Abstract
Glucosinolates are secondary components characteristic for the Brassicaceae with complex biological functions. Glucosinolates in the seeds are anti-nutritive when feeding animals and their inheritance have been extensively investigated. Much less is known about the genetics of glucosinolates in leaves and stems, which may attract some insects, while repelling others. They may also inhibit bacterial processes of importance when using green biomass for the production of biogas. The objective of this study was to analyse the genetic variation of total and individual glucosinolates in the green material of rapeseed. For this 28 resynthesized winter rapeseed lines were tested at two locations. There was a large variation in leaf glucosinolate content between 0.10 and 4.75 μmol/g dry matter. The predominant leaf glucosinolates are the alkenyle glucosinolates progoitrin, gluconapin and glucobrassicanapin. The line R53 is exceptional, while combining a relative high content of the indole glucosinolate glucobrassicin with low alkenyle glucosinolates in the leaves. The total glucosinolate concentration in the stems and leaves is not correlated with the seed glucosinolate concentrations. Heritabilities are above h² = 0.60 for progoitrin, h² = 0.65 for gluconapin, h² = 0.30 for glucobrassicanapin and h² = 0.52 for total glucosinolate content in the leaves. In conclusion, resynthesized rapeseed is an important genetic resource to modify the leaf glucosinolate content and composition of rapeseed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glucosinolates with more than 100 different side chain structures have been described (Mithen 2001). In the Brassicaceae, the main groups are the aliphatic or alkenyle glucosinolates (derived from methionine), the phenyl or aromatic glucosinolates (from phenylalanine or tyrosine) and indole glucosinolates (from tryptophane). Depending on structural differences, alkenyle, aromatic and indole glucosinolates produce different toxic end-products after cleavage by the myrosinase enzyme (Fenwick et al. 1983). The alkenyle glucosinolates are dominant in Brassica napus L. and are systematically classified as 2-propenyl (sinigrin = SIN), 3-butenyl (gluconapin = GNA), 2-hydroxy-3-butenyl (progoitrin = PRO), 4-pentenyl (glucobrassicanapin = GBN) and 2-hydroxy-4-pentenyl (gluconapoleiferin = GNL) glucosinolates (Mithen 2001), also the aromatic glucosinolate NAS (gluconasturtiin), and GBC, NEO, 4OH which belong to the indole glucosinolate group is classified (Hopkins et al. 2009), see systematic names in Table 1.
The genetic variation and inheritance of seed glucosinolates is well known. Compared to this, the knowledge on glucosinolates in leaves and stems is still rather limited. Therefore the objective of this study is to investigate the genetic variation of glucosinolate content and composition in green material of rapeseed. As material, resynthesized rapeseed lines from interspecific hybridization between cabbage (B. oleracea L.) and turnip rape (B. rapa L.) (Gland et al. 1981) are used, because in such material the maximum amount of genetic variation available in Brassica napus L. can be expected.
Materials and methods
Materials
The material consisted of 28 resynthesized lines with very broad genetic background both for the B. oleracea L. and the B. rapa L. parent (Table 2). For comparison, the common German winter rapeseed cultivar ‘Express’ was included as check.
Field experiments
The resynthesized lines were sown in two row plots of 2.5 m length with 10 cm plant distance, at two locations, Einbeck and Göttingen in the 2007/2008 season. At beginning of May, the leaves and stems were harvested as random sample of 10 green fresh leaves and stems from each plot, cooled during transport, and dried in an oven at 55°C (McGregor and Love 1978). At maturity, the pods of three open pollinated plants were harvested; more than 100 seeds were stored for further analysis.
Glucosinolate analysis
Glucosinolate profiles of stems, leaves and seeds were analyzed by HPLC (High Pressure Liquid Chromatography). After heating 200 mg of milled material twice for 10 min at 75°C glucosinolates were extracted and hydroxylated using concentrates of both 70 and 10% methanol. After decantation the extract was passed through Sephadex micro-columns. After rinsing the columns with 1 ml of water and addition of a sulphatase, these were incubated over night at 40°C. The desulfo-glucosinolates were eluated by 500 μl of water. An ultraviolet detector (190–400 nm) was used for peak detection. Glucosinolates are expressed in μmol/g dry matter (D.M.). For seed meal containing SIN, glucotropaeolin (200 μl 6 mM) was used as an internal standard. For leaf and stem material, SIN (200 μl, 6 mM) was used as an internal standard (Spinks et al. 1984). The HPLC analyses were performed at least three times for each sample, and the results were averaged.
