Abstract
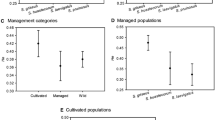
We studied populations of Stenocereus pruinosus throughout Mexico, a species important for its edible fruit. The Tehuacán Valley is setting of ancient and the currently most active management of S. pruinosus; we hypothesized Tehuacán as the original area of domestication of S. pruinosus and expected to find there its highest genetic variation and original source of genes of cultivated plants. Through nuclear microsatellite loci we studied spatial distribution of genetic variation and population differentiation. We sampled wild and managed populations of the Central-western (Tehuacán, Central Valleys and Tehuantepec, Oaxaca), north-eastern (Huasteca) and south-eastern (Chiapas) regions. Differences among regions and populations were compared through homogeneity and exact test for F IS , AMOVA, Bayesian analysis, and genetic barriers. A niche analysis allowed corroborating taxonomic identity of populations. The highest genetic diversity was in Tehuantepec (H E = 0.841), decreasing towards the extremes of distribution (H E = 0.242 in Huasteca, H E = 0.254 in Chiapas). Genetic structure is significantly high among populations and regional groups, differentiating one group formed by northern and southern populations and other formed by populations of the Central-western region. Differences among groups suggested that populations from Huasteca could be species different to S. pruinosus, but the niche analysis did not support such hypothesis. Populations from Tehuantepec were different but genetically interconnected with those of Tehuacán. Tehuantepec is the main reservoir of genetic diversity of wild populations of S. pruinosus, but Tehuacán is the principal current area of domestication of S. pruinosus and probably where its domestication originated. Conclusions would be stronger by analyzing DNAc lineages.







Similar content being viewed by others
References
Arnaud-Haond S, Teixeira SI, Mssa C, Billot P, Saenger G, Coupland CM, Duarte A, Aerraos A (2006) Genetic structure at range edge: low diversity and high inbreeding in Southeast Asian mangrove (Avicennia marina) populations. Mol Ecol 15:3515–3525
Barrier E, Velasquillo L, Chávez M, Gaulon R (1998) Neotectonic evolution of the Isthmus of Tehuantepec (southeastern Mexico). Tectonophysics 287:77–96
Beerli P, Felsenstein J (1999) Maximum-likelihood estimation of migration rates and effective population numbers in two populations using a coalescent approach. Genet 152:763–773
Beerli P, Felsenstein J (2001) Maximum likelihood estimation of a migration matrix and effective population sizes in n subpopulations by using a coalescent approach. Proc Natl Acad Sci USA 98:4563–4568
Blancas J, Casas A, Rangel-Landa S, Moreno-Calles A, Torres I, Pérez-Negrón E, Solís L, Delgado-Lemus A, Parra F, Arellanes Y, Caballero J, Cortés L, Lira R (2010) Plant management in the Tehuacán-Cuicatlán Valley, Mexico. Econ Bot 64:287–302
Blancas J, Casas A, Pérez-Salicrup D, Caballero J, Vega E (2013) Ecological and socio-cultural factors influencing plant management in Náhuatl communities of the Tehuacán Valley, Mexico. J Ethnobiol Ethnomed 9:39
Boege E (2008) El patrimonio biocultural de los pueblos indígenas de México: Hacia la conservación in situ dela biodiversidad y agrobiodiversidad de los territorios indígenas. Instituto Nacional de Antropología e Historia, México
Bravo-Hollis E (1978) Las Cactáceas de México. Vol. I. Universidad Nacional Autónoma de México, México
Brunet J (1967) Geologic studies. In: Byers DS (ed) The prehistory of the Tehuacan Valley. Environment and subsistence. Texas University of Press, Austin, pp 66–90
Buckler E, Pearsall D, Holtsford T (1998) Climate, plant ecology, and central Mexican archaic subsistence. Curr Anthrop 39:152–164
