Abstract
Vaccination with meningococcal glycoconjugate vaccines has decreased the incidence of invasive meningitis worldwide. These vaccines contain purified capsular polysaccharides attached to a carrier protein. Because of derivatization chemistries used in the process, conjugation of polysaccharide to protein often results in heterogeneous mixtures. Well-defined vaccines are needed to determine the relationship between vaccine structure and generated immune response. Here, we describe efforts to produce well-defined vaccine candidates by chemoenzymatic synthesis. Chemically synthesized lactosides were substrates for recombinant sialyltransferase enzymes from Camplyobacter jejuni and Neisseria meningitidis serogroup C. These resulting oligosialic acids have the same α(2-9) sialic acid repeat structure as Neisseria polysaccharide capsule with the addition of a conjugatable azide aglycon. The degree of polymerization (DP) of carbohydrate products was controlled by inclusion of the inhibitor CMP-9-deoxy-NeuNAc. Polymers with estimated DP < 47 (median DP 25) and DP < 100 (median DP 51) were produced. The receptor binding domain of the tetanus toxin protein (TetHc) was coupled as a carrier to the enzymatically synthesized oligosialic acids. Recombinant TetHc was derivatized with an alkyne squarate. Protein modification sites were determined by trypsin proteolysis followed by LC/MS-MSE analysis of peptides. Oligosialic acid azides were conjugated to modified TetHc via click chemistry. These chemoenzymatically prepared glycoconjugates were reactive in immunoassays with specific antibodies against either group C polysaccharide or TetHc. Sera of mice immunized with oligosialic acid-TetHc glycoconjugates contained much greater levels of polysaccharide-reactive IgG than the sera of control mice receiving unconjugated oligosialic acids. There was no apparent difference between glycoconjugates containing oligosaccharides of DP < 47 and DP < 100. These results suggest that chemoenzymatic synthesis may provide a viable method for making defined meningococcal vaccine candidates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive bacterial meningitis is primarily caused by three species of encapsulated bacteria: Haemophilus influenzae, Streptococcus pneumoniae, and Neisseria meningitidis [1]. The bacterial capsules consist of extracellular polysaccharide and are essential for virulence. Bacteria lacking the polysaccharide capsule are more susceptible to complement-mediated killing [2–4]. Capsular polysaccharides provide a useful target for vaccine development because of their role in virulence. There are a number of glycoconjugate vaccines currently licensed against H. influenzae type b, 13 or more S. pneumoniae serotypes, and N. meningitidis serogroups A, C, Y and W135 [5]. Glycoconjugate vaccines are comprised of purified capsular polysaccharides covalently attached to a carrier protein such as diphtheria or tetanus toxoid. This class of vaccines is more effective than those vaccines containing polysaccharide alone. Attachment of polysaccharide antigens to a carrier protein switches the immune response from a short lived T-cell independent response to a longer lasting T-cell dependent response [6]. Glycoconjugate vaccines have led to a substantial decrease in meningococcal disease worldwide [1].
While these conjugate vaccines have been successful, the structural elements of polysaccharide-protein conjugates required to elicit a protective antibody response are not known. This is partly a consequence of the non-specific coupling methods used for attachment. Glycoconjugate vaccines are usually prepared by size reduction and activation of the polysaccharide prior to chemical coupling to derivatized carrier protein [7–9]. Vaccines prepared from these methods are heterogeneous and often contain random, multipoint attachment of polysaccharide to protein. A method to make homogenous and easily characterized vaccines would provide a way to investigate the impact of vaccine structure on immunogenicity. These well-defined vaccines should (a) contain glycan antigen of determined polymer length and (b) have known sites of attachment to carrier protein.
One recently developed method to produce glycoproteins for potential use as conjugate vaccines has been exploitation of bacterial N-glycosylation systems. N-linked protein glycosylation, once thought to be a strictly eukaryotic process, has recently been found in the prokaryote Camplyobacter jejuni [10] . The glycosylation machinery of C. jejuni, which includes Pgl enzymes responsible for glycosyltransferase and flippase activity, has been successfully transferred to E. coli [11–13]. This glycoengineering approach was used to produce typhoid glycoconjugate vaccine candidates [14].
We have previously described a chemoenzymatic method to develop well-defined glycoconjugates as potential vaccine candidates against Neisseria meningitidis [15]. Using our knowledge of Neisseria sialic acid biosynthesis, we have developed a potentially controllable, chemoenzymatic approach to obtain conjugatable oligosialic acids (Fig. 1a). A lactoside substrate was chemically synthesized (Fig. 2) for enzymatic polymerization by sialyltransferase enzymes from Camplyobacter jejuni [16] and Neisseria meningitidis serogroup C [17]. This lactoside contains an azido group at the reducing end of the sugar. Sialylation occurs at the non-reducing end. Conjugation of oligosialic acids to alkyne-modified carrier protein occurs by click 1,3 cycloaddition chemistry [18] (Fig. 1b). We previously applied this scheme to produce immunoreactive glycoconjugates of bovine serum albumin and chemoenzymatically-produced oligosialylated azidolactosides as proof-of-concept [15].