Statistical analysis
An analysis of variance was performed with location and genotype as factors. For comparison of glucosinolate content between lines least significant differences (P = 0.05) were calculated. The software Plabstat (Utz 1996) was used for all statistical analyses.
Results
To explore the genetic resources of Brassica napus L. seeds, leaves and stems from resynthesized lines was analyzed for their glucosinolate content. The glucosinolate concentrations are subdivided into their main components. The seed glucosinolates of the resynthesized lines are given in Table 3. The results for leaves and stem are given in Table 4. The mean level of total glucosinolates in the seeds is 64.23 μmol/g D.M., for the leaves 1.06 μmol/g D.M., and for stems 1.99 μmol/g D.M. The dominant glucosinolates belong to the alkenyles (PRO, GBN, GNL and GNA) in seeds as well as in stems and leaves, SIN and 4OH are only present in the seeds. Total leaf glucosinolate values range from 0.10 to 4.75 μmol/g D.M. Alkenyles are the most dominant glucosinolate group in the seeds (70–80%) followed by the indole glucosinolate GBC (10%) and the phenyl type NAS (10%). Leaves and stems have dominant concentrations of PRO and GNA. In the leaves the most prevalent individual glucosinolate was PRO (0.06–2.00 μmol/g D.M.) followed by GBN (0.00–0.81 μmol/g D.M). NAS was the major glucosinolate type in the phenyl group (0.00–0.81 μmol/g D.M.). The indole group was dominated by GBC (0.01–0.31 μmol/g D.M.).
The genotype S3 has the highest content of leaf glucosinolates associated with high seed glucosinolate content. Least significant differences showed in the leaves of S3 a significantly higher total glucosinolate content and levels of PRO and GNA compared with the rest of the resynthesized lines. H4 has the lowest leaf glucosinolate content; in this line alkenyle glucosinolates are almost absent. H327 has the highest seed glucosinolate content, whereas H19 had the lowest seed glucosinolate content. This corresponds both with a high and low leaf glucosinolate content respectively 2.02 μmol/g D.M. and 0.30 μmol/g D.M. The line R53 combines a very low leaf alkenyle content (PRO, GNA, GNL, GBN) and high leaf indole (GBC) glucosinolate content. Express is the standard cultivar chosen for comparison with the resynthesized rapeseed lines. Express has the lowest seed glucosinolate content, but average leaf glucosinolate content.
An analysis of variance for leaves, stems and seeds shows highly significant differences for total glucosinolates among locations and genotypes (Table 5). Depending on the genotype the level of PRO and GNA varies significantly in the leaves and the stems. In the stem, also GBN, GBC, and NAS show significant genotypic differences. In the seeds, for all glucosinolates except GBC significant genotypic variance was observed. The heritability estimates are high for total and major glucosinolate types of the alkenyles group (PRO, GNA, GBN) within the leaves and stems (Table 5). For total glucosinolate content heritability is very high for seeds (h2 = 0.90), and lower for leaves (h2 = 52) and stems (h2 = 0.58).
The correlation between the different leaf glucosinolate types are significant for GNA, PRO (0.87**) and GNA, GBN (0.69**), which are alkenyle glucosinolates related with each other (Table 6). The minor glucosinolate types NAS and GBC, belong to the aromatic and indole glucosinolate groups are also significantly correlated [0.76**]. However, significant correlations between the main indole glucosinolate type (GBC) and alkenyle types are absent.
A clear difference between glucosinolate composition if seeds, stems, and leaves is observed (Fig. 1). Increasing relative amounts of PRO in the leaves, stems and in the seeds are observed (24% in the leaves, 48% in the stems and 56% in the seeds). GNL contributes in a smaller amount to the total glucosinolate content, with values from 11% in the leaves, 7% in the stems and 3% in the seeds. The same is true for GBN (leaves = 18%, stems = 16% and seeds 4%) and the indole glucosinolate GBC (8% in the leaves, 6% in the stems and almost absent in the seeds).
