Abstract
Anthoxanthum aristatum Boiss. is native to southern and western Europe and North Africa. In Central Europe this species is recognized as invasive. Its ability to colonize various habitats may result from a high level of polymorphic isozyme loci and high morphological variability. The aim of this study was to recognize the degree of genetic diversity of the studied invasive species at its non-native range limit using molecular markers. The examined plant material consisted of 70 samples collected from 7 populations originating from various habitats: arable fields, recently abandoned field and sandy grassland. Ninety-six the Directed Amplification of Minisatellite-region DNA loci and 82 Sequence-Related Amplified Polymorphism loci were detected. The genetic diversity at the species level was high. The majority of genetic variation was present within populations (68% and 66%), while the remaining molecular variation was distributed among populations within and among habitat types. Specimens originating from sandy grassland populations were grouped together in one cluster that was genetically distinct from the arable field and fallow populations. The high genetic diversity populations at the A. aristatum non-native range limit in Central Europe and their marked diversity in arable field and sandy grassland habitats suggest that this species may systematically cross both geographical and ecological barriers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Knowledge of the variation and genetic structure of weed populations occurring at the borders of the non-native ranges of species may be crucial to understanding the biology of these species and planning measures to prevent their invasion. Interesting subjects for this type of research are the populations of Anthoxanthum aristatum Boiss. located at the species eastern non-native range limit. A. aristatum is an annual with loose tufts, flowering from May to June and sometimes again in summer and autumn. This species is distributed naturally in West Europe and North Africa in the Mediterranean and Atlantic regions (Kästner et al. 2001). After accidental introductions to Germany during the Napoleonic period (Hegi 1909), by the late nineteenth century, it had spread into the Netherlands and all over Germany. In subsequent decades, A. aristatum spread throughout western and central Poland and migrated towards the country’s eastern border, which it finally reached in the 1990s (Ciosek and Skrzyczyńska 1997). Beyond Europe, A. aristatum has spread to North America, Australia, New Zealand (Howell and Sawyer 2006; Randall 2007), and Asia (e.g., Mito and Uesugi 2004).
Within its natural range, A. aristatum occupies habitats of differing fertility composed of mesophilic deciduous forests (Rivas-Martínez et al. 1986), fertile grasslands (Fernández-Moya et al. 2010), mountain swards and pastures (Santa-Bárbara et al. 2003), communities connected to the edges of periodic ponds (Brullo et al. 2007; Fraga i Arguimbau 2008), grapevine fields and ruderal habitats (pers. observ.). Within its non-native range, A. aristatum mainly occupies oligotrophic crop fields, sandy grasslands, roadsides, fallow lands and wastelands (Schneider et al. 1994; Ryves et al. 1996; Niemann and Zwerger 2006; Pinke et al. 2006).
In Poland, annual vernal-grass is recognized as an invasive species (Tokarska-Guzik 2005). It has the highest number of localities in western and central Poland (Zając and Zając 2001). The spread of A. aristatum in Poland and its invasion into new habitats pose an increasing threat to the country’s crops. The number of its localities is rising, which indicates that the species should be monitored. Particularly alarming is its spread to new, more fertile habitats (Skrajna and Skrzyczyńska 2007). In addition, this species can be found in sandy grasslands and along roadsides (Jackowiak et al. 1990). Less frequently, it grows in open pine, birch or pine-oak forests of post-agricultural lands, at the forest-field borders and on sandy forest roads and roadsides (Woziwoda 2010; Bomanowska et al. 2014).
A. aristatum shows considerable morphological variability and genetic differentiation in both its native and non-native ranges (Pimentel et al. 2010; Drapikowska 2013; Drapikowska et al. 2013). Its ability to colonize various non-native habitats in Poland may result from a high level of polymorphic isozyme loci and high morphological variability previously observed in A. aristatum populations from Central and western Poland (Drapikowska et al. 2012; Drapikowska 2013).
In this study, we used molecular markers to better understand the variability and genetic structure of A. aristatum populations in different habitats at the non-native range limit of the species. Molecular biology offers many types of genetic markers that can be used in population genetic studies. PCR-based Single Primer Amplification Reaction (SPAR) markers, e.g., Random Amplification of Polymorphic DNA (RAPD), Inter Simple Sequence Repeats (ISSR) and Intron-Splice Junction (ISJ), are useful because they enable simultaneous detection of multiple polymorphic loci across the entire genome and do not require knowledge of the genome to design primers. These markers have been applied to the analysis of genetic variation in many groups of plants, including grasses (Vanderpoorten and Engels 2003; Alwala et al. 2008; Buczkowska et al. 2010). This group of markers also includes the Directed Amplification of Minisatellite-region DNA (DAMD), introduced by Heath et al. (1993) and, more recently, Sequence-Related Amplified Polymorphism (SRAP) developed by Li and Quiros (2001), which target the coding regions of the genome. These markers should be employed for research addressing new hypotheses in plant systematics, biogeography, conservation and ecology (Robarts and Wolfe 2014).