Chemoenzymatic approach to oligosialic acid production. a The reaction scheme includes lactoside acceptors with a conjugatable aglycon with initial sialylation performed by Camplyobacter jejuni CST II. Subsequent sialylation of the disialylated product is catalyzed by the Neisseria meningitidis serogroup C polysialyltransferase (NmC PST). b Schematic of click conjugation of 9-azidononanyllactoside to alkyne modified TetHc carrier protein or alkyne-containing dye for product visualization
Synthetic scheme for 9-azidononanyllactoside. Conditions are described further in Materials and Methods
In this work, we have used a recombinant carrier protein, the receptor binding domain (TetHc) of the Clostridium tetani tetanus toxin, to make glycoconjugate vaccine candidates [19, 20] and mapped the sites of protein modification. The full length tetanus toxoid protein has been a common carrier in conjugate vaccines. The structural effect of formaldehyde inactivation of the protein has not been fully characterized. However, recent work suggests that formaldehyde can produce Schiff bases on lysine residues leading to oligomers [21]. We have focused on using the TetHc domain as a carrier protein because it is a non-toxic protein, is easily purified, has a published crystal structure [22] and has the ability to elicit production of antibodies that are protective against tetanus [23–26]. Additionally, in this report we have used chain-terminating inhibitors to modulate oligosialic acid chain length. We have formed glycoconjugates with these oligosialic acids and immunized mice to determine whether oligosaccharide chain length has any impact on immune response.
Materials and methods
Preparation of CMP-9-deoxy-NeuNAc and CMP-9-fluoro-NeuNAc
The ManNAc analogue, 6-deoxy-ManNAc, was a generous gift of Dr. Y.C. Lee from Johns Hopkins University [27]. The sialic acid analogue, 9-deoxy-NeuNAc was prepared by reaction of 30 μmoles of 6-deoxy-ManNAc with recombinant C. jejuni sialic acid synthase [28] (500 μL) in the presence of phosphoenolpyruvate (30 μmoles) in 50 mM Bicine, 5 mM MnCl2 buffer at pH 8.0 (1 mL total reaction volume). The reaction was incubated for 18 h at 37 °C. The reaction was monitored periodically by thiobarbituric acid (TBA) assay [29], to detect sialic acid synthesis, and by thin layer chromatography (TLC) using 1-butanol:28 % ammonium hydroxide:H2O (6:2:2 v/v) as a solvent system.
The 9-deoxy-NeuNAc produced from the reaction was converted into CMP-9-deoxy-NeuNAc by addition of Neisseria meningitidis CMP-NeuNAc synthetase [30] (750 μL of a stock solution, 19,176 U/mL) to the same reaction solution along with CTP (75 μmoles in H2O pH 7.0) and 50 mM MgCl2 (pH 8.0) to give a 2 mL final solution. The reaction was incubated at 37 °C and synthesis was monitored by TBA to detect total sialic acid and CMP-bound sialic acid present in the reaction. When the reaction was complete, the solution was applied to a column containing Sephadex G-10 resin (GE Healthcare, Pittsburgh, PA). Fractions were collected using gravity flow column chromatography with H2O as the eluent. All fractions collected were assayed by TBA for total sialic acid and CMP-sialic acid. Additionally, the absorbance at 260 nm for each fraction was measured. Appropriate fractions were pooled, dried, and stored at −80 °C.
The sialic acid analogue, 9-fluoro-NeuNAc, was commercially synthesized (TCI, Inc., Shanghai, China). The compound was converted to CMP-9-fluoro-NeuNAc using Neisseria CMP-NeuNAc synthetase. The compound (25 μmoles) was reacted with cytidine triphosphate, CTP (20 μmoles) in the presence of 100 mM HEPES pH 8.0, 50 mM MgCl2, and 0.2 mM DTT. Neisseria CMP-NeuNAc synthetase (25.6 U) was added to start the reaction. The final volume of the reaction was 150 μL. The reaction was incubated at 37 °C. Synthesis was monitored by TBA and isolated as described for CMP-9-deoxy-NeuNAc.
Synthesis of the acceptor 9-azidononanyllactoside
Peracetylated lactosyl trichloroacetimidate (1) [31] (2.2 g) and 9-bromononanol (1.2 eq) were dissolved in 25 mL dry dichloromethane under argon and over molecular sieves. The mixture was stirred for 15 min at −42 °C. TMS-triflate (1.5 eq.) was added to the stirring mixture dropwise and after addition, the mixture was left to react for 3–4 h with stirring. The reaction mixture was neutralized with triethylamine and filtered through Cellite. The filtered solution was concentrated and the reaction product purified by column chromatography on silica gel with chloroform:methanol (40:1) as a solvent. Yield: 900 mg of 9-bromononanyl peracetylated lactoside (2).
The 9-bromononanyl peracetylated lactoside (2) (800 mg) was reacted with 10 equivalents NaN3 in 10 mL dimethyl formamide at 85 °C for 3 days under argon with stirring. The reaction mixture was concentrated by azeotrope with toluene and fractionated on silica gel chromatography. The column was developed by first washing with hexane:acetone (3:1) then eluting the desired product with a 1.5:1 mixture of hexane:acetone. Yield: 650 mg of 9-azidononanyl peracetylated lactoside (3). ESI M.S. – Calculated m/z value for C35H53N3O18Na [M + Na]+: 826.3222; found 826.3235.
The 9-azidononanyl peracetylated lactoside (3) (650 mg) was deacetylated with sodium methoxide in methanol for 4 h. The reaction mixture was neutralized and the H+ form of the IR resin, filtered, concentrated and purified on silica gel using chloroform:methanol (3:1 to 2:1). Yield: 590 mg of 9-azidononanyllactoside (4). ESI M.S. – Calculated m/z value for C21H40N3O11 [M + H]+: 510.2657; found 510.2656. The structure was confirmed by HSQC NMR (see Supplementary Fig. 2).
Enzymatic sialylation of 9-azidononanyllactoside
The CST II enzyme was prepared and the activity was determined as described previously [15]. For the CST II enzyme preparation used in this work, 1 U = 20 μg. Recombinant expression and purification of Neisseria group C polysialyltransferase (NmC PST) was prepared as described previously [17].