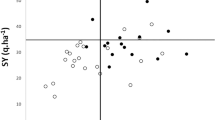
Highly significant (P = 0.01) correlations are found between the content of glucosinolates in the stems and leaves (r² = 0.65; Fig. 2). Lower correlations were observed between seed glucosinolate content and glucosinolates in stems (r2 = 0.47, data not shown) and leaves (r2 = 0.39, Fig. 3). Though leave and seed glucosinolates are not highly correlated, there is a relationship in so far, that low leaf glucosinolate content was only observed in genotypes with low seed glucosinolate content.
Discussion
Glucosinolate content in leaves and stems is low in comparison with the content in seeds. The leaves are quite fragile material, which differ within the season, within and between the years and during their development, and even during the day (Rosa 1997).
Jürges (1982) who did a comparable research on winter rapeseed cultivars before flowering measured leaf concentrations ranging from 1.0 to 15.5 μmol/g D.M. According to Clossais-Besnard and Larher (1991), the concentration of glucosinolates in dry seeds is about five to ten times higher as in stems and leaves; however, this is not always the case (Mithen 2004). Therefore manipulation of 0 and 00 lines with low and high seed glucosinolate content independently from the leaf glucosinolates (Mithen 2004) is rather challenging. The distribution of the glucosinolates varies depending on plant part, with both quantitative and qualitative differences among leaves, stems and seeds (Velasco et al. 2007). A low (<4.8 μmol/g D.M.) total glucosinolate content in the leaves of winter rapeseed was observed.
A further explanation for the low glucosinolate content in the leaves and stems in comparison with the seeds could be found in the dilution of glucosinolates during plant growth (Clossais-Besnard and Larher 1991). This starts already after germination, where a mixture of enzymatic reactions causes the further turn-over of glucosinolates. Because of the existence of seed-specific glucosinolates, it is suggested that vegetative parts mainly provide precursors and that the final steps for glucosinolate synthesis occur in the seed (Clossais-Besnard and Larher 1991). Secondly while the tissue in the seeds is morphologically protected, a lower decomposition of instable glucosinolates types due to environmental reasons in the seeds as in the green material is caused. Thirdly a possible explanation could be differences in transport between the different plant organs. Transport properties of glucosinolates within Brassica napus L. are of interest as identification of the mechanism leading to lower levels obtained in specific tissues such as seeds (Brudenell et al. 1999). This is particularly observed for PRO, which is highest in the seeds and leaves of Brassica napus L.
The correlation between total seed and leaf glucosinolates is low. This is most probable caused by differences in biochemical reactions due to different gene actions in the tissue of the green material compared to the seeds. Seed glucosinolate concentrations cannot be used for indirectly predict the concentration of the glucosinolates in the leaves. A triangle shaped plot is shown in Fig. 3. This means that low seed glucosinolate lines always have low leaf glucosinolate content, whereas high seed glucosinolate lines may have low or high leaf glucosinolate content. For the relation of the total glucosinolate contents in the different plant organs, earlier observations on the presence or absence of correlations are until now rather contradictory (Jürges 1982). It has been suggested that weak correlations between seed and leaf glucosinolates content might be caused by the dependence of leaf glucosinolate content on environmental effects and growing stage (Schilling and Friedt 1991).
This study describes the genetic variation of alkenyle, indole and phenyl glucosinolates occurring in low but measurable quantities. Genetic variation in leaves and stems of rapeseed is high for alkenyle glucosinolate types (PRO, GNA and GBN). Glucosinolate variability has been observed within leaves of the Brassicaceae, which are distinct for their alkenyle glucosinolate composition. An assumption is that a difference in gene action causes this methionine side chain elongation. This makes it possible to further investigate gene controlled variation in leaves within Brassica napus L. (Kroymann et al. 2000). In leaves of Brassica napus L., this is expressed in significant correlated levels of PRO and GNA (Gland et al. 1981). GBC, which is synthesized from tryptophane (Kutácek and Králová 1971) is the indole glucosinolate with the highest level. The causes of high glucobrassicin levels are possibly enzymatic and absence can be explained by a genetic block for direct glucosinolate synthesis from tryptophane (Kutácek and Králová 1971).