The aim of this study was: (1) to determine the degree of genetic diversity of A. aristatum at the species level at the limit of its non-native range, (2) to investigate significant genetic differences among populations representing arable field and sandy grassland habitats.
Materials and methods
The studied populations of A. aristatum are located in western Poland, near the eastern limit of its non-native range in Central Europe (Fig. 1). The plant material used in this study consisted of 70 samples collected from 7 populations (10 samples per populations) originating from two type of habitats: periodically disturbed habitats (sandy grassland near field roadside—FR, sandy grassland adjacent arable field—GA and sandy grassland on the edge of a pine forest—GF) and regularly disturbed habitats (arable fields—AF, recently abandoned field—RF) (Table 1). From each collected plant, all leaves were placed in silica gel or in a plastic bag and frozen upon arrival at the laboratory.
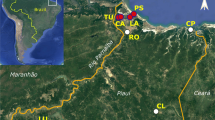
Distribution of Anthoxanthum aristatum in Europe based on the authors’ investigations and numerous literature sources (incl. Valdés et al. 2009). 1—native range, 2—primary localities beyond the native range, 3—non-native range, 4—secondary localities beyond the non-native range, 5—research localities
Localities in which plant material was collected for genetic analyses were investigated in floristic and habitat (ecological) terms. In each locality, a phytosociological relevé was made using the Braun-Blanquet method (Oberdorfer et al. 2001). Next, based on the Ellenberg method (Ellenberg et al. 1992), the mean ecological indicator values for each studied area and two distinguished groups of habitats: arable field and sandy grassland, were calculated.
Total genomic DNA was extracted from approximately 1 cm2 of frozen or silica gel dried plants. In the case of frozen samples, the leaves were ground with steel beads in a Bioprep-24 Homogenizer (BioEquip) for 35 s, and the standard CTAB procedure (Murray and Thompson 1980), downscaled to fit 1.5 ml Eppendorf tubes, was used for DNA extraction. For the extraction of DNA from silica gel material, the Novabeads Plant DNA Kit (Novazym) was used. Isolated DNA was dissolved in TE buffer and stored at − 20 °C. The quality of isolated DNA was evaluated by electrophoresis in 0.8% agarose gels, and the concentration and purity of DNA samples were determined using the Epoch™ Multi-Volume Spectrophotometer System (BioTek).
A set of 23 DAMD primers (Karaca et al. 2008; Karaca and Ince 2008; Sharma et al. 2011) was screened, which enabled us to choose six primers that amplified and produced reproducible and clear banding patterns for further analysis (Table 2). A preliminary screen was carried out using an annealing temperature gradient to optimize the PCR conditions. PCR was performed in 25 µl reaction volumes containing 40 ng of genomic DNA, 0.5 µM primer (Sigma), 0.5 mg/ml of BSA (Sigma), 1.5 mM MgCl2, 200 µM of each dNTP (Novazym), 1 × PCR buffer (20 mM (NH4)SO4, 50 mM Tris-HCl, pH 9.0 at 25 °C), and 1 U of Taq Allegro polymerase (Novazym). The amplifications were carried out in a BIO-GENER Thermocycler with a heated lid under the following amplification conditions: 94 °C for 4 min followed by 45 cycles at 94 °C for 60 s, 56 °C for 90 s, and 72 °C for 2 min, and a final elongation at 72 °C for 7 min.
The protocol for SRAP analysis was based on Li and Quiros (2001). Initially, a total of 27 different SRAP primers were tested according to Li and Quiros (2001), Nie et al. (2014) and Zitouna et al. (2015). Finally, eight primer pairs that produced clear bands were chosen for further analysis (Table 3). These pairs were selected from the 27 combinations of 11 forward (Me1—Me11) and 12 reverse (Em1—5, 7–9, 11, 12, 14, 15) primers. PCR was performed in 25 µl reaction mixtures with a final composition of 40 ng of genomic DNA, 0.5 µM primer (Sigma), 0.5 mg/ml of BSA (Sigma), 1.5 mM MgCl2, 200 µM of each dNTP (Novazym), 1 × PCR buffer (20 mM (NH4)SO4, 50 mM Tris-HCl, pH 9.0 at 25 °C), and 1 U of Taq Allegro polymerase (Novazym). Amplifications were carried out in a BIO-GENER Thermocycler with a heated lid under the following block temperature cycle: an initial heat denaturation at 94 °C for 5 min, five cycles were run at 94 °C for 1 min, 35 °C for 1 min and 72 °C for 1 min, for denaturation, annealing and extension, respectively. Then, the annealing temperature was raised to 48 °C for another 35 cycles, followed by final extension step of 10 min at 72 °C.
The PCR products of both types of markers were separated in 2% agarose gels containing ethidium bromide at 150 V for 2 h at room temperature. The amplified products were visualized using the ethidium bromide staining method under UV light and were documented using the Kodak (1D v. 3.5.4) gel documentation system. Amplified bands were manually scored as present (1) or absent (0); only clear and distinct bands were scored. The Nova 100 bp DNA ladder (Novazym) was used to estimate the size of the bands. PCR was conducted in duplicate to confirm the reproducibility of the banding patterns. One negative control in each amplification was included to exclude contamination.
