) to 18.05% (GvRxCR-GEF) (Table 2) was higher than 2.58% (Lockett et al. 2000) and 3.86% (Jacob et al. 2016) recorded in S. spinosa fruits from Nigeria, as well as Malawi (4.1%) (Sanka and Msonthi 1994) and Botswana (4.6%) (Amarteifio and Mosasae 2006). Ash content indicates fruits’ mineral composition and underscores their mineral potency (Karwacka et al. 2022). The diversity in mineral content observed within the species is attributed to variations in phenotype (Ngadze et al. 2017), as was evident with S. spinosa morphotypes. The high ash content in S. spinosa fruits from KwaZulu-Natal makes them potentially valuable in addressing micronutrient deficiencies often faced by rural communities. Generally, fruits with lower ash content often contains higher moisture content, as minerals are concentrated within the dry matter (Marles 2017). This clearly correlates in current study, where morphotype with high moisture content have reduced ash content (GSR-GEO).
Among all the morphotypes, PRxCP-GEO produced the highest (9.19%) crude protein content and PRR-dGRF had the lowest (2.85%) (Table 2). Strychnos spinosa morphotypes from KwaZulu-Natal that are identified as producing high crude protein (> 6.00%) in fruit had values comparable to 8.72% (Jacob et al. 2016) and 11.70% (Lockett et al. 2000) in fruits from Nigeria. Crude protein content of 5.4% in Strychnos spinosa fruits from Malawi (Sanka and Msonthi 1994) was similar to the moderate crude protein content (> 4.00–6.00%) recorded in the majority of morphotypes. However, the crude protein content of 3.3% in S. spinosa fruits from Zimbabwe (Amarteifio and Mosasae 2006) was aligning with the lowest levels (≤ 4.00%) of crude protein content among the morphotypes. Plant-based protein offers several advantages such as being abundantly available, affordability, absence of cholesterol, and potential disease prevention (Messina et al. 2023). As a sustainable and safe food source, plant protein serves as a viable alternative to animal protein, helping to address potential shortages in the supply of dietary protein (Hu et al. 2022). The protein content available in S. spinosa morphotypes is much more than that which is available in commercial fruits such as Ananas comosus L. Merr. (0.4%), Carica papaya L. (0.61%), Citrus limon (L.) Burm. F. (1.1%), Citrus × sinensis (L.) Osbeck. (0.7%), Fragaria × ananassa Duch. (0.61%), Musa sp (1.09%), Litchi chinensis Sonn. (0.8%), Malus domestica Borkh. (0.26%), Mangifera indica L. (0.51%), Psidium guajava L. (2.54%), Punica granatum L. (0.95%), Vitis sp (0.63%), Prunus avium L. (1.06%), and Pyrus sp (0.5%) (Mahapatra et al. 2012). Strychnos spinosa fruit is a good dietary source of protein (Omotayo and Aremu 2021).
The carbohydrate content ranged from 37.39 (GSR-GEF) to 42.24% (GRxCP-GEF) among S. spinosa morphotypes (Table 2). The variability of carbohydrate content (37.39–42.24%) among S. spinosa morphotypes was lower than 59.82% (Locket et al. 2000) and 62.47% (Jacob et al. 2016) reported in Nigeria. However, the highest carbohydrate content of 42.24% in morphotype GRxCP-GEF was similar to 42.1% found in S. spinosa fruits from Malawi (Saka and Msonthi 1994). Carbohydrates are a fundamental source of energy for the body, serving as a fuel for various physiological processes (Biswas et al. 2022). The carbohydrate content in fruits encompasses natural sugars, dietary fibre, and sometimes small amounts of starch (Yuan et al. 2023). Carbohydrate is the most abundant macronutrient in the human diet, providing 45–70% of daily calorie which closely aligns with morphotype GRxCP-GEF (Isenmann et al. 2021). The carbohydrate content available in S. spinosa morphotypes is much more than that which is available in commercial fruits such as Ananas comosus L. Merr. (11.82%), Carica papaya L. (9.81%), Citrus limon (L.) Burm. F. (9.32%), Citrus × sinensis (L.) Osbeck. (11.54%), Fragaria × ananassa Duch. (7%), Litchi chinensis Sonn. (16.5%), Malus domestica Borkh. (13.81%), Mangifera indica L. (17%), Musa sp (22.84), Psidium guayava L. (14.3%), Punica granatum L. (17.17%), Prunus avium L. (16.01%), and Pyrus sp (10.65%), Vitis sp (17.15%) (Mahapatra et al. 2012). Strychnos spinosa fruit are a good dietary source of carbohydrates (Omotayo and Aremu 2021).