The CST II-mediated sialylation of two azidolactosides were compared: commercially available azidoethyllactoside (Carbosynth, Compton, Berkshire, UK) and the chemically synthesized 9-azidononanyllactoside. Both lactosides (1 mM final concentration) were incubated with CST II (0.04 U) in two separate 1.5 mL reaction tubes in the presence of 5.0 mM CMP-NeuNAc and 40 mM MnCl2 in 200 mM sodium cacodylate buffer, pH 8.0 (50 μL final volume). For the control reaction, no enzyme was added. The reaction progressed for 4 h at 37 °C. Reactions were quenched by addition of ethanol to 25 % by volume.
Sialylation of the 9-azidononanyllactoside acceptor by both CST II and NmC PST was assessed. In two separate 1.5 mL reaction tubes, the compound (1 mM) was incubated at 37 °C with CST II (0.06 U) in the presence of 2.5 mM CMP-NeuNAc and 40 mM MnCl2 in 200 mM sodium cacodylate buffer, pH 8.0 (50 μL final volume) for 3.5 h. NmC PST (5 μg in 16.7 μL) and sodium cacodylate buffer was added to one reaction tube giving a final reaction volume of 100 μL. For the control reaction with CST II only, 50 μL of buffer was added instead of NmC PST.
Inhibition of polymerization
The effect of sialic acid derivatives on the overall sialylation was investigated concurrently. In four separate 1.5 mL reaction tubes, CST II (0.06 U) and lactoside (1 mM) were incubated in the presence of 2.5 mM CMP-NeuNAc and 40 mM MnCl2 in 200 mM sodium cacodylate buffer, pH 8.0 (50 μL final volume each tube). After 3.5 h, NmC PST (5 μg in 16.7 μL), and CMP-9-fluoro-NeuNAc or CMP-9-deoxy-NeuNAc (125 or 250 μM), and sodium cacodylate buffer were added to give 100 μL total. All six reactions were incubated an additional 18 h at 37 °C after addition of NmC PST (or buffer in the case of the control). Reactions were then quenched to give a final ethanol concentration of 25 %.
Detection of oligosialic acid azides
To facilitate fluorescence detection of reaction products by HPLC or TLC, the reactions were conjugated to an alkyne-containing fluorescent dye, 4-ethynyl-N-ethyl-1,8-naphthalimide [32], excitation wavelength 365 nm, emission wavelength 460 nm. The click chemistry reaction was initiated by addition of DMSO (20 % of reaction volume), with subsequent addition of TBTA (1 mM), dye (1 mM), sodium ascorbate (5 mM), and Cu(I)Br (1 mM) [15]. Stock solutions of click reagents were made at 10 mM concentration in DMSO except sodium ascorbate (50 mM in H2O). The reaction was incubated at 37 °C for 1 h. Samples were run on TLC using an ethyl acetate:methanol:H2O:acetic acid (4:2:1:0.1 v/v) solvent system and spots were visualized using a hand-held UV light. For further visualization of products, these samples were evaporated to dryness by SpeedVac and brought up to 50 μL in H2O. Each sample was subjected to HPLC analysis using a Dionex DNAPac PA200 anion exchange column on an Agilent 1200 series system equipped with a fluorescence detector. Elution was performed at 1 mL/min with a NaNO3 gradient similar to that of Inoue and Inoue [33, 34]. For elongation reactions containing NmC PST, product samples were run on the following gradient: at 1, 3, 7, 14, 21, 47 min the sodium nitrate concentration was 2, 3, 10, 20, 25, and 35 % of 1.0 M NaNO3. For reactions with CST II only, this gradient was shortened: at 1, 3, 7, 14, 21 min the sodium nitrate concentration was 2, 3, 10, 20, and 25 % of 1.0 M NaNO3.
The amount of sialylated product for later large-scale vaccine use was optimized as follows. Three amounts (0.5 U (10 μg), 1.0 (20 μg), 2.5 (50 μg)) of CST II were reacted with acceptor (1 mM) in the presence of 5 mM CMP-NeuNAc and 40 mM MnCl2 in 200 mM sodium cacodylate buffer, pH 8.0 (50 μL final volume each reaction). To determine how much inhibitor to use for large scale reactions, 1 mM acceptor was incubated with CST II (1.2 μg, 0.06 U) in the presence of 2.5 mM CMP-NeuNAc and 40 mM MnCl2 in 200 mM sodium cacodylate buffer pH 8.0 in 50 μL total. Reactions were prepared in four separate 1.5 mL tubes and incubated at 37 °C for 3.5 h. NmC PST, (5 μg in 16.7 μL) CMP-9-deoxy-NeuNAc, (125 or 250 μM) and buffer were added to give 100 μL total. Reactions were quenched after 18 h to give a final ethanol concentration of 25 %. Products were then subjected to click conjugation as described above and monitored by HPLC.
Isolation of oligosialic acids for conjugation to tetanus toxin C fragment (Tet Hc)
Sialylated oligosaccharides were chemoenzymatically synthesized in 500 μL volume. Two reactions were performed in the absence and presence of CMP-9-deoxy-NeuNAc (62.5 μL). For both reactions, CST II (2.5 U) was reacted with 9-azidononanyllactoside (1 mM) in the presence of 5 mM CMP-NeuNAc and 40 mM MnCl2 in 200 mM sodium cacodylate buffer, pH 8.0 for 4 h at 37 °C. To one reaction tube, NmC PST (50 μg in 145 μL), CMP-NeuNAc (5 mM final) and cacodylate buffer were added to give a final volume of 750 μL and the reaction was allowed to proceed at 37 °C for 20 h. In the other reaction, NmC PST (25 μg in 72.5 μL), CMP-NeuNAc (5 mM final), cacodylate buffer and 150 μM CMP-9-deoxy-NeuNAc (62.5 μL) were added to give 750 μL total. A small volume of both reactions (10 μL) were removed at 4 h after CST II addition and after 20 h of incubation with NmC PST. These samples were quenched with 10 μL of 50 % ethanol and reacted with alkyne dye to visualize products via HPLC. The degree of polymerization (DP) of 9-azidononanyllactoside sialylated by NmC PST was determined by counting the number of observable peaks in the HPLC chromatogram. The median DP is expressed as the point at which 50 % of the peak distribution lies above or below that value.