Statistical analysis was based on 178 loci (96 DAMDs and 82 SRAPs). The obtained binary data matrices were analysed using GenAlEx 6.5 (Peakall and Smouse 2012). The following parameters were used to estimate the genetic diversity of the studied populations: the percentage of polymorphic loci (P), number of different alleles (A), number of effective alleles (Ae), Nei’s (1973) gene diversity or the expected heterozygosity (He) and Shannon’s information index of diversity (Is). The patterns of the population structure were determined according to the Bayesian clustering method implemented in STRUCTURE v.2.3.4 (Pritchard et al. 2000; Falush et al. 2007). STRUCTURE analysis was performed using a burn-in of 10,000 generations, followed by 100,000 MCMC repetitions and 10 iterations for 1–8 clusters (K) and default settings for other parameters. The optimal number of genetic groups (K) in the dataset was determined based on the ΔK method (Evanno et al. 2005) using the web-based program Structure Harvester (Earl and von Holdt 2012). The results of STRUCTURE analysis were visualized using the CLUMPAK server (Kopelman et al. 2015).
Analysis of molecular variance (AMOVA) was hierarchically calculated to estimate the allocation of genetic variation for the following configurations: (1) among and within the studied population and (2) at three levels: among two types of habitats (sandy grassland habitats, FR, GA, GF and arable field habitats, AF, RF), among populations within habitats and within populations. The overall and pairwise genetic differentiation between populations were also estimated by AMOVA using ΦPT statistics (an analogue to FST). The statistical significance of the ΦPT statistic was tested by a permutation test with 1000 permutations. Gene flow as measured by Nm was approximated from the ΦPT statistic (Cockerham and Weir 1993). Additionally, principal coordinate analysis (PCoA) based on the Nei’s (1978) genetic distance matrix was used to illustrate the relationships between individuals and populations. The above analyses were carried out using GenAlEx 6.5. Cluster analysis for all individuals was performed using Mega 6.06 (Tamura et al. 2013) based on the Nei’s genetic distances and the Unweighted Pair Group Method with Arithmetic Mean (UPGMA).
The primer banding characteristics, such as the number of total bands (NTB), number of polymorphic bands (NPB), number of monomorphic bands (NMB) and percentage of polymorphic bands (PPB), were calculated from the binary data matrix. To evaluate the usefulness of each primer to distinguish between the studied genotypes, the following measures were computed: marker index (MI) and polymorphic information content (PIC) according to Powell et al. (1996), resolving power (RP) and mean resolving power (MRP) according to Prevost and Wilkinson (1999). The parameters of the genetic diversity of A. aristatum were calculated at the species level.
Results
Floristic and ecological characteristics of studied habitats
A. aristatum occupies two types of habitats at the eastern limit of its non-native range: field habitats, where regular agrotechnical treatment is conducted (P4, P5, P6, P7), and relatively stable grassland habitats that develop at the edges of coniferous forests and along forest and field roadsides (P1, P2, P3) (Table 4). This species most often occurs in winter crop fields, mainly in rye fields, and recently abandoned fallow lands. These habitats are well lit (L = 6.9), warm (T = 5.8), relatively dry to periodically moist (F = 3.9), moderately to highly acidic (R = 4.1) and poor in nitrogen (N = 3.9). A. aristatum frequently dominates in species-poor agrocoenoses that occupy this type of habitats, where it covers up to 40% of the area. More and more often, A. aristatum migrates also into habitats that are less-disturbed than crop fields, i.e., into species-poor sandy grasslands. Some of them are related to Corynephorus-Agrostis grasslands, protected by the Habitat Directive—habitat code: 2330 (Interpretation Manual of EU Habitats 2007). Ecological parameters of grassland habitats are similar to agrocoenosis parameters, although the former are even more insolated (L = 7.8), dryer (F = 3.3) and distinctly poorer in nitrogen (N = 2.7). In the conditions of much stronger competition with grassland species, including perennial plants, A. aristatum forms less dense populations that cover up to 20% of the area.
Polymorphism of DAMD and SRAP markers
A total of 178 bands were detected in 70 examined individuals of A. aristatum, with 96 bands amplified by 6 DAMD primers and 82 by 8 SRAP primers. The parameters that characterized each DAMD and SRAP primer are listed in Tables 2 and 3, respectively. The DAMD primers produced from 9 to 22 bands (average = 16). Most bands were amplified by the primer URP13R, whereas the least efficient primer was URP9F. The resolving power of the analysed DAMD primers ranged from 3.51 to 14.54. The highest RP value was noted for the primer URP13R, whereas the lowest was recovered for the primer URP9F. The primer URP13R also had the highest values for PIC (0.31) and MI (6.82), while URP9F had the lowest values for both of these parameters. The SRAP primers amplified between 5 and 17 bands, with an average of 10.25. The highest number of bands was amplified by the primer pair Me7–Em8, and the fewest were amplified by the primer pair Me8–Em14. The resolving power of the analysed SRAP primers ranged from 2.69 (Me8–Em14) to 11.23 (Me7–Em8). The highest PIC value (0.43) was from the primer pair Me7–Em4, whereas the maximum MI value (5.95) was found for the primer pair Me7–Em8. As each individual examined by both types of these markers had its own genotype, seventy genotypes were detected.