Caballero J, Casas A, Cortés L, Mapes C (1998) Patrones en el conocimiento, uso y manejo de plantas en pueblos indígenas de México. Revista Estudios Atacameños 16:181–196
Casas A, Pickersgill B, Caballero J, Valiente-Banuet A (1997) Ethnobotany and domestication in xoconochtli, Stenocereus stellatus (Cactaceae), in the Tehuacán Valley and la Mixteca Baja, México. Econ Bot 51:279–292
Casas A, Caballero J, Valiente-Banuet A (1999) Use, management and domestication of columnar cacti in the South-Central México: a historical perspective. J Ethnobiol 19:71–95
Casas A, Cruse J, Morales E, Otero-Arnaiz A, Valiente-Banuet A (2006) Maintenance of phenotypic and genotypic diversity of Stenocereus stellatus (Cactaceae) by indigenous peoples in Central Mexico. Biodivers Conserv 15:879–898
Casas A, Otero-Arnaiz A, Pérez-Negrón E, Valiente-Banuet A (2007) In situ management and domestication of plants in Mesoamerica. Ann Bot 100:1101–1115
Casas A, Rangel-Landa S, Torres-García I, Pérez-Negrón E, Solís L, Parra F, Delgado A, Blancas JJ, Farfán B, Moreno AI (2008) In situ management and conservation of plant resources in the Tehuacán-Cuicatlán Valley, Mexico: an ethnobotanical and ecological approach. In: Albuquerque UP, Alves-Ramos M (eds) Current topics in ethnobotany. Research Signpost, Kerala, pp 1–25
Colunga-GarcíaMarín P, Zizumbo-Villareal D (2004) Domestication of plants in Mayan lowlands. Econ Bot 58:101–110
Colunga-GarcíaMarín P, Estrada-Loera E, May-Pat F (1996) Patterns of morphological variation, diversity, and domestication of wild and cultivated populations of Agave in Yucatán, Mexico. Am J Bot 83:1069–1082
Cruse-Sanders JM, Parker KC, Friar EA, Huang DI, Mashayekhi S, Prince LM, Otero-Arnaiz A, Casas A (2013) Managing diversity: Domestication and gene flow in Stenocereus stellatus Riccob. (Cactaceae) in Mexico. Ecol Evol 3:1340–1355
Darwin C (1859) The origins of species by means in natural selection or the preservation of favoured races in the struggle for life. Wiley, London
Dávila P, Arizmendi MC, Valiente-Banuet A, Villaseñor JL, Casas A, Lira R (2002) Biological diversity in the Tehuacán-Cuicatlán Valley, Mexico. Biodivers Conserv 11:421–442
Dieringer D, Schotterer C (2003) Microsatellite analyzer (MSA): a platform independent analysis tool for large microsatellite data sets. Mol Ecol Notes 3:167–169
Doebley J, Gaut B, Smith B (2006) The molecular genetics of crop domestication. Cell 127:1309–1321
Dyer RJ, Nason JD (2004) Population graphs: the graph theoretic shape of genetic structure. Mol Ecol 13:1713–1727
Eckert CG (2002) The loss of sex in clonal plants. Evol Ecol 15:501–520
Eckert CG, Samis KE, Lougheed SC (2008) Genetic variation across species’ geographical ranges: the central–marginal hypothesis and beyond. Mol Ecol 17:1170–1188
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Excoffier L, Laval G, Schneider S (2005) Arlequin (version 3.0): An integrated software package for population genetics data analysis. Evol Bioinform 1:47–50
Falush D, Stephens M, Pritchard JK (2003) Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genet 164:1567–1587
Flannery KV (1986) Guilá Naquitz. Academic Press, New York
Gapare WJ, Aitken SN (2005) Strong spatial genetic structure in peripheral but not core populations of Sitka spruce [Picea sitchensis (Bong.) Carr.]. Mol Ecol 14:2659–2667
García E (1981) Modificaciones al sistema de clasificación climática de Köeppen para adaptarlo a las condiciones de la República Mexicana. Instituto de Geografía, Universidad Nacional Autónoma de México, México
Gepts P, Bettinger R, Brush S, Damania A, Famula T, McGuire P, Qualset C (2012) Introduction: the domestication of plants and animals: ten unanswered questions. In: Gepts P, Bettinger R, Brush S, Damania A, Famula T, McGuire P, Qualset C (eds) Biodiversity in agriculture: domestication, evolution, and sustainability. Cambridge University Press, Cambridge, pp 1–8