References
Brudenell AJP, Griffiths H, Rossiter JT, Baker DA (1999) The phloem mobility of glucosinolates. J Exp Bot 50:745–756
Clossais-Besnard N, Larher F (1991) Physiological role of glucosinolates in Brassica napus. Concentration and distribution pattern of glucosinolates among plant organs during a complete life cycle. J Sci Food Agric 56:25–38
Fenwick GR, Heaney RK, Mullin WJ (1983) Glucosinolates and their breakdown products in food and food plants. Crit Rev Food Sci Nutr 18:123–201
Gland A, Röbbelen G, Thies W (1981) Variation of alkenyle glucosinolates in seeds of Brassica species. Z Pflanzenzüchtg 87:96–110
Hopkins RJ, Griffiths DW, Birch ANE, McKinlay RG (2009) Influence of increasing herbivore pressure on modification on modification of glucosinolate content of swedes (Brassica napus spp. rapifera). J Chem Ecol 24:2003–2019
Jürges K (1982) Möglichkeiten einer Auslese auf Glucosinolat-Armut in der Grünmasse von B. napus L. und B. campestris L. Z Pflanzenzüchtg 89:74–87
Kroymann J, Textor S, Tokuhisa JG, Kimberly LF, Bartram S, Gershenzon J, Mitchell-Olds T (2000) A gene controlling variation in Arabidopsis glucosinolate composition is part of the methionine chain elongation pathway. Plant Physiol 127:1077–1088
Kutácek M, Králová M (1971) Biosynthesis of the glucobrassicin aglycone from 14C and 15N labeled l-tryptophan precursors. Biologia Plantarum 14:279–285
McGregor DI, Love HK (1978) Analysis of vegetative tissue as a means of facilitating selection for seed glucosinolate composition in Brassica, vol 7, pp 1514–1519. In: Proceedings of the 7th international rapeseed congress, Poznan
Mithen R (2001) Glucosinolates- biochemistry, genetics and biological activity. Plant Growth Regul 34:91–103
Mithen R (2004) Leaf glucosinolate profiles and their relationship to pest and disease resistance in oilseed rape. Euphytica 63:71–83
Rosa EAS (1997) Daily variation in glucosinolate concentration in the leaves and roots of cabbage seedlings in two constant temperature regimes. J Sci Food Agric 73:364–368
Schilling W, Friedt W (1991) Breeding of 00-rapeseed (Brassica napus L.) with differential glucosinolate content in the leaves, pp. 250–255. In: Proceedings of the 8th international rapeseed conference, Saskatoon, Canada
Spinks EA, Sones K, Fenwick GR (1984) The quantitative analysis of glucosinolates in cruciferous vegetables, oilseeds and forage crops using high performance liquid chromatography. Fette Seifen Anstrichmittel 86:228–231
Utz FH (1996) PLABSTATT. Plant breeding institute, University of Hohenheim. http://www.unihohenheim.de/˜ipspwww/soft.html
Velasco L, Becker HC (2000) Variability for seed glucosinolates in a germplasm collection of the genus Brassica. Genet Resour Crop Evol 47:231–238
Velasco P, Cartea ME, González C, Vilar M, Ordás A (2007) Factors affecting the glucosinolate content of kale (Brassica oleracea acephala). J Agric Food Chem 55:955–962
Acknowledgments
The results are the outcome of a project funded by the Deutsche Bundesstiftung Umwelt (DBU). The authors express their gratitude to the KWS SAAT AG for establishing the field experiments.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Cleemput, S., Becker, H.C. Genetic variation in leaf and stem glucosinolates in resynthesized lines of winter rapeseed (Brassica napus L.). Genet Resour Crop Evol 59, 539–546 (2012). https://doi.org/10.1007/s10722-011-9701-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10722-011-9701-x