Genetic variation within populations
Of the 96 detected DAMD bands, 67 were polymorphic in all of the studied populations. The highest number of polymorphic loci was recovered in populations P7—81.25%, P6—73.96% and P4—71.88%. The mean percentage of polymorphic loci in populations was 70.09%. The average number of alleles per locus was 1.50. The mean gene diversity within populations (He) for all loci was 0.210 and Shannon’s information index (Is) was 0.33 (Table 5). In SRAP, 4 bands were monomorphic in all of the studied populations of A. aristatum. The highest number of polymorphic loci was found in population P6—76.83%, whereas the lowest in population P5, this number was 57.32%. The mean percentage of polymorphic loci in the populations was 65.33%. The average number of alleles per locus was 1.50. The mean gene diversity within populations (He) for all loci was 0.217 and Shannon’s information index (Is) was 0.33. In general, for both types of markers, all of the statistics of genetic variation were higher in populations originating from regularly disturbed habitats (RDH) compared to populations from periodically disturbed habitats (PDH) (Table 5). However, as the Mann–Whitney U test demonstrated, the differences are not statistically significant.
Genetic structure of populations
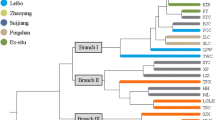
The analysis performed with STRUCTURE v2.3.4 based on a Bayesian clustering method suggested the presence of two genetic groups. The optimum number of genetic clusters was estimated using the ΔK criterion from the Evanno method. The maximal value of ΔK was 2 in both DAMD and SRAP markers, indicating that the studied individuals can be divided into 2 clusters. Bar plots showed the inclusion of both genetic groups in the studied populations (Fig. 2). Individual populations differed in their share of these genetic groups; populations P1 and P2 from sandy grassland (GA, FR) predominate in the first genetic group (orange cluster), while populations P4 from recently abandoned field (RF) and P5–P7 from arable fields (AF) formed the second group (blue cluster). Depending on the marker used, population P3 from sandy grassland (GF) fell within the first (in DAMD) or second (in SRAP) genetic group (Fig. 2).
The genetic groups defined using the Bayesian method (Fig. 2) were generally confirmed by PCoA (Fig. 3), UPGMA clustering (Fig. 4). To determine whether the genetic structure of the studied population was affected by the outlier loci, we repeated the analysis using only putative neutral loci. Removing the outlier loci from the analysis did not significantly affect the genetic structure of the populations (not shown).
UPGMA dendrogram of Anthoxanthum aristatum samples based on Nei’s genetic distances for DAMD (a) and SRAP (b) data. The studied populations are marked by different symbols. Samples: 1–10—population P1 (filled circle), 11–20—P2 (open circle), 21–30—P3 (filled square), 31–40—P4 (open square), 41–50—P5 (filled diamond), 51–60—P6 (open diamond), 61–70—P7 (filled triangle). Blue-orange bars to the right of the graphs indicate populations belonging to the groups highlighted in STRUCTURE analysis
Analysis of molecular variance (AMOVA) conducted without the division of the A. aristatum populations into habitat types revealed that most of the genetic variation (72% and 70%) was located within populations, regardless of the type of marker used (Table 6). AMOVA with the division of populations into two types of habitats showed that 68% and 66% of total genetic variation was still within populations, while the remaining molecular variation was distributed among populations within the habitats (20% and 21%) and among habitat types (12% and 13%) in DAMD and SRAP markers, respectively (Table 6). The genetic differentiation (Φ statistic) at all hierarchical levels was statistically significant (P = 0.001) (Table 6). The gene flow Nm among all of the studied populations estimated from DAMD and SRAP markers was 1.18 and 1.06 migrants per generation, respectively.
The pairwise genetic differentiation ΦPT between the studied populations ranged from 0.086 to 0.427 in DAMD and 0.039 to 0.428 in SRAP markers. All of the ΦPT values, except for the lowest value in SRAP, were statistically significant at P = 0.001 or P = 0.002. The highest ΦPT values (0.427 and 0.428) were between populations 3 and 5 for DAMD markers and 2 and 5 for SRAP markers (Table 7).
Discussion
Preliminary studies on the genetic diversity of A. aristatum populations occurring at the border of the species non-native range lead to several significant conclusions.
Firstly, our study showed that the polymorphism of invasive populations of A. aristatum at the eastern border of its non-native range was relatively high: P = 70.9% for DAMD and P = 65.33% for SRAP markers. Additionally, the heterozygote frequency and PIC were lower. Depending on the markers used, He was 0.210 for DAMD markers and 0.217 for SRAP, and the PIC values were 0.31 and 0.43, respectively.