Isolation of sialylated lactosides from remaining reaction volume was performed using a column containing 1.0 mL of Q-Sepharose anion exchange resin (Amersham Biosciences, Uppsala, Sweden) equilibrated with 10 mL of 20 mM Tris, pH 8.0. Sialylation reaction products were adjusted to 7.5 mL with H2O and loaded onto the Q-Sepharose column by gravity flow. The column was then washed with 5 mL of 20 mM Tris, pH 8.0 buffer. Sialylated 9-azidononanyllactosides were eluted with 5 mL of 20 mM Tris, 500 mM NaCl, pH 8.0 buffer.
To determine which fractions contained sialylated 9-azidononanyllactoside, a portion of the elution fractions (50 μL) was conjugated to fluorescent dye using click reagents. Samples were run on TLC using an ethyl acetate:methanol:H2O:acetic acid (4:2:1:0.1 v/v) solvent system and spots were visualized using a hand-held UV light. For further analysis, these samples were dried and analyzed by HPLC. Fractions containing oligosialylated lactosides were dried by Speedvac and redissolved in 500 μL of H2O. Sialic acid concentration was determined using TBA assay. Final concentrations of uninhibited oligosialic acids (DP < 100, median DP 51) were 32.7 μg/mL and inhibited oligosialic acids (DP < 47, median DP 25) were 23.0 μg/mL.
Alkyne modification of the tetanus toxin C fragment (TetHc)
Purification of the TetHc protein was slightly modified from a previously published method [17]. After ammonium sulfate precipitation, the protein pellet was dissolved in 20 mM Tris buffer, pH 8.0 and desalted on a PD-10 column equilibrated with the same buffer while collecting 0.5 mL fractions. Fractions containing protein were pooled and protein concentrations were determined by BioRad assay. Pooled protein was dialyzed against 10 mM ammonium carbonate and lyophilized.
Activated TetHc preparation 1: Propargyl methyl squarate ester was synthesized using a previously published procedure [15]. Alkyne incorporation into carrier protein was adapted from Hou et al. [35]. Solid TetHc protein (6.6 mg, 0.13 μmol protein, 4.4 μmol lysine residues) and propargyl squarate (0.3 mg, 1.8 μmol) were dissolved in 1.0 mL of 0.5 M borate buffer pH 9.0 pre-warmed to 35 °C. The reaction was stirred at this temperature for 24 h and then 3 mL of 50 mM potassium phosphate pH 7.0 was added to quench the reaction. The solution was exchanged into 10 mM ammonium carbonate by dialysis and lyophilized.
Activated TetHc preparation 2: In another preparation of modified protein, solid TetHc (2.6 mg, 0.05 μmol protein, 1.7 μmol lysine residues) and propargyl squarate (1.1 mg, 6.4 μmol) was dissolved in 1.5 mL of warmed 0.5 M borate buffer pH 9.0. The reaction progressed for 17 h and then 3 mL of 500 mM potassium phosphate pH 7.0 was added to assure quenching of the reaction. The solution was dialyzed and lyophilized as before.
Incorporation of squarate residues into all preparations of TetHc was confirmed by MALDI-TOF mass spectrometry. MALDI-TOF analysis was conducted using a Voyager-DE RP BioSpectrometry Workstation in the positive ion mode. TetHc protein samples were prepared for MALDI by diluting 1:4 with 0.1 % trifluoroacetic acid (TFA). Wash and elution procedures were followed according to manufacturer directions for C4 ZipTips (Millipore, Billerica, MA). Samples were eluted using a matrix solution of 30 mg/mL sinapinic acid (Sigma, St. Louis, MO) in 0.1 % TFA/acetonitrile (1:1, v/v). The degree of substitution was estimated by determining the difference in molecular weight between unmodified and modified protein and dividing this value by the molecular weight of the squarate incorporation (Δ133 g).
TetHc protein digestion
Solutions of TetHc and squarate-modified TetHc from preparations 1 and 2 (0.5–1.0 mg/mL in 30 μL) were prepared either in 50 mM ammonium bicarbonate or in 1X phosphate buffered saline. Proteins were dissolved in 0.1 % RapiGest (Waters Corp., Milford, MA) in 50 mM ammonium bicarbonate. Dithiothreitol (DTT) was added to reduce the disulfide bonds at a final concentration of 5 mM for 30 min at 60 °C, then cooled to room temperature. The reduced cysteine residues were alkylated with 15 mM iodoacetamide (IAA) for 30 min in the dark at room temperature. Trypsin was added (enzyme:protein, 1:50, w/w) to the protein solution and the samples incubated at 37 °C for 18 h. After digestion, the samples were adjusted to 0.5 % trifluoroacetic acid (TFA) and incubated at 37 °C for 45 min to degrade the RapiGest surfactant. Samples were centrifuged at 13,000 rpm for 10 min to remove the insoluble by-products and the solutions were vacuum dried for downstream analysis.