Gibson AC, Horak KE (1978) Systematic anatomy and phylogeny of Mexican columnar cacti. Ann Mo Bot Gard 65:999–1057
Gibson AC, Nobel P (1990) The cactus primer. Harvard University Press, Harvard
Goldstein DB, Ruiz-Linares A, Cavalli-Sforza LL, Feldman MW (1995) An evaluation of genetic distances for use with microsatellite loci. Genetics 139:463–471
Goudet J (1995) Fstat version 1.2: a computer program to calculate F statistics. J Hered 86:485–486
Gross B, Olsen K (2010) Genetic perspectives on crop domestication. Trend Plan Sci 15:529–537
Guillén S, Terrazas T, De la Barrera E, Casas A (2011) Germination differentiation patterns of wild and domesticated columnar cacti in a gradient of artificial selection intensity. Genet Resour Crop Evol 58:409–423
Gutiérrez-Rodríguez C, Ornelas JF, Rodríguez-Gómez F (2011) Chloroplast DNA phylogeography of a distylous shrub (Palicourea padifolia, Rubiaceae) reveals past fragmentation and demographic expansion in Mexican cloud forests. Mol Phylogen Evol 61:603–615
Harlan J (1975) Crops and man. American Society of Agronomy, Madison
Horner MA, Fleming TH, Sahey CT (1998) Foraging behaviour and energetics of a nectar-feeding bat, Leptonycteris curasoae (Chiroptera: Phyllostomidae). J Zool 244:575–586
Lonn M, Prentice HC (2002) Gene diversity and demographic turnover in central and peripheral populations of the perennial herb Gypsophila fastigiata. Oikos 99:489–498
Luna-Morales C, Aguirre R, Peña C (2001) Cultivares tradicionales mixtecos de Stenocereus pruinosus y S. stellatus (Cactaceae). An Instit Biol 72:131–155
MacNeish RS (1967) A summary of the subsistence. In: Byers DS (ed) The prehistory of the Tehuacán Valley. Environment and subsistence. University of Texas Press, Austin, pp 290–331
Manni F, Guérard E, Heyer E (2004) Geographic patterns of (genetic, morphologic, and linguistic) variation: how barriers can be detected by using Monmonier´s algorithm. Hum Biol 72:173–190
McAuliffe JR, Sundt PC, Valiente-Banuet A, Casas A, Viveros JL (2001) Pre-Columbian soil erosion, persistent ecological changes, and collapse of a subsistence agricultural economy in the semi-arid Tehuacán Valley, Mexico’s ‘Cradle of Maize’. J Arid Environ 47:47–75
McCormack JE, Zellmer AJ, Knowles LL (2009) Does niche divergence accompany allopatric divergence in Aphelocoma jays as predicted under ecological speciation? Insights from tests with niche models. Evolution 64:1231–1244
Miller MP (1997) Tools for population genetics analyses (TFPGA) 1.3. A windows program for the analysis of allozymes and molecular population genetic data. Computer software distributed by author
Miller A, Schaal B (2006) Domestication and the distribution of genetic variation in wild and cultivated populations of the Mesoamerican fruit tree Spondias purpurea L. (Anacardiaceae). Mol Ecol 15:1467–1480
Morrone JJ (2006) Biogeographic areas and transition zones of Latin America and the Caribbean islands based on a biogeographic and cladistics analyses of the entomofauna. Ann Rev Entom 51:467–494
Nei M (1972) Genetic distance between populations. Am Nat 106:283–292
Otero-Arnaiz A, Cruse-Sanders J, Casas A, Hamrick JL (2004) Isolation and characterization of microsatellites in the columnar cactus: Polaskia chichipe and cross-species amplification within the Tribe Pachycereeae (Cactaceae). Mol Ecol Notes 4:265–269
Otero-Arnaiz A, Casas A, Hamrick JL, Cruse-Sanders J (2005) Genetic variation and evolution of Polaskia chichipe (Cactaceae) under domestication in the Tehuacán Valley, Central Mexico. Mol Ecol 14:1603–1611
Parra F, Casas A, Peñaloza-Ramírez JM, Cortés-Palomec A, Rocha-Ramírez V, González-Rodríguez A (2010) Process of domestication of Stenocereus pruinosus (Cactaceae) in the Tehuacán Valley, Central Mexico. Ann Bot 106:483–496
Parra F, Blancas J, Casas A (2012) Landscape management and domestication of Stenocereus pruinosus (Cactaceae) in the Tehuacán Valley: human guided selection and gene flow. J Ethnobiol Ethnomed 8:32