Although the indigenous populations of this species were studied with the help of other molecular markers, it is worth noting that their level of polymorphism is even higher. A study of Iberian populations using AFLP markers showed that the proportions of polymorphic loci were P = 87.9% (Pereira et al. 2007) and P = 87.96% (Pimentel et al. 2010), whereas the heterozygote frequency was He = 0.66 (Pereira et al. 2007). In turn, investigations using microsatellite markers showed that He ranged from 0.36 to 0.82, while the polymorphism information content (PIC) ranged from 0.33 to 0.80 (Lema-Suárez et al. 2016). Significant differences in genetic variability among populations located in the centre and at the species natural range limit have already been repeatedly demonstrated (e.g., Eckert et al. 2008; Zhang et al. 2015).
Secondly, our study of A. aristatum invasive populations at the eastern limit of the species non-native range showed a higher level of intrapopulation than interpopulation variability. AMOVA demonstrated that 72% and 70% of genetic variability occurs within populations for DAMD and SRAP markers, respectively, while only 28% and 30% results from interpopulation differences. Genetic variability of native populations of A. aristatum presents a different pattern. Investigations using AFLPs conducted in the Iberian Peninsula indicated that the interpopulation variability for this species was 77.34%, whereas the intrapopulation variability was 22.66% (Pereira et al. 2007; Pimentel et al. 2010).
Thirdly, our study demonstrated that habitat differentiation is reflected in the variation and genetic structure of A. aristatum populations occurring at the non-native range limit. These results refer to the often-discussed problem of interactions between an alien species and habitats it colonizes beyond its natural range limit (among others, Dietz and Edwards 2006; Riis et al. 2010; Erfmeier et al. 2011; Pahl et al. 2013; Tunaitienė et al. 2017). Previous studies of plants occurring in habitats differing in use intensity indicate three possible patterns of population genetic diversity: (1) plants in disturbed habitats have higher genotypic and genetic diversity than in stable habitats (Koppitz et al. 2000; Xie et al. 2001; Lambertini et al. 2008); (2) there are no significant differences between the genotypic and genetic diversity of populations occurring in habitats with different disturbance regimes (Solé et al. 2004); and (3) populations in stable habitats are more differentiated than populations in disturbed habitats (Trtikova et al. 2011; Tunaitienė et al. 2017).
In our study AMOVA with the division of populations into two types of habitats (field and non-field) shows that 68% and 66% of the total genetic variation remains within populations based on DAMD and SRAP markers, respectively, while the remaining molecular variation was distributed among populations within habitats (20% and 21%) and among habitat types (12% and 13%). Genetic differentiation (Φ statistic) at all hierarchical levels was statistically significant (P = 0.001).
In the case of A. aristatum occurring at its non-native range limit, our study demonstrated that despite a short period of adaptation, habitat differentiation reflects the variation and genetic structure of populations.
It is worth stressing that, irrespective of marker type used, two groups of populations can be distinguished according to Bayesian clustering, PCoA (Fig. 3) and cluster analysis based on Nei’s distance. According to the analyses, most groups consisted of populations from similar habitats. The first group comprises populations that inhabit relatively stable habitats (semi-natural sandy grasslands). The second group includes populations connected to field habitats that are regularly disturbed. The affiliation of the population occurring near pine forest is not clear and belongs to either the first or second group depending on the marker used (DAMD or SRAP). This may result from the instability of the habitat and the short-term, transitory character of this type of population. During colonization, A. aristatum is subject to selective pressure, which was supported by a positive alpha factor value (BayeScan analysis), probably favouring genotypes adapted to oligotrophic habitats. This is in accordance with the post-invasion evolution hypothesis (Müller-Schärer and Steinger 2004). A higher level of genetic variability was found in field populations, which may be due to two reasons: (1) a relatively longer time of occurrence in a given habitat compared to sandy grassland populations and necessity of adapting to more dynamic and competitive conditions and (2) more selective ecological barriers separating sandy grasslands from A. aristatum migration routes that permit only some ecotypes to enter.
The high genetic diversity populations at the A. aristatum non-native range limit in Central Europe and their marked diversity in arable field and sandy grassland habitats suggest that the studied species may systematically cross both geographical and ecological barriers.