Reverse phase LC-MSE analysis of peptides
The peptides were reconstituted in 0.1 % formic acid in water and injected onto a C-18 column (HSS T3 75 μm i.d. × 100 mm, 1.8 μm particle, Waters Corp., Milford, MA) for nanoLC-MSE analysis. The solvent system consisted of solvent A: 100 % water/0.1 % formic acid, and solvent B: 100 % acetonitrile/0.1 % formic acid. A Waters nanoAcquity UPLC system was used for automatic sample loading and flow control. The gradient was as follows: 3–50 % of solvent B in 48 min, 85 % of solvent B for 7 min, and 3 % of solvent B for 25 min. The effluent was introduced into a Waters SYNAPTTM G2 HDMS system (Waters Corp., Milford, MA) via an uncoated 15-μm i.d. PicoTip Emitter (New Objective Inc., Woburn, MA). The spray voltage was 3000 V. The mass spectrometer was operated in the positive resolution mode. The instrument was set to perform MSE experiment, a data independent acquisition, which used a low collision energy (4 V) scan for precursor ions followed by an elevated collision energy (ramping from 15 to 45 V) scan for fragment ions. Scan time was 0.9 s. An auxiliary pump was used to spray a solution of 200 fmol/μL Glu-fibrinopeptide B in 50/50 methanol/water with 1 % acetic acid for mass calibration (lockmass channel), at a flow rate of 500 nL/min and sampling every 30 s. The system was tuned for a minimum resolution of 20,000 FWHM and calibrated using a 5 mM sodium formate infusion.
Data analysis for peptide modification identification
The LC-MSE data were processed by BiopharmaLynx 1.3 to identify the modification sites on TetHc. The algorithm processed the MSE spectra based on the following parameters: 1) the precursor monoisotopic ion intensity greater than 100 counts and mass tolerance less than 30 ppm; 2) the fragment monoisotopic ion intensity greater than 50 counts and mass tolerance less than 30 ppm. The search used trypsin digestion with one missed cleavage on the known sequences of TetHc. Cysteine carbamidomethylation was set as a fixed modification, while oxidation on methionine was set as a variable modification. The squarate group (Δm = 133.0) was set as a variable modification on lysine sidechain. Identified modified peptides were confirmed by MSE spectra manually. Modified TetHc from preparation 1 (64 % sequence coverage) contained 9 modified sites and modified protein from preparation 2 (90 % sequence coverage) contained these same 9 with an additional 5 for a total of 14 sites.
Click conjugation of propargyl modified TetHc and oligosialic acids
Squarate-modified TetHc from preparation 1 (9 modified sites, 15 μg in 73.7 μl of 340 μg/mL solution in 1X PBS) was conjugated to an oligosialic acid pool estimated to be DP < 100 [36] (3.4 μg in 2.5 μL). The total reaction was 100 μL total. Click reagents were added: 20 μL DMSO, 10 μL each TBTA, sodium ascorbate, and Cu(I)Br. All reactions were incubated at 37 °C overnight.
To investigate the effect of oligosialic acid chain length on immune response, two glycoconjugates were made. One conjugate was prepared from squarate-modified TetHc (13 modified sites, 10 μg in 13.7 μL of 730 μg/mL solution in PBS) and oligosialic acids from the DP < 47 pool (4 μg in 173.9 μL) in 200 mM sodium cacodylate buffer (200 μL total). The other conjugate was prepared from TetHc (10 μg) and DP < 100 oligosialic acids (4 μg in 127.3 μL). Click reagents were added to all tubes to start the conjugation reactions: 20 μL DMSO, 10 μL each TBTA, sodium ascorbate, and Cu(I)Br. All reactions were incubated at 37 °C overnight.
Immunoblot analysis of TetHc-oligosialic acid conjugates
Formation of meningococcal group C polysaccharide-TetHc conjugates was confirmed by SDS-PAGE followed by Western blot. Polysialylated TetHc protein samples were resolved on a 3–8 % SDS-PAGE gel (Invitrogen). Proteins were transferred to a nitrocellulose membrane and developed as previously described [15]. The immunoblots were developed with 1:10,000 dilution of the mouse monoclonal antibody C2/706.12 against N. meningitidis de-O-acetylated group C polysialic acid [37] and 1:5,000 dilution of rabbit hyperimmune antiserum against TetHc protein.
Mice immunization with TetHc-oligosialic acid conjugates
The TetHc-oligosialic acid conjugates were tested in two separate immunization experiments. Each group of mice (5 mice/group) received 0.2 μg sialic acid/dose with an injection every 2 weeks for 4 weeks total.
Experiment 1 - Two groups of mice were used. One group was injected with free oligosialic acids (DP < 100) and free TetHc. The other group was injected with a glycoconjugate of modified TetHc (9 squarate residues) and DP < 100 oligosialic acids.
Experiment 2 - Oligosialic acid chain length in the glycoconjugate was investigated in 4 groups of mice. Of the 4 groups, one group was injected with free oligosialic acids (DP < 100) and free modified TetHc (14 squarate residues), one group was injected with the corresponding TetHc-oligosialic acids conjugate, one group was injected with free oligosialic acids (DP < 47) and free modified TetHc, and one group was injected with the corresponding conjugate. One week after the final dose, mice were sacrificed, sera were collected and ELISA was performed using native serogroup C polysaccharide to coat plates as described previously [9].
Results and discussion
9-azidononanyllactoside is a substrate of CSTII and NmC PST
We have previously shown that lactoside acceptors containing conjugatable groups at the aglycon, can be sialylated by the enzymes in a chemoenzymatic method for oligosialic acid synthesis [15]. The first step in our chemoenzymatic scheme is sialylation of acceptor by C. jejuni CST II (Fig. 1a). It is critical that the lactoside be disialylated. Once two or more NeuNAc groups are added, the acceptor can be further sialylated by NmC PST. The rate of formation of CST II-sialylated product was dependent upon the structure of the aglycon lactoside acceptor. Lactosides containing short aliphatic chains at the aglycon required hours to react. Alternatively, lactosides containing a large aromatic, hydrophobic aglycon were readily sialylated by CST II in under an hour (Supplemental Fig. 1). Similarly, Willis et al. observed increased reactivity with aminophenylglycoside-based synthetic acceptors [38]. We have chemically synthesized an acceptor containing a 9 carbon linker between the lactoside and azido group in order to increase hydrophobicity of the aglycon (4, Fig. 2). This acceptor was found to be a more robust substrate for CST II when compared to a previously used lactoside containing a 2 carbon linker (Fig. 3).