A range for acid detergent fibre (ADF) (7.94–21.75%) (Table 2) was lesser than that reported for Vitis species which range from 37.0–53.9% (Correddu et al. 2023). The majority of S. spinosa morphotypes which represented the moderate category (> 14.00) aligned with ADF of 23.96% in fruits from Nigeria (Locket et al. 2000). The neutral detergent fibre (NDF) ranges from 16.46–42.55% was closely related to a range of 15.1–49% in Punica granatum L. (Correddu et al. 2023).
The ADF is a measure of plant material resistant to acid hydrolysis, including components like cellulose and lignin found in the cell wall (Nansikombi et al. 2019). It serves as an indicator of the less digestible and less nutritious parts of the plant (Lee 2018). On the other hand, NDF measures the total fibre content in plant material, encompassing cellulose, hemicellulose, and lignin (Vélez-Terranova et al. 2022). Higher percentages of ADF and NDF displayed by morphotypes with a rough pericarp texture (Fig. 1) can be attributed to thicker and fibrous pulp that is present rough-textured fruit pericarp (Mbhele et al. 2022). Elevated ADF and NDF content in fruits generally indicates a more fibrous composition, with a greater presence of structural elements such as cellulose, hemicellulose, and lignin, which can influence the fruit's texture (Sood et al. 2010). The high neutral detergent fibre, which is easily digestible, makes the S. spinosa ideal to aid against constipation and avoiding haemorrhoids (Barot et al. 2015). Moreover, consuming fruits with higher fibre content can contribute to a feeling of stomach fullness and promote healthy digestion (Dhingra et al. 2012). Furthermore, together with its high moisture content in morphotype such as GSR-GEO (60.53%), S. spinosa could promote hydration and maintain healthy bowel movements (Dreher 2018).
Macronutrients
The range of calcium from 3 to 35 mg/100 g recorded among fruits of S. spinosa morphotypes from KwaZulu-Natal (Table 2) was lower than 52.47 mg/100 g (Jacob et al. 2016) and 130 mg/100 g (Lockett et al. 2000) of S. spinosa fruits in Nigeria, as well as by fruits from Zimbabwe (56 mg/100 g) (Amarteifio and Mosasae 2006). However, majority of morphotypes with fruit calcium in low category (≤ 18) (Table 3) was similar to 14.9 mg/100 g determined in fruits from Malawi (Saka and Msonthi 1994). Calcium plays a critical role in bone health, and it is involved in numerous intracellular signal transduction processes and is essential for functions such as muscle contraction, blood coagulation, regulation of the hormonal system, nerve impulse transmission, and energy and fat metabolism (Jones and Hazlehurst 2021).
The calcium content available in S. spinosa morphotypes is much more than that which is available in commercial fruits such as Ananas comosus Duch. (13 mg/100 g), Citrus limon (L.) Burm. F. (7 mg/100 g), Citrus × sinensis (L.) Osbeck. (11 mg/100 g), Litchi chinensis Sonn. (5 mg/100 g), Malus domestica Borkh. (5 mg/100 g), Mangifera indica L. (10 mg/100 g), Musa sp (5 mg/100 g), Punica granatum L. (10 mg/100 g), Vitis sp (14 mg/100 g), Prunus avium L. (12 mg/100 g), and Pyrus sp (4 mg/100 g) (Mahapatra et al. 2012).
The potassium content range of 810–2510 mg/100 g (Table 3) was higher than that of S. spinosa fruits in Nigeria (764.68 mg/100 g) (Jacob et al. 2016), but within a range of those in Zimbabwe (1370 mg/100 g) (Amarteifio and Mosasae 2006) and Malawi (1968.3 mg/100 g) (Saka and Msonthi 1994). This places potassium content of S. spinosa fruits from Nigeria in the low category, whereas those from Zimbabwe and Malawi were similar to the moderate category among morphotypes in current study. Potassium is an essential mineral that plays a crucial role in maintaining various bodily functions, including heart health, muscle contraction, nerve signalling, and fluid balance (Yamada and Inaba 2023). The potassium content available in S. spinosa morphotypes is much more than that which is available in commercial fruits such as Ananas comosus L. Merr. (109 mg/100 g), Carica papaya L. (257 mg/100 g), Citrus limon (L.) Burm. F. (124 mg/100 g), Citrus × sinensis (L.) Osbeck. (200 mg/100 g), Fragaria × ananassa Duch. (292 mg/100 g), Litchi chinensis Sonn. (171 mg/100 g), Malus domestica Borkh. (90 mg/100 g), Mangifera indica L. (156 mg/100 g), Musa sp (358 mg/100 g), Psidium guayava L. (417 mg/100 g), Punica granatum L. (236 mg/100 g), Vitis sp (191 mg/100 g), Prunus avium L. (146 mg/100 g), and Pyrus sp (121 mg/100 g) (Mahapatra et al. 2012).