References
Alwala S, Kimbeng CA, Veremis JC, Gravois KA (2008) Linkage mapping and genome analysis in a Saccharum interspecific cross using AFLP, SRAP and TRAP markers. Euphytica 164:37–51. https://doi.org/10.1007/s10681-007-9634-9
Bomanowska A, Ferchmin M, Kirpluk I, Otręba A (2014) Invasive plant species in the flora of the Kampinos National Park. In: Otręba A, Michalska-Hejduk D (eds) Invasive plant species in the flora of the Kampinos National Park and its surroundings. Kampinos National Park, Izabelin, pp 25–35 (in Polish)
Brullo S, Cormaci A, Guarino R, Musarella CM (2007) Syntaxonomical survey of Geranio-Cardaminetalia hirsutae: semi-natural therophytic vegetation of the Mediterranean region. Ann Bot 7:183–216
Buczkowska K, Sawicki J, Szczecińska M, Klama H, Milewicz M, Bączkiewicz A (2010) Genetic variation in the liverwort Bazzania trilobata inferred from ISSR markers. J Bryol 32:265–274. https://doi.org/10.1179/037366810X12814321877660
Ciosek TM, Skrzyczyńska J (1997) Anthoxanthum aristatum (Poaceae) in the Nizina Południowopodlaska and its neighborhood (Poland). Fragm Florist Geobot 42(2):44–48 (in Polish)
Cockerham CC, Weir BS (1993) Estimation of gene flow from F-statistics. Evolution 47:855–863. https://doi.org/10.1111/j.1558-5646.1993.tb01239.x
Dietz H, Edwards PJ (2006) Recognition that causal processes change during plant invasion helps explain conflicts in evidence. Ecology 87:1359–1367. https://doi.org/10.1890/0012-9658(2006)87%5b1359:rtcpcd%5d2.0.co;2
Drapikowska M (2013) Variability of Anthoxanthum species in Poland in relation to geographical-historical and environmental conditions. Biodivers Res Conserv 30:1–93. https://doi.org/10.2478/biorc-2013-0010
Drapikowska M, Celka Z, Szkudlarz P, Jackowiak B (2012) Variability of the alien species Anthoxanthum aristatum Boiss. (Poaceae) in the Wielkopolska Lowland (Western Poland). Ukr Bot J 69(3):385–392
Drapikowska M, Leśniewska K, Hasterok R, Szkudlarz P, Celka Z, Jackowiak B (2013) Variability of stomata and 45S and 5S rDNAs loci characteristics in two species of Anthoxanthum genus: a. aristatum and A. odoratum (Poaceae). Acta Biol Hung 64(3):352–363. https://doi.org/10.1556/abiol.64.2013.3.8
Earl DA, von Holdt BM (2012) Structure Harvester: a website and program for visualizing Structure output and implementing the Evanno method. Conserv Genet Resour 4(2):359–361. https://doi.org/10.1007/s12686-011-9548-7
Eckert CG, Samis KE, Lougheed SC (2008) Genetic variation across species’ geographical ranges: the central-marginal hypothesis and beyond. Mol Ecol 17(5):1170–1188. https://doi.org/10.1111/j.1365-294X.2007.03659.x
Ellenberg H, Weber HE, Düll R, Wirth V, Werner W, Paulissen D (1992) Zeigerwerte von Pflanzen in Mitteleuropa; indicator values of plants of Central Europe. Scr Geobot 18:1–258 (in German)
Erfmeier A, Böhnke M, Bruelheide H (2011) Secondary invasion of Acer negundo: the role of phenotypic responses versus local adaptation. Biol Invasions 13:1599–1614. https://doi.org/10.1007/s10530-010-9917-2
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14(8):2611–2620. https://doi.org/10.1111/j.1365-294X.2005.02553.x
Falush D, Stephens M, Pritchard JK (2007) Inference of population structure using multilocus genotype data: dominant markers and null alleles. Mol Ecol Notes 7:574–578. https://doi.org/10.1111/j.1471-8286.2007.01758.x
Fernández-Moya J, San Miguel-Ayanz A, Cañellas I, Gea-Izquierdo G (2010) Variability in Mediterranean annual grassland diversity driven by small-scale changes in fertility and radiation. Plant Ecol 212(5):865–877. https://doi.org/10.1007/s11258-010-9869-8
Fraga i Arguimbau P (2008) Vascular flora associated to Mediterranean temporary ponds on the island of Minorca. Ann Jard Botánico Madrid 65(2):393–414. https://doi.org/10.3989/ajbm.2008.v65.i2.302
Heath DD, Lawma GK, Devlin RH (1993) PCR primed with VNTR core sequences yields species specific patterns and hypervariable probes. Nucleic Acids Res 21(24):5782–5785
Hegi G (1909) Illustrated flora of Central Europe. I. Carl Hanser Verlag, München (in German)
Howell C, Sawyer JWD (2006) New Zealand naturalised vascular plant checklist. New Zealand Plant Conservation Network, Wellington
Interpretation Manual of EU Habitats (2007) European Commission Interpretation Manual of European Union Habitats. EUR 27. European Commission, Brussels, Belgium
Jackowiak B, Chmiel J, Latowski K (1990) Segetal communities in winter cereal crops of Wielkopolska. Part 1. Bad Fizjogr Pol Zach Seria B Botanika 40:107–120 (in Polish)