Peakall R, Smouse PE (2006) GENALEX 6, genetic analysis in Excel: population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Peck JR, Yearsley JM, Waxman D (1998) Explaining the geographic distributions of sexual and asexual populations. Nature 391:889–892
Pickersgill B (2007) Domestication of plants in the Americas: insights from Mendelian and molecular genetics. Ann Bot 100:925–940
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. http://www.R-project.org/
Raymond M, Rousset F (1995) GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J Hered 86:248–249
Red Mundial de Información sobre Biodiversidad (REMIB) (2011) México: Comisión Nacional de para el Conocimiento y Uso de la Biodiversidad (CONABIO). http://www.conabio.gob.mx/remib/doctos/remib_esp.html
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Santa Anna H, Contreras-Medina R, Luna-Vega I (2009) Biogeographic analysis of endemic cacti of the Sierra Madre Oriental, Mexico. Biol J Linn Soc 97:373–389
Servicio Meteorológico Nacional (2012) México. http://smn.cna.gob.mx/
Silvertown J (2008) The evolutionary maintenance of sexual reproduction: evidence from the ecological distribution of asexual reproduction in clonal plants. Int J Plant Sci 169:157–168
Slatkin M (1995) A measure of population subdivision based on microsatellite allele frequencies. Genetics 139:457–462
Smith EC (1967) Plant remains. In: Byers DS (ed) The prehistory of the Tehuacán Valley. Environment and subsistence. University of Texas Press, Austin, pp 220–255
Stenberg P, Lundmark M, Saura A (2003) MLGsim: a program for detecting clones using a simulation approach. Mol Ecol Notes 3:329–331
Twyford A, Kidner C, Harrison N, Ennos RA (2013) Population history and seed dispersal in widespread Central American Begonia species (Begoniaceae) inferred from plastome-derived microsatellite markers. Bot J Linn Soc 171:260–276
Valiente-Banuet A, Arizmendi MC, Rojas-Martinez A, Domínguez-Canseco L (1996) Ecological relationships between columnar cacti and nectar-feeding bats in México. J Trop Ecol 12:103–119
Van Oosterhout C, Hutchinson WF, Wills DP, Shipley P (2004) MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Program Note. Mol Ecol Notes 4:535–538
Vavilov NI (1951) The Origen, variation, immunity and breeding of cultivated plants. Chron Bot 13:1–366
Wallace R (2002) The phylogeny and systematic of columnar cacti: an overview. In: Fleming T, Valiente- Banuet A (eds) Columnar cacti and their mutualists. evolution, ecology and conservation. The University of Arizona Press, Tucson
Zeder M (2006) Central questions in the domestication of plants and animals. Evol Anthrop 15:105–117
Zohary D (1996) The mode of domestication of the founder crops of Southwest Asian agriculture. In: Harris DR (ed) The origins and spread of agriculture and pastoralism in Eurasia. University College London Press, London, pp 142–158
Zohary D (1999) Monophyletic vs. polyphyletic origin of the crops on which agriculture was founded in the Near East. Genet Resour Crop Evol 46:133–142
Acknowledgments
The authors thank the Posgrado en Ciencias Biológicas, UNAM and CONACYT, Mexico for ease PhD studies of FP and HRC. We also thank DGAPA UNAM (Project IN209214) CONACYT (research project CB-2013-01-221800) and partial fellowship for Postgraduate Thesis of Red Latinoamericana de Botánica for financial support, as well as Edgar Pérez-Negrón for field work assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parra, F., Casas, A., Rocha, V. et al. Spatial distribution of genetic variation of Stenocereus pruinosus (Otto) Buxb. in Mexico: analysing evidence on the origins of its domestication. Genet Resour Crop Evol 62, 893–912 (2015). https://doi.org/10.1007/s10722-014-0199-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10722-014-0199-x