Morphotype GRR-dGRO had trees with the higher (69.0 mg/100 g) phosphorus levels, whereas GRP-dGRO had the lower (09.0 mg/100 g) (Table 3). The largest portion of the morphotypes had high phosphorus content, which closely aligned with that of fruits from Zimbabwe (66 mg/100 g) (Amarteifio and Mosasae 2006), but lower than in S. spinosa fruits from Malawi (108.1 mg/100 g) (Saka and Msonthi 1994) and Nigeria (168 mg/100 g) (Locket et al. 2000). Phosphorus is an essential nutrient and a constituent of the human body, playing a pivotal role in both structural composition and metabolic regulation (Li et al. 2022). Phosphorus, in the form of phosphate, contributes to key metabolic processes, including the metabolism of carbohydrates, fats, and proteins (Ling et al. 2020).
The magnesium content ranged from 9 to 55 mg/100 g among S. spinosa morphotypes (Table 3) is relatively lower than the S. spinosa fruits (141 mg/100 g) from Nigeria (Locket et al. 2000). However, magnesium content of morphotypes GRP-dGEO (46 mg/100 g), GSR-GEO (50 mg/100 g), GvRR-dGRO (54 mg/100 g), PRR-dGRO (54 mg/100 g), and GSxCR-dGRF (55 mg/100 g) (Table 3), are comparable to that of S. spinosa from Malawi (43 mg/100 g) (Saka and Msonthi 1994), Nigeria (45.39 mg/100 g) (Jacob et al. 2016), and Zimbabwe (49 mg/100 g) (Amarteifio and Mosase 2006). Magnesium is a vital nutrient that plays a pivotal role in various physiological functions within the body such as encompassing enzymatic reactions, protein synthesis, nucleic acid synthesis, and energy metabolism (Ciosek et al. 2021).
Fruit sodium concentration varied between 7.0 (GSR-GEO) and 54.0 mg/100 g (GRR-dGEO) among S. spinosa morphotypes (Table 3). Strychnos spinosa morphotypes categorized as having fruits with high sodium content (> 28 mg/100 g), namely, GRR-dGEO, GRR-dGRO, GRR-GRO, GRxCP-dGEF, GRxCP-GEO, GvRR-dGEO, GvRR-dGRO, GvRR-GRO, PRR-dGEF and PRR-dGRO (Table 3), had concentrations that were comparable to that of Fragaria × ananassa Duch. at 37 mg/100 g (Mahapatra et al. 2012). Sodium is essential for electrolyte balance, healthy heart functioning, metabolic activities, and nerve transition (Bernal et al. 2023).
Micronutrients
Almost all the morphotypes in current study showed similar copper content to that recorded in S. spinosa fruits in Nigeria where copper content is 0.24 mg/100 g (Locket et al. 2000) and 0.53 mg/100 g (Jacob et al. 2016) (Table 4). Fruits with copper content contribute to red blood cell production, immune system function, bone health, energy production, antioxidant activity, iron absorption, arthritis prevention, and wound healing (Liu et al. 2022; Djoko et al. 2015).
Iron content in S. spinosa morphotypes, GRP-GEO (4.00 mg/100 g), GRxCR-dGRO (5.50 mg/100 g), and GvRR-dGRO (5.50 mg/100 g), within moderate and high iron concentration categories (Table 4), which were comparable with 4.39 mg/100 g in fruits from Nigeria (Locket et al. 2000). However, S. spinosa fruits in Malawi had higher levels of iron at 13.6 mg/100 g (Saka and Msonthi 1994). Further, fruits within low iron content category (< 2.25 mg/100 g) were related to 1.72 mg/100 g (Jacob et al. 2016) recorded in fruits from Nigeria. Consuming fruits that are high in iron can offer numerous health benefits, such as preventing iron deficiency (anaemia), which can lead to symptoms like fatigue and weakness (Liberal et al. 2020).