HPLC chromatogram of sialylation products of the reaction of 2-azidoethylactoside and 9-azidononanyllactoside with CST II. Lactoside acceptors (1 mM) were incubated with C. jejuni CST II (0.04 U) in the presence of 5.0 mM CMP-NeuNAc and 40 mM MnCl in 200 mM sodium cacodylate buffer pH 8.0 for 4 h. For the control reactions, no enzyme was added. Reactions were clicked to fluorescent dye for visualization by HPLC (see Materials and Methods)
CMP-9-deoxy-NeuNAc and CMP-9-fluoro-NeuNAc inhibit oligosialic acid synthesis
Our main objective is to produce vaccines that are well-defined to better understand how glycoconjugate vaccine structure relates to the immune response generated. We sought to modulate the degree of polymerization of oligosialic acids in our synthesis. The approach that proved most successful was use of inhibitors that are similar in structure to the donor molecule CMP-NeuNAc. These inhibitors were added to the reaction after the CST II reaction to allow sufficient accumulation of sialylated acceptor for NmC PST. Initial studies used the competitive inhibitor cytidine diphosphate, CDP. However, large concentrations of CDP (up to 4 mM, results not shown) did not show a significant shift in product distribution from long to shorter length products. This led us to alter our strategy by switching to inhibitors that lead to termination of the growing sialic acid chain. Neisseria serogroup C polysialyltransferase catalyzes growth of the sialic acid chain in an α(2-9) linked fashion. Therefore, donor molecules that contain groups at the 9 position of sialic acid block formation of this glycosidic linkage [36]. We used a sialic acid derivative that contains a fluorine group at this position as well as a derivative that lacks an oxygen group at C-9. These derivatives were converted to their CMP-bound forms by in vitro incubation with the Neisseria CMP-NeuNAc synthetase (see Materials and Methods).
Small scale reactions (volume = 50 μL) were performed to observe the effects of these inhibitors on oligosialic acid production. In the absence of inhibitors, the oligosialic acid distribution included DP 100 [36] with the largest peak eluting at DP 47. When either inhibitor was included in the reaction, CMP-9-fluoro-NeuNAc (Fig. 4a) or CMP-9-deoxy-NeuNAc (Fig. 4b) led to a change in product distribution towards oligosialic acids of shorter length (DP < 47, median DP 25). At both concentrations used in the reactions, CMP-9-deoxy-NeuNAc had a more dramatic effect on product distribution. Thus, this inhibitor was selected for use in larger scale syntheses for vaccine use.
Sialylation of 9-azidononanyllactoside in the absence and presence of donor-like inhibitors. The 9-azidononanyllactoside acceptor (1 mM) was incubated with CST II (0.06 U) in the presence of 2.5 mM CMP-NeuNAc and 40 mM MnCl in 200 mM sodium cacodylate buffer pH 8.0. After 3.5 h, NmC PST (5 μg in 16.7 μL), sodium cacodylate buffer, and either a) CMP-9-fluoro-NeuNAc or b) CMP-9-deoxy-NeuNAc (125 μM or 250 μM) were added to appropriate reactions. Reactions were quenched after 20 h
We scaled up production of oligosialic acids in order to make glycoconjugate vaccines. We first wanted to determine the optimal CST II concentration to use for production of a large amount of sialylated lactoside acceptor. Three enzyme concentrations (0.5, 1.0, and 2.5 U) were tested. The reaction containing 2.5 U CST II gave more highly sialylated material compared to the reaction containing 0.5 U which was primarily di- and tri-sialylated material. We decided to use 2.5 U of CST II in the large scale reaction (Fig. 5a) which was done at 10× the volume of the small scale reaction (500 μL). In order to assure, shorter and longer chain length oligosialic acids were produced, the concentrations of NmC PST were modulated in both reactions. To favor conversion of CST II-sialylated lactosides to longer chain oligosialic acids, 50 μg (10× the small scale amount) of NmC PST enzyme was used (Fig. 5b). To favor production of shorter oligosialic acids, 25 μg NmC PST and 150 μM CMP-9-deoxy-NeuNAc were used (Fig. 5b). There is an expected shift in reaction products toward shorter chain length (DP < 47) in the presence of CMP-9-deoxy-NeuNAc. Oligosialic acids from both reactions were isolated for conjugation with carrier protein, the alkyne-modified tetanus toxin Hc fragment (TetHc).
