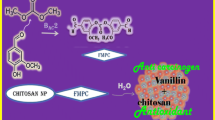
Abstract
In this study, we synthesized a novel water-soluble low molecular chitosan (LMC) derivative through Vilsmeier reaction and reductive amination reaction. The derivative was characterized by UV-visible spectroscopy, 1H NMR, FTIR and SEM techniques. The results showed that the derivative effectively reduced the cell viability rate, inhibited cell metastasis, induced cell apoptosis and dissipated mitochondrial membrane potential (ΔΨm). Moreover, the antitumor activity was strengthened with the increase of the degree of substitution of tanshinone I (TanI). These findings provided important support for developing new water-soluble antitumor agent and expand the scope of application of LMC.










Similar content being viewed by others
References
Rinaudo M.: Chitin and chitosan: properties and applications. Prog. Polym. Sci. 31(7), 603–632 (2006)
Kumar M.N.R.: A review of chitin and chitosan applications. React. Funct. Polym. 46(1), 1–27 (2000)
Pillai C., Paul W., Sharma C.P.: Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 34(7), 641–678 (2009)
Jeon Y.-J., Kim S.-K.: Production of chitooligosaccharides using an ultrafiltration membrane reactor and their antibacterial activity. Carbohydr. Polym. 41(2), 133–141 (2000)
Xia W., Liu P., Zhang J., Chen J.: Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll. 25(2), 170–179 (2011)
Xu J., Zhao X., Han X., Du Y.: Antifungal activity of oligochitosan against Phytophthora capsici and other plant pathogenic fungi in vitro. Pestic. Biochem. Physiol. 87(3), 220–228 (2007)
Lu Y., Slomberg D.L., Schoenfisch M.H.: Nitric oxide-releasing chitosan oligosaccharides as antibacterial agents. Biomaterials. 35(5), 1716–1724 (2014)
He Q., Gong K., Ao Q., Ma T., Yan Y., Gong Y., Zhang X.: Positive charge of chitosan retards blood coagulation on chitosan films. J. Biomater. Appl. 27(8), 1032–1045 (2013)
Huang R., Mendis E., Rajapakse N., Kim S.-K.: Strong electronic charge as an important factor for anticancer activity of chitooligosaccharides (COS). Life Sci. 78(20), 2399–2408 (2006)
Zhou L., Zuo Z., Chow M.S.S.: Danshen: an overview of its chemistry, Pharmacology, pharmacokinetics, and clinical use. J. Clin. Pharmacol. 45(12), 1345–1359 (2005)
Dong Y., Morris-Natschke S.L., Lee K.-H.: Biosynthesis, total syntheses, and antitumor activity of tanshinones and their analogs as potential therapeutic agents. Nat. Prod. Rep. 28(3), 529–542 (2011)
Liu, Q.y., Zhang, Z.h., Jin, X., Jiang, Y.R., Jia, X.B.: Enhanced dissolution and oral bioavailability of tanshinone IIA base by solid dispersion system with low-molecular-weight chitosan. J. Pharm. Pharmacol. 65(6), 839–846 (2013).
Zeng L.-W., Zhou C.-X., Liu J.-D., Liu C.-H., Mo J.-X., Hou A.-F., Yao W., Wang Z.-Z., Gan L.-S.: Design, synthesis, and antimicrobial activities of new tanshinone IIA esters. Nat. Prod. Res. 1, 1–7 (2016)
Su W., Weng Y., Jiang L., Yang Y., Zhao L., Chen Z., Li Z., Li J.: Recent progress in the use of Vilsmeier-type reagents. Org. Prep. Proced. Int. 42(6), 503–555 (2010)
Yalpani, M., Hall, L.D.: Some chemical and analytical aspects of polysaccharide modifications. III. Formation of branched-chain, soluble chitosan derivatives. Macromolecules 17(3), 272–281 (1984).