Strychnos spinosa morphotype GRP-dGEO (2.20 mg/100 g), GRxCP-GEO (2.00 mg/100 g), and PRR-dGRO (2.43 mg/100 g) (Table 4) produced fruits with a high manganese content, which aligned with 2.24 mg/100 g (Jacob et al. 2016) and 2.74 mg/100 g (Locket et al. 2000) in S. spinosa fruits from Nigeria. Manganese plays a critical role in the activation of enzymes that are essential for various metabolic processes and these enzymes facilitate the breakdown of proteins, amino acids, cholesterol, and carbohydrates, enhancing the body's ability to efficiently utilize these nutrients (Awol 2014; Zipkin et al. 2017).
Zinc content in current study was ranged from 0.10 to 0.80 mg/100 g (Table 4), which was lower than 1.08 mg/100 g (Locket et al. 2000) and 2.71 mg/100 g (Jacob et al. 2016) recorded in S. spinosa fruits from Nigeria. The majority (69%) of morphotypes in this study demonstrated low zinc content levels (< 0.40 mg/100 g), which was comparable to 0.22 mg/100 g recorded in fruits from Zimbabwe (Amarteifio and Mosasae 2006). Zinc is one of the crucial micronutrients for growth and the immune system. Its deficiency, being the fifth leading risk factor for diseases is associated with several disorders and infections, especially diarrhoea (Sangeetha et al. 2022).
Principal component and cluster analyses
Principal component analysis showed that calcium, phosphorus, magnesium, copper, iron, manganese, fat, acid detergent fibre, neutral detergent fibre, sodium and crude protein were the key indicators identified because of their significant association with different principal components (Supplementary Data 1) and should be prioritized for nutritional variation research among S. spinosa morphotypes, as breeding traits are identified by the most important traits that contribute to genetic variation (Kumar et al. 2022).
The majority of morphotypes within sub-cluster IB had rough pericarp texture (Fig. 2) and were associated with higher quantities of acid detergent fibre, neutral detergent fibre, calcium, magnesium, potassium, iron, and manganese (Tables 2, 3 and 4). The same morphotypes with a rough pericarp are characterized as having small-sized fruits and few, small-sized seeds (Mbhele et al. 2022b), which might attribute to the dilution effect of nutrients in large-sized fruits (Davies 2009). The slow migration of nutrients from the soil into plant roots could result in limited supply of nutrients to large, rapidly growing fruits (Marles 2017), and thus result in lower nutritional content in large fruits but higher in small-sized fruits (Choi et al. 2019).
Morphotypes GRR-dGEO, GRxCP-GEF, GvRxCR-GEF, GRxCP-dGEF, PRR-dGRF, PRxCP-GEO, GvRR-dGEO, GvRR-GRO, GRxCR-dGEF, and GRxCR-dGEO that formed Cluster I in the biplot (Fig. 1) and Sub-cluster IIA of the dendrogram (Fig. 3), had moderate or high amounts of ash, fat, crude protein, sodium, and potassium (Tables 2 and 3).
Biplot based on the first principal components (PC) for nutritional components for Sspinosa morphotypes. Note: Morphotypes are explained in Table 1. Variables: CP, crude protein; ADF, acid detergent fibre; NDF, neutral detergent fibre; Ca, calcium; K, potassium; P, phosphorus; Mg, magnesium; Na, Sodium; Cu, copper; Fe, Iron; Mn, manganese, Zn, zinc.
Further, morphotypes GRR-GEO, GRP-GEF, GSR-dGRF, GSR-GEF, GRP-GRO, GRP-dGRO, GRP-dGEF, GRP-dGEO that were in Cluster II of the biplot (Fig. 2) and sub-cluster IIB of the dendrogram (Fig. 3), had the least content in proximate and mineral composition (Tables 2, 3, and 4). The latter biplot and dendrogram clusters that were associated with morphotypes that had smooth pericarp and also produced many and large fruits with numerous and large seeds (Tables 4 and Supplementary Data 1; Mbhele et al. 2022b), showed the evidence of dilution effect in nutritional status of large-sized fruits (Choi et al. 2019).
Dendrogram grouping of Strychnos spinosa morphotypes based on Euclidean distances. Note: The description for morphotypes is in Table 1
Conclusion
This is the first study to report on the variation in nutritional composition among S. spinosa morphotypes. Previous studies have focused on the general fruit nutritional status of the species. The study showed that fruits of S. spinosa morphotypes have nutritional value that is either comparable or better than that of other commercially available fruits. This comprehensive nutritional characterization of the wild fruits of S. spinosa morphotypes is essential to promote their inclusion in the improved dietary patterns and promote their consumption for better health outcomes; and incorporation into breeding programs, Therefore, these morphotypes are promising for domestication and commercialization in product development which may also be considered as good alternatives for many commercialized fruits. The notable differences in nutrient quantities among these morphotypes from the same geographical location suggests the possibility of genetic divergence among these morphotypes. Therefore, studies on genetic diversity among these morphotypes are necessary.