Large scale production of oligosialylated lactoside acceptor for vaccine use. The chemoenzymatic reaction was performed in a volume of 500 μL. a 9-azidononanyllactoside (1 mM) was incubated with CST II (2.5 U) in the presence of 5.0 mM CMP-NeuNAc and 40 mM MnCl in 200 mM sodium cacodylate buffer pH 8.0 for 3.5 h at 37 °C. b To produce longer chain-length oligosialic acids, NmC PST (50 μg), CMP-NeuNAc (5 mM final), and sodium cacodylate buffer were added to the reaction to give a final volume of 750 μL. b For shorter products, NmC PST (25 μg), CMP-NeuNAc (5 mM final), CMP-9-deoxy-NeuNAc (150 μM), and sodium cacodylate buffer were added to the other reaction. Both reactions progressed for 20 h
Propargyl squarate is incorporated at random sites in the TetHc protein structure
Alkyne groups have been introduced into the carrier protein using squaric acid chemistry. Squaric acid chemistry is a useful method for incorporating desired groups onto proteins via the ε-amino of lysine residues [35, 39] of which TetHc has 34. TetHc protein was incubated with propargyl squarate, an alkyne-containing squaric acid ester. This is the corresponding group for later click chemistry conjugation to sialylated azidolactosides. Initial studies determined that long incubation times and high concentrations of propargyl squarate led to protein aggregation. This suggests that incorporation of too many propargyl squarate residues can affect protein solubility. We prepared alkynyl TetHc under 2 conditions that resulted in a soluble derivatized protein: (a) Soluble protein was prepared using a 2.4:1 excess of lysine to squarate with a 24 h incubation. This preparation was subjected to trypsin digestion and LC-MS/MSE analysis in order to determine the locations of these modifications more precisely. There was an average of 9 propargyl squarate residues incorporated at sites dispersed throughout the structure of the protein suggesting a random reaction with available lysines; (b) Preparation 2 of TetHc was done using a shorter incubation time (17 h) but a 3.7 excess of squarate to lysine, yielding a soluble protein with 14 lysines modified. LC-MS/MSE analysis revealed the same 9 sites of incorporation in both preparations, but with an additional 5 modified sites (Fig. 6, Supplemental Table 1) modified in the condition with excess squarate. It is possible that these 9 modified sites may be the most reactive lysines in the TetHc protein. This represents the first report of the mapping of reactive lysines in a tetanus Hc carrier protein.
Propargyl squarate modification sites of TetHc protein. TetHc protein was subject to trypsin digestion followed by LC-MS/MSE analysis to determine modified lysine residues (see Materials and Methods). The 9 shared sites of lysine modification between TetHc preparations are highlighted in blue while the 5 additional sites are highlighted in grey on the crystal structure (pdb ID: 1a8d). Peptide sequence is shown with observed tryptic fragments underlined. Modified lysine residues are highlighted in bold type. Total sequence coverage is 91.6 %
Immunoreactivity of the TetHc glycoconjugate
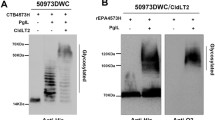
Alkyne modified-TetHc protein (containing 14 squarate residues) was conjugated to the DP < 47 and DP < 100 oligosialic acids by incubation of 10 μg modified Hc protein and 4 μg of sialic acid overnight with click reagents. The glycoconjugates were assessed by SDS-PAGE as well as Western blot using monoclonal antibodies against TetHc and against serogroup C polysaccharide. After the click reaction, the TetHc band has completely disappeared suggesting full incorporation of sialylated azidolactosides to alkyne-squarate modified sites (Fig. 7a). In the lanes containing glycoconjugates, there is visible smearing at higher molecular weight indicating conjugate formation. The glycoconjugates were reactive to antibodies against and TetHc (Fig. 7b) and serogroup C polysaccharide (Fig. 7c). The Western blots clearly demonstrate two important facts. First, the chemoenzymatic reactions form α(2-9) linked polysialic acid that is reactive with specific antibody regardless of the presence of CMP-NeuNAc analogues. Secondly, although TetHc has been subjected to alkyne-squarate modification, it is still recognized by the antibody against unmodified TetHc suggesting that the epitopes are still exposed.
Assessment of glycoconjugate formation by SDS-PAGE Western blot analysis. Conjugation of chemoenzymatically-produced oligosialic acids and propargyl-squarate modified TetHc was performed by the click reaction (see Materials and Methods). SDS-PAGE followed by Western blot was performed using antibody against TetHc protein and serogroup C polysaccharide. a Coomassie blue stained SDS-PAGE gel: Western blot using TetHc-specific antibody: Lane 1 = MW marker, Lane 2 = unmodified TetHc, Lane 3 = modified TetHc, Lane 4 = Glycoconjugate containing DP < 100 oligosialic acids, Lane 5 = Glycoconjugate containing DP < 47 oligosialic acids. b Western blot using TetHc-specific antibody with same configuration as A. c Western blot using group C polysaccharide-specific antibody: Lane 1 = unmodified TetHc, Lane 2 = MW marker, Lane 3 = modified TetHc, Lane 4 = Glycoconjugate containing DP < 100 oligosialic acids, Lane 5 = Glycoconjugate containing DP < 47 oligosialic acids
Immunogenicity of sialylated 9-azidononyllactoside-TetHc glycoconjugates
Chemoenzymatically-produced glycoconjugates were used in immunization studies to assess the ability of these vaccines to elicit an immune response. In one study of a glycoconjugate of modified TetHc (9 squarate residues) and DP < 100 oligosialic acids, 2 groups of mice were used. Each group, containing 5 mice, received 0.2 μg sialic acid per dose with an injection every 2 weeks (4 weeks total). One group was injected with free oligosialic acids (DP < 100) and free TetHc while the other group was injected with the conjugate of modified TetHc-oligosialic acids. One week after the final dose, sera were collected from sacrificed mice and assayed by ELISA. Sera from mice immunized with the glycoconjugate showed higher reactivity against serogroup C polysaccharides than sera from mice receiving unconjugated oligosaccharides (geometric mean 170.4 vs. 2.6, Fig. 8a). For the control dosage, 3 out of 5 mice had no detectable antibody response.