Zhang A., Mu H., Zhang W., Cui G., Zhu J., Duan J.: Chitosan coupling makes microbial biofilms susceptible to antibiotics. Sci. Report. 3, (2013). doi:10.1038/srep03364
Zhang W., Dong D., Li P., Wang D., Mu H., Niu H., Duan J.: Novel pH-sensitive polysialic acid based polymeric micelles for triggered intracellular release of hydrophobic drug. Carbohydr. Polym. 139, 75–81 (2016)
He G., Chen X., Yin Y., Zheng H., Xiong X., Du Y.: Synthesis, characterization and antibacterial activity of salicyloyl chitosan. Carbohydr. Polym. 83(3), 1274–1278 (2011)
Amoozgar Z., Park J., Lin Q., Yeo Y.: Low molecular-weight chitosan as a pH-sensitive stealth coating for tumor-specific drug delivery. Mol. Pharm. 9(5), 1262–1270 (2012)
Leane M., Nankervis R., Smith A., Illum L.: Use of the ninhydrin assay to measure the release of chitosan from oral solid dosage forms. Int. J. Pharm. 271(1), 241–249 (2004)
Tan S.C., Khor E., Tan T.K., Wong S.M.: The degree of deacetylation of chitosan: advocating the first derivative UV-spectrophotometry method of determination. Talanta. 45(4), 713–719 (1998)
Wang M., Zhang L., Han X., Yang J., Qian J., Hong S., Samaniego F., Romaguera J., Yi Q.: Atiprimod inhibits the growth of mantle cell lymphoma in vitro and in vivo and induces apoptosis via activating the mitochondrial pathways. Blood. 109(12), 5455–5462 (2007)
Liu M.-C., Yang S.-J., Jin L.-H., Hu D.-Y., Xue W., Song B.-A., Yang S.: Synthesis and cytotoxicity of novel ursolic acid derivatives containing an acyl piperazine moiety. Eur. J. Med. Chem. 58, 128–135 (2012)
Xin H., Liu X.H., Zhu Y.Z.: Herba leonurine attenuates doxorubicin-induced apoptosis in H9c2 cardiac muscle cells. Eur. J. Pharmacol. 612(1), 75–79 (2009)
Parashar S., Cheishvili D., Arakelian A., Hussain Z., Tanvir I., Khan H.A., Szyf M., Rabbani S.A.: S-adenosylmethionine blocks osteosarcoma cells proliferation and invasion in vitro and tumor metastasis in vivo: therapeutic and diagnostic clinical applications. Cancer medicine. 4(5), 732–744 (2015)
Liu X., Xia W., Jiang Q., Xu Y., Yu P.: Synthesis, characterization, and antimicrobial activity of kojic acid grafted chitosan oligosaccharide. J. Agric. Food Chem. 62(1), 297–303 (2013)
Simons W.W.: Sadtler handbook of infrared spectra. Sadtler research laboratories (1978)
Lavertu M., Xia Z., Serreqi A., Berrada M., Rodrigues A., Wang D., Buschmann M., Gupta A.: A validated 1 H NMR method for the determination of the degree of deacetylation of chitosan. J. Pharm. Biomed. Anal. 32(6), 1149–1158 (2003)
Badawy M.E.: Chemical modification of chitosan: synthesis and biological activity of new heterocyclic chitosan derivatives. Polym. Int. 57(2), 254–261 (2008)
Sashiwa, H., Aiba, S.-i.: Chemically modified chitin and chitosan as biomaterials. Prog. Polym. Sci. 29(9), 887–908 (2004).
Curotto E., Aros F.: Quantitative determination of chitosan and the percentage of free amino groups. Anal. Biochem. 211(2), 240–241 (1993)
Zhang Y., Jiang P., Ye M., Kim S.-H., Jiang C., Lü J.: Tanshinones: sources, pharmacokinetics and anti-cancer activities. Int. J. Mol. Sci. 13(10), 13621–13666 (2012)
Ly J.D., Grubb D., Lawen A.: The mitochondrial membrane potential (Δψm) in apoptosis; an update. Apoptosis. 8(2), 115–128 (2003)
Na Y.: Recent cancer drug development with xanthone structures. J. Pharm. Pharmacol. 61(6), 707–712 (2009)
Pecere T., Gazzola M.V., Mucignat C., Parolin C., Dalla Vecchia F., Cavaggioni A., Basso G., Diaspro A., Salvato B., Carli M.: Aloe-emodin is a new type of anticancer agent with selective activity against neuroectodermal tumors. Cancer Res. 60(11), 2800–2804 (2000)
Salvioli S., Ardizzoni A., Franceschi C., Cossarizza A.: JC-1, but not DiOC 6 (3) or rhodamine 123, is a reliable fluorescent probe to assess ΔΨ changes in intact cells: implications for studies on mitochondrial functionality during apoptosis. FEBS Lett. 411(1), 77–82 (1997)
Chaffer C.L., Weinberg R.A.: A perspective on cancer cell metastasis. Science. 331(6024), 1559–1564 (2011)
Twentyman P., Luscombe M.: A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br. J. Cancer. 56(3), 279 (1987)
Acknowledgments
This project was supported by the National Natural Science Foundation of China (NSFC) (Grant31570799) and Program for New Century Excellent Talents in University (NCET-13-0480).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The manuscript did not contain animal experiments, and all other experiments were compliance with ethical standards.
Electronic supplementary material
ESM 1
(PDF 471 kb)
Rights and permissions
About this article
Cite this article
Wang, D., DanXu, Sun, Y. et al. Synthesis, characterization and anticancer activity of tanshinone I grafted low molecular chitosan. Glycoconj J 34, 3–12 (2017). https://doi.org/10.1007/s10719-016-9712-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-016-9712-0