Data Availability
No datasets were generated or analysed during the current study.
References
AACC (1983) Approved methods of the american association of cereal chemists; method 71–10; In: AACC International: St. Paul, MN, USA. http://methods.aaccnet.org/summaries/08-10-01.aspx
AACC (1999) Approved Methods of the American Association of Cereal Chemists; Method 08–01; In: AACC International: St. Paul, MN, USA. http://methods.aaccnet.org/summaries/30-25-01.aspx
Achaglinkame MA, Aderibigbe RO, Hensel O, Sturm B, Korese JK (2019) Nutritional characteristics of four underutilized edible wild fruits of dietary interest in Ghana. Foods 8(104):1–12. https://doi.org/10.3390/foods8030104
Akweni AL, Sibanda S, Zharare GE, Zimudzi C (2020) Fruit-based Allometry of Strychnos madagascariensis and S spinosa (Loganiaceae) in the Savannah Woodlands of the Umhlabuyalingana Municipality, KwaZulu-Natal South Africa. Trees for People. https://doi.org/10.1016/j.tfp.2020.100025
Amarteifio JO, Mosasae MO (2006) The chemical composition of selected indigenous fruits of botswana. J Appl Sci Environ Manag 10(2):43–47. https://doi.org/10.4314/jasem.v10i2.43659
AOAC—Association of Official Analytical Chemists (2005) Official Methods of Analysis of AOAC International, 18th ed. AOAC, Arlington, TX, USA
Awol A (2014) Phytochemical screening, proximate and mineral composition of sweet potato leaves grown in tepi provision, South-west of Ethiopia. Sci J Sci Technol Arts 3(3):112–115
Barot A, Pinto S, Balakrishnan S, Prajapati JP (2015) Composition, functional properties and application of bottle gourd in food products. Dairy Sci Technol 4(1):15–27. https://doi.org/10.37591/RRJODST.V4I1.407
Bernal A, Zafra MA, Simón MJ, Mahía J (2023) Sodium homeostasis, a balance necessary for life. Nutrients 15:395. https://doi.org/10.3390/nu15020395
Biswas SC, Kumar P, Kumar R, Das S, Misra TK, Dey D (2022) Nutritional composition and antioxidant properties of the wild edible fruits of tripura. Northeast India Sustain 14:12194. https://doi.org/10.3390/su141912194
Bvenura C, Sivakumar D (2017) The role of wild fruits and vegetables in delivering a balanced and healthy diet. Food Res Int 99:15–30. https://doi.org/10.1016/j.foodres.2017.06.046
Chemane SSI, Ribeiro M, Pinto E, Pinho SCM, Martins ZS, Almeida A, Ferreira IMPLVO, Khan M, Pinho O, Casal S, Viegas O (2022) Nutritional characterization of Strychnos madagascariensis fruit flour produced by mozambican communities and evaluation of its contribution to nutrient adequacy. Foods 11(616):1–17. https://doi.org/10.3390/foods11040616
Choi S, Ahn G, Kim E, Son J, Park Y, Joung W (2019) Fruit characteristics and mineral nutrient concentrations depending on different sizes of “Fuyu” persimmon fruits. Agric Sci 10:1015–1022. https://doi.org/10.4236/as.2019.108076
Ciosek Z, Kot K, Kosik-Bogacka D, Łanocha-Arendarczyk N, Rotter I (2021) The effects of calcium, magnesium, phosphorus, fluoride, and lead on bone tissue. Biomolecules 11(506):1–26. https://doi.org/10.3390/biom11040506
Correddu F, Caratzu MFM, Lunesu F, Carta S, Pulina G, Nudda A (2023) Grape pomegranate, olive, and tomato by-products fed to dairy ruminants improve milk fatty acid profile without depressing milk production. Foods 12(865):1–21. https://doi.org/10.3390/foods12040865
Davies DR (2009) Declining fruit and vegetable nutrient composition: what is the evidence? HortScience 44(1):15–19. https://doi.org/10.21273/HORTSCI.44.1.15
Dhingra D, Michael M, Rajput H, Patil RT (2012) Dietary fibre in foods: a review. J Food Sci Technol 49(3):255–266
Djoko KY, Ong CL, Walker MJ, McEwan AG (2015) The role of copper and zinc toxicity in innate immune defence against bacterial pathogens. JBC 290(31):18954–18961
Dreher ML (2018) Whole fruits and fruit fiber emerging health effects. Nutrients 8(10):1833. https://doi.org/10.3390/nu10121833