Karaca M, Ince AG (2008) Minisatellites as DNA markers to classify bermudagrasses (Cynodon spp.): confirmation of minisatellite in amplified products. J Genet 87:83–86
Karaca M, Ince AG, Ay ST, Turgut K, Onus AN (2008) PCR-RFLP and DAMD-PCR genotyping for Salvia species. J Sci Food Agric 88(14):2508–2516. https://doi.org/10.1002/jsfa.3372
Kästner A, Jäger EJ, Schubert R (2001) Handbook of segetal plants of Central Europe. Springer, Wien (in German)
Kopelman NM, Mayzel J, Jakobsson M, Rosenberg NA, Mayrose I (2015) Clumpak: a program for identifying clustering modes and packaging population structure inferences across K. Mol Ecol Resour 15(5):1179–1191. https://doi.org/10.1111/1755-0998.12387
Koppitz H, Kühl H, Kohl JG (2000) Differences in morphology and C/N-balance between clones of Phragmites australis within a plantation at a degraded fen. Folia Geobot 35(4):389–402. https://doi.org/10.1007/BF02803551
Lambertini C, Gustafsson MHG, Frydenberg J, Speranza M, Brix H (2008) Genetic diversity patterns in Phragmites australis at the population, regional and continental scales. Aquat Bot 88:160–170. https://doi.org/10.1016/j.aquabot.2007.10.002
Lema-Suárez I, Sahuquillo E, Marí-Mena N, Pimentel M (2016) Polymorphic microsatellite markers in Anthoxanthum (Poaceae) and cross-amplification in the Eurasian complex of the genus. Appl Plant Sci 4(10):1600070. https://doi.org/10.3732/apps.1600070
Li G, Quiros CF (2001) Sequence-related amplified polymorphism (SRAP), a new marker system based on a simple PCR reaction: its application to mapping and gene tagging in Brassica. Theor Appl Genet 103:455–461. https://doi.org/10.1007/s001220100570
Mito T, Uesugi T (2004) Invasive alien species in Japan: the status quo and the new regulation for prevention of their adverse effects. Glob Environ Res 8(2):171–191
Müller-Schärer H, Steinger T (2004) Predicting evolutionary change in invasive, exotic plants and its consequences for plant–herbivore interactions. In: Ehler LE, Sforza R, Mateille T (eds) Genetics, evolution and biological control. CAB International, Wallingford, pp 137–162
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8(19):4321–4325
Nei M (1973) Analysis of gene diversity in subdivided populations. Proc Natl Acad Sci USA 70:3321–3323
Nei M (1978) Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89(3):83–590
Nie G, Zhang XQ, Huang LK, Xu WZ, Wang JP, Zhang YW, Ma X, Yan YH, Yan HD (2014) Genetic variability and population structure of the potential bioenergy crop Miscanthus sinensis (Poaceae) in Southwest China based on SRAP markers. Molecules 19:12881–12897. https://doi.org/10.3390/molecules190812881
Niemann P, Zwerger P (2006) Population dynamics and competition of annual vernal grass Anthoxanthum puelii Lecoq et Lamotte. Nachrichtenblatt des Deutschen Pflanzenschutzddienstes 58(9):225–227
Oberdorfer E, Schwabe A, Müller T (2001) Phytosociological flora of Germany and adjacent areas. Ulmer, Stuttgart (in German)
Pahl AT, Kollmann J, Mayer A, Haider S (2013) No evidence for local adaptation in an invasive alien plant: field and greenhouse experiments tracing a colonization sequence. Ann Bot 112:1921–1930. https://doi.org/10.1093/aob/mct246
Peakall R, Smouse PE (2012) GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research—an update. Bioinformatics 28:2537–2539. https://doi.org/10.1093/bioinformatics/bts460
Pereira MP, Pérez GE, Balbuena ES (2007) European sweet vernal grasses (Anthoxanthum: Poaceae, Pooideae, Aveneae): a morphometric taxonomical approach. Syst Bot 32(1):43–59. https://doi.org/10.1600/036364407780360201
Pimentel M, Catalan P, Sahuquillo E (2010) Morphological and molecular taxonomy of the annual diploids Anthoxanthum aristatum and A. ovatum (Poaceae) in the Iberian Peninsula. Evidence of introgression in natural populations. Bot J Linn Soc 164:53–71. https://doi.org/10.1111/j.1095-8339.2010.01068.x
Pinke G, Pál R, Király G, Szendrödi V, Mesterházy A (2006) The occurrence and habitat conditions of Anthoxanthum puelii Lecoq & Lamotte and other Atlantic-Mediterranean weed species in Hungary. J Plant Dis Protect 20:587–596
Powell W, Morgante M, Andre C, Hanafey M, Vogel J, Tingey S, Rafalski A (1996) The comparison of RFLP, RAPD, AFLP, and SSR (microsatellite) markers for germplasm analysis. Mol Breed 2:225–238. https://doi.org/10.1007/BF00564200
Prevost A, Wilkinson M (1999) A new system of comparing PCR primers applied to ISSR fingerprinting of potato cultivars. Theor Appl Genet 98(1):107–112. https://doi.org/10.1007/s001220051046