Fanzo J, Rudie C, Sigman I, Grinspoon S, Benton TG, Brown ME, Covic N, Fitch K, Golden CD, Grace D, Hivert MF, Huybers P, Lindsay M, Jaacks LM, Masters WA, Nisbett N, Richardson RA, Chelsea R, Singleton CR, Webb P, Willett WC (2020) Sustainable food systems and nutrition in the 21st century: a report from the 22nd annual Harvard Nutrition Obesity Symposium. AJCN, 115:18–33. Doi: https://doi.org/10.1093/ajcn/nqab315
Fukalova FT, García-Martínez MD, Raigón MD (2022) Nutritional composition, bioactive compounds, and volatiles profile characterization of two edible undervalued plants: portulaca oleracea L and Porophyllum ruderale (Jacq) Cass. Plants 11:377. https://doi.org/10.3390/plants11030377
Hassan LG, Abdulmumin U, Umar KJ, Ikeh PO, Aliero AA (2014) Nutritional and anti-nutritional composition of Strychnos Innocua Del. (Monkey orange) Pulp Grown in Zuru. Nigeria Nig J Basic Appl Sci 22:33–37. https://doi.org/10.4314/njbas.v22i1.6
Hu G-G, Liu J, Wang Y-H, Yang Z-N, Shao H-B (2022) Applications of plant protein in the dairy industry. Foods 11(1067):1–14. https://doi.org/10.3390/foods11081067
Isenmann E, Dissemond J, Geisler S (2021) The effects of a macronutrient-based diet and time-restricted feeding (16:8) on body composition in physically active individuals—A 14-week randomised controlled trial. Nutrients 13(3122):1–14. https://doi.org/10.3390/nu13093122
Jacob JO, Mann A, Adeshina OI, Ndamitso MM (2016) Nutritional composition of selected wild fruits from Minna Area of Niger State. Nigeria International JNHFE 10(1):37–42
Jiru NA, Gemede HF, Keyata EO (2023) Nutritional composition and antioxidant properties of selected underutilized wild edible fruits in east wollega zone. West Ethiop Int J Fruit Sci 23(1):34–45. https://doi.org/10.1080/15538362.2023.2166649
Jones CA, Hazlehurst LA (2021) Role of calcium homeostasis in modulating EMT in cancer. Biomedicines 9(1200):1–13. https://doi.org/10.3390/biomedicines9091200
Karwacka M, Rybak K, ´Swieca M, Galus S, Janowicz M, (2022) The effect of the addition of selected fruit pomace powders and pectin as carrier agents on the nutritional value of freeze-dried snacks. Sustainability 14(13012):1–16. https://doi.org/10.3390/su142013012
Kiani AK, Dhuli K, Donato K, Aquilanti B, Velluti V, Matera G, Iaconelli A, Connelly ST, Bellinato F, Gisondi P, Bertelli M (2022) Main nutritional deficiencies. JPMH 63(3):93–101. https://doi.org/10.15167/2421-4248/jpmh2022.63.2S3.2752
Kumar K, Anjoy P, Sahu S, Durgesh K, Das A, Tribhuvan KU, Sevanthi AM, Joshi R, Jain PK, Singh NK, Rao AR, Gaikwad K (2022) Single trait versus principal component based association analysis for flowering related traits in Pigeonpea. Sci Rep 12:10453. https://doi.org/10.1038/s41598-022-14568-1
Lee M (2018) A global comparison of the nutritive values of forage plants grown in contrasting environments. J Plant Res 131:641–654. https://doi.org/10.1007/s10265-018-1024-y
Li N, He J, Chen H, Chen Y, Chen L, Yang H, Xu L, Wang Z (2022) Effects of dietary phosphorus deficiency and high phosphorus content on the growth performance, serum variables, and tibia development in goslings. Agriculture 12(1908):1–17. https://doi.org/10.3390/agriculture12111908
Liberal Â, Pinela J, Vívar-Quintana AM, Ferreira ICFR, Barros L (2020) Fighting iron-deficiency anaemia: innovations in food fortificants and biofortification strategies. Foods 9(1871):1–19
Ling MP, Huang JD, Hsiao HA, Chang YW, Kao YT (2020) Risk assessment of the dietary phosphate exposure in taiwan population using a total diet study. Foods 9(15740):1–13. https://doi.org/10.3390/foods9111574
Liu Y, Zhu J, Xu L, Wang B, Lin W, Luo Y (2022) Copper regulation of immune response and potential implications for treating orthopaedic disorders. Front Mol Biosci 9(1065265):1–6. https://doi.org/10.3389/fmolb.2022.1065265
Lockett CT, Calvert CC, Grivetti LE (2000) Energy and micronutrient composition of dietary and medicinal wild plants consumed during drought. Study of rural Fulani, Northeastern Nigeria. Int J Food Sci Nutr 51:195–208
Mahapatra AK, Mishra S, Basak UC, Panda PC (2012) Nutrient analysis of some selected wild edible fruits of deciduous forests of india: an explorative study towards non-conventional bio-nutrition. Adv J Food Sci Technol 4(1):15–21
Manson AD, Roberts VG (2000) Analytical Methods Used by the Soil Fertility and Analytical Services Section; KZN Agri-Report No. N/A/2001/4; KwaZulu-Natal Department of Agriculture and Rural Development: Pietermaritzburg, South Africa, 2000
Marles RJ (2017) Mineral nutrient composition of vegetables, fruits and grains: The context of reports of apparent historical declines. J Food Compos Anal 56:93–103. https://doi.org/10.1016/j.jfca.2016.11.012
Mbhele Z, Zharare GE, Zimudzi C, Ntuli NR (2022a) Indigenous knowledge on the uses and morphological variation among Strychnos spinosa Lam. at Oyemeni Area, KwaZulu-Natal. South Africa Sustain 14(6623):1–20. https://doi.org/10.3390/su14116623
Mbhele Z, Zharare GE, Zimudzi C, Ntuli NR (2022b) Morphological variation of strychnos spinosa lam. Morphotypes: a case study at bonamanzi game reserve, KwaZulu-Natal, South Africa. Diversity 14:1094. https://doi.org/10.3390/d14121094
Messina M, Duncan AM, Glenn AJ, Mariotti F (2023) Perspective: plant-based meat alternatives can help facilitate and maintain a lower animal to plant protein intake ratio. Adv Nutr 14(3):392–405. https://doi.org/10.1016%2Fj.advnut.2023.03.003
Militao EMA, Salvador EM, Uthman OA, Vinberg S, Macassa G (2022a) Food insecurity and health outcomes other than malnutrition in Southern Africa: a descriptive systematic review. IJERPH 19:5082. https://doi.org/10.3390/su14148710
Militao EMA, Salvador EM, Silva JP, Uthman OA, Vinberg S, Macassa G (2022b) Coping strategies for household food insecurity, and perceived health in an urban community in Southern Mozambique: a qualitative study. Sustainability 14:8710. https://doi.org/10.3390/ijerph19095082
Mudzielwana R, Mafongoya P, Mudhara M (2022) An analysis of the determinants of irrigation farmworkers’ food security status: a case of tshiombo irrigation scheme. South Africa Agri 12:999. https://doi.org/10.3390/agriculture12070999
Nansikombi N, Muyonga JH, Byaruhanga YB (2019) Association between fruit characteristics and postharvest stability of different pumpkin (Cucurbita) species. J Food Res 8(4):131–145. https://doi.org/10.5539/jfr.v8n4p131
Ngadze RT, Linnemann AR, Nyanga LK, Fogliano V, Verkerk R (2017) Local processing and nutritional composition of indigenous fruits: the case of monkey orange (Strychnos spp) from Southern Africa. Food Rev Int 33(2):123–142. https://doi.org/10.1080/87559129.2016.1149862
Ngemakwe PHN, Remize F, Thaoge ML, Sivakumar D (2017) Phytochemical and nutritional properties of underutilised fruits in the southern african region. SAJB 113:137–149. https://doi.org/10.1016/j.sajb.2017.08.006
Nkosi NN, Morstert THC, Dzikiti S, Ntuli NR (2020) Prioritization of indigenous fruit tree species with domestication and commercialization potential in KwaZulu-Natal, South Africa. Genet Resour Crop Evol 67:1567–1575. https://doi.org/10.1007/s10722-020-00932-5
Ntshidi Z, Dzikiti S, Mobe NT, Ntuli NR, Du Preez R, Nkosi NN, Buthelezi LN, Ncapai L, Wilkens L, Gush MB, Mostert TCH (2022) Water use efficiency of indigenous fruit trees in South Africa. In: Conference paper 6th International Congress on Water, Waste and Energy Management, Rome, Italy
Omotayo AO, Aremu AO (2021) Undervalued spiny monkey orange (Strychnos spinosa Lam): an indigenous fruit for sustainable fruit nutrition and economic prosperity. Plants. https://doi.org/10.3390/plants10122785
Rodrigues S, de Brito ES, de Oliveira SE (2018) Maboque/Monkey Orange-Strychnos spinosa. In: Fruits E (ed) Sueli Rodrigues; Ebenezer de Oliveira Silva, Edy Sousa de Brito. Academic press, pp 293–296