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155(2):945–959
Randall RP (2007) The introduced flora of Australia and its weed status. CRC for Australian Weed Management, Glen Osmond
Riis T, Lambertini C, Olesen B, Clayton JS, Brix H, Sorrell BK (2010) Invasion strategies in clonal aquatic plants: are phenotypic differences caused by phenotypic plasticity or local adaptation? Ann Bot 106:813–822. https://doi.org/10.1093/aob/mcq176
Rivas-Martínez S, de la Fuente V, Sánchez-Mata D (1986) Alisedas mediterráneo-iberoatlánticas en la Península Ibérica. Stud Botánica 5:9–38
Robarts DWH, Wolfe AD (2014) Sequence-related amplified polymorphism (SRAP) markers: a potential resource for studies in plant molecular biology. Appl Plant Sci 2(7):1400017. https://doi.org/10.3732/apps.1400017
Ryves TB, Clement EJ, Foster MC (1996) Alien grasses of the British Isles. Botanical Society of the British Isles City, London
Santa-Bárbara C, Valdés B, Pina FJ (2003) New floristic records for Andevalo area (Huelva Province, SW Spain). IV. Acta Bot Malacit 28:237–246 (in Spanish)
Schneider Ch, Sukopp U, Sukopp H (1994) Biological and ecological foundations of threatened segetal plant protection. Schriftenreihe für Vegetationskunde 26:1–356 (in German)
Sharma SK, Kumaria S, Tandon P, Rao SR (2011) Single primer amplification reaction (SPAR) reveals inter- and intra-specific natural genetic variation in five species of Cymbidium (Orchidaceae). Gene 483:54–62. https://doi.org/10.1016/j.gene.2011.05.013
Skrajna T, Skrzyczyńska J (2007) Selected biological features and occurrence of Anthoxanthum aristatum Boiss. in the Kałuszyska Upland. Ann Univ Mariae Curie-Skłodowska Lub Pol 62(2):145–155 (in Polish)
Solé M, Durka W, Eber S, Brandl R (2004) Genotypic and genetic diversity of the common weed Cirsium arvense (Asteraceae). Int J Plant Sci 165:437–444. https://doi.org/10.1086/382801
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) Mega 6.06: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729. https://doi.org/10.1093/molbev/mst197
Tokarska-Guzik B (2005) The establishment and spread of alien plant species (kenophytes) in the flora of Poland, vol 2372. Scientific works of the Silesian University in Katowice, Katowice, pp 1–192
Trtikova M, Güsewell S, Baltisberger M, Edwards PJ (2011) Distribution, growth performance and genetic variation of Erigeron annuus in the Swiss Alps. Biol Invasions 13:413–422. https://doi.org/10.1007/s10530-010-9835-3
Tunaitienė V, Patamsytė J, Naugžemys D, Kleizaitė V, Čėsnienė T, Rančelis V, Žvingila D (2017) Genetic and allelopathic differences between populations of daisy fleabane Erigeron annuus (L.) Pers. (Asteraceae) from disturbed and stable habitats. Biochem Syst Ecol 70:294–303. https://doi.org/10.1016/j.bse.2016.12.015
Valdés B, Scholz H, von Raab-Straube E, Parolly G (2009) Poaceae (pro parte majore). Euro + Med Plantbase—the information resource for Euro-Mediterranean plant diversity. http://ww2.bgbm.org/EuroPlusMed/. Accessed 15 Nov 2018
Vanderpoorten A, Engels P (2003) Patterns of bryophyte diversity and rarity at a regional scale. Biodivers Conserv 12:545–553. https://doi.org/10.1023/A:1022476902547
Woziwoda B (2010) Variety of flora species of vascular plants in various aged forests on former agricultural soils on the example of grass. Stud Mater Nat For Educ Centre Rogów 12, 2(25):405–416 (in Polish)
Xie ZW, Lu YQ, Ge S, Hong DY, Li FZ (2001) Clonality in wild rice (Oryza rufipogon, Poaceae) and its implications for conservation management. Am J Bot 88:1058–1064
Zając A, Zając M (eds) (2001) Distribution atlas of vascular plants in Poland. Laboratory of Computer Chorology, Institute of Botany, Jagiellonian University, Cracow
Zhang YH, Zhang JW, Li ZM, Sun H (2015) Genetic diversity of the weed species, Stellera chamaejasme, in China inferred from amplified fragment length polymorphism analysis. Weed Biol Manag 15(4):165–174. https://doi.org/10.1111/wbm.12084
Zitouna N, Gharbi M, Chennaoui H, Touati A, Fadhlaoui I, Trifi-Farah N, Marghali S (2015) Characterization of Sulla species via SRAP markers: polymorphism and systematic analyses. Merit Res J Agric Sci Soil Sci 3:089–097
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Drapikowska, M., Buczkowska, K., Celka, Z. et al. Genetic variability of Anthoxanthum aristatum Boiss. (Poaceae) at the non-native range limit. Genet Resour Crop Evol 67, 163–176 (2020). https://doi.org/10.1007/s10722-019-00850-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10722-019-00850-1