Saka JDK, Msonthi JD (1994) Nutritional value of edible fruits indigenous wild tree in malawi. For Ecol Manag 64(2):245–248. https://doi.org/10.1016/0378-1127(94)90298-4
Sangeetha V, Dutta J, Moses JA, Anandharamakrishnan C (2022) Zinc nutrition and human health: overview and implications, eFood, vol 3(5): p e17. doi: https://doi.org/10.1002/efd2.17
Savarino G, Corsellon A, Corsello G (2021) Macronutrient balance and micronutrient amounts through growth and development. Ital J Pediatr 47(109):2–14. https://doi.org/10.1186/s13052-021-01061-0
Shahid M, Singh RK, Thushar S (2023) Proximate composition and nutritional values of selected wild plants of the United Arab Emirates. Molecules 28:1504. https://doi.org/10.3390/molecules28031504
Sharma SP, Chung HJ, Kim HJ, Hong ST (2016) Paradoxical effects of fruit on obesity. Nutrients 8(633):1–16
Sibiya NP, Kayitesi E, Moteetee AN (2021) Proximate analyses and amino acid composition of selected wild indigenous fruits of Southern Africa. Plants 10(721):1–20. https://doi.org/10.3390/plants10040721
Siddiqui F, Salam RA, Lassi ZS, Das JK (2020) The intertwined relationship between malnutrition and poverty. Front Public Health 8:453. https://doi.org/10.3389/fpubh.2020.00453
Sood P, Modgil R, Sood M (2010) Physico-chemical and nutritional evaluation of indigenous wild fruit Kasmal, Berberis lycium Royle. IJNPR 1:362–366
Thaxton GE, Melby PC, Manary MJ, Preidis GA (2018) New insights into the pathogenesis and treatment of malnutrition. Gastroenterol Clin North Am 47(4):813–827
Vélez-Terranova M, Salamanca-Carreño A, Bejarano-Sánchez AM, González-Castro DA, Higuera-Pedraza RD, Giraldo LA (2022) Nutritional characteristics and digestibility of woody and herbaceous native plants from tropical flooded savannas ecosystems. Agriculture 12(1613):1–8. https://doi.org/10.3390/agriculture12101613
Venn BJ (2020) Macronutrients and human health for the 21st century. Nutrients 12:2363. https://doi.org/10.3390/nu12082363
Yamada S, Inaba M (2023) Potassium metabolism and management in patients with CKD. Nutrients 13(1751):1–19. https://doi.org/10.3390/nu13061751
Yuan X, Zheng H, Fan J, Liu F, Li J, Zhong C, Zhang Q (2023) Comparative study on physicochemical and nutritional qualities of kiwi fruit varieties. Foods 12(108):1–15. https://doi.org/10.3390/foods12010108
Zharare GE, Akweni AL, Mostert M, Opoku AR (2022) The potential of strychnos madagascariensis (poir) as a source of vegetable oil. Food Biosci 48(101719):1–12. https://doi.org/10.1016/j.fbio.2022.101719
Zipkin FB, Falciglia GA, Kuhnell P, Haynes EN (2017) Development and evaluation of a manganese and iron food frequency questionnaire for paediatrics. Int J Environ Res Public Health 14(1060):1–9. https://doi.org/10.3390/ijerph14091060
Funding
Open access funding provided by University of Zululand. This research was funded by the University Capacity Development Program (UCDP).
Author information
Authors and Affiliations
Contributions
Conceptualization, Z.M.; N.R.N.; G.E.Z.;C.N.M and C.Z.; methodology, Z.M.;C.N.M and N.R.N.; software, Z.M.; validation, N.R.N.; G.E.Z. and C.Z.; formal analysis, Z.M.; investigation, Z.M.; resources, Z.M.; data curation, Z.M.; writing—original draft preparation, Z.M.; writing—review and editing, N.R.N.; supervision, N.R.N.; G.E.Z. and C.Z.; project administration, Z.M.; funding acquisition, N.R.N. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the author(s).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mbhele, Z., Zharare, G.E., Zimudzi, C. et al. Variation in nutritional composition of Strychnos spinosa Lam. morphotypes in KwaZulu-Natal, South Africa. Genet Resour Crop Evol (2024). https://doi.org/10.1007/s10722-024-01982-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10722-024-01982-9