Glycoconjugates are immunogenic in mice. The geometric means of polysaccharide-specific antibody levels are shown on a logarithmic plot with T-bars indicating 95 % confidence intervals. a In the first trial, 2 groups of mice (n = 5) were immunized. One group received a control dosage containing unconjugated oligosaccharides and native Hc protein. The second group was immunized with glycoconjugates of modified TetHc (9 squarate modifications) and oligosialic acids of DP < 100. b In the second trial, 4 groups of mice (n = 5) were immunized. One group received a control dosage of containing unconjugated DP < 100 oligosialic acids and free modified TetHc (14 squarate residues). The second group was injected with the corresponding modified TetHc-oligosialic acid conjugate. The third group was injected with a control dosage containing free DP < 47 oligosialic acids and free modified TetHc. The fourth group was immunized with the corresponding conjugate. Mice received injections (0.2 μg sialic acid/dose) every 2 weeks for 4 weeks total. One week after the final dose, mice were sacrificed, sera were collected, and ELISA was performed
In another experiment, the effect of oligosialic acid chain length in the glycoconjugate was investigated in 4 groups of mice (5 mice/group). Of the 4 groups, 1 group was injected with free uninhibited oligosialic acids (DP < 100) and free modified TetHc (14 squarate residues), 1 group was injected with the corresponding TetHc-oligosialic acids conjugate, 1 group was injected with free inhibited oligosialic acids (DP < 47) and free modified TetHc, and 1 group was injected with the corresponding conjugate. Each group received 0.2 μg sialic acid per dose with an injection every 2 weeks (4 weeks total). Sera from mice immunized with glycoconjugates containing DP < 100 oligosialic acids were more reactive to group C polysaccharide than the control mice receiving free DP < 100 oligosialic acids (geometric mean 827.3 vs. 52.4) (Fig. 8b). For the control group immunized with average DP < 47 oligosialic acids, 4 out of 5 mice had no detectable antibody response. In the case of the glycoconjugate containing DP < 47 oligosialic acids, there is still a marked difference in immune response compared to control (geometric mean 838.4 vs. 1.5). Comparison of the antibody titers produced by glycoconjugates in this trial suggests no substantial difference in immune response between mice receiving longer or shorter saccharide length glycoconjugates. Overall, polysaccharide- reactive IgG levels were higher for the glycoconjugate containing 14 modification sites compared to the glycoconjugate containing less. Taken together, both immunization trials indicate that these chemoenzymatically produced glycoconjugates are capable of eliciting an immune response in vivo.
Conclusions and future work
Our overall goal is to produce well-defined glycoconjugate vaccine candidates that can be used to explore the relationship between vaccine structure and immune response. The chemoenzymatic methods described in this work are a significant step in this direction. This work has shown that our methods can be used to make glycoconjugates that elicit an immune response in vivo. We have used CMP-NeuNAc analogs to modulate the chain length of the enzymatically produced oligosialic acids. The approach outlined here allows for the construction of oligosialic acids modified at positions other than C-9 as tools to investigate the role of sialic acid structure in interaction with the immune system. Pozsgay, Robbins, and Schneerson observed that the immunogenicity of glycoconjugates of human serum albumin (HSA) and synthetic O-specific polysaccharide domain of Shigella lipopolysaccharide was dependent on both oligosaccharide chain length and the number of modification sites [40]. Our chemoenzymatic strategy should allow further investigation of the impact of these two characteristics on immunogenicity.
One possible alternative for alkyne incorporation into the carrier protein is the use of non-natural amino acids. At the very beginning of this work, our goal was to incorporate azidohomoalanine into TetHc and chemoenzymatically produce oligosialic acids on an alkyne-containing lactoside acceptor. However, this alkynyl lactoside proved to be a poorer substrate for CST II than azidononanyllactosides. In this work, alkyne groups were incorporated into the carrier protein chemically and the sites of modification where mapped through proteolytic cleavage and mass spectrometry. An examination of suitable ways to incorporate an alkyne-containing non-natural amino acid into TetHc may be useful in future experiments.
References
McIntyre, P.B., O’Brien, K.L., Greenwood, B., van de Beek, D.: Effect of vaccines on bacterial meningitis worldwide. Lancet 380(9854), 1703–1711 (2012)
Horwitz, M.A., Silverstein, S.C.: Influence of the Escherichia coli capsule on complement fixation and on phagocytosis and killing by human phagocytes. J. Clin. Invest. 65(1), 82–94 (1980)
Pluschke, G., Mayden, J., Achtman, M., Levine, R.P.: Role of the capsule and the O antigen in resistance of O18:K1 Escherichia coli to complement-mediated killing. Infect. Immun. 42(3), 907–913 (1983)
Alvarez, D., Merino, S., Tomas, J.M., Benedi, V.J., Alberti, S.: Capsular polysaccharide is a major complement resistance factor in lipopolysaccharide O side chain-deficient Klebsiella pneumoniae clinical isolates. Infect. Immun. 68(2), 953–955 (2000)
Astronomo, R.D., Burton, D.R.: Carbohydrate vaccines: developing sweet solutions to sticky situations? Nat. Rev. Drug. Discov. 9(4), 308–324 (2010)
Mitchison, N.A.: The carrier effect in the secondary response to hapten-protein conjugates. II. Cellular cooperation. Eur. J. Immunol. 1(1), 18–27 (1971)
Bardotti, A., Averani, G., Berti, F., Berti, S., Carinci, V., D’Ascenzi, S., Fabbri, B., Giannini, S., Giannozzi, A., Magagnoli, C., Proietti, D., Norelli, F., Rappuoli, R., Ricci, S., Costantino, P.: Physicochemical characterisation of glycoconjugate vaccines for prevention of meningococcal diseases. Vaccine 26(18), 2284–2296 (2008)
Lees, A., Nelson, B.L., Mond, J.J.: Activation of soluble polysaccharides with 1-cyano-4-dimethylaminopyridinium tetrafluoroborate for use in protein-polysaccharide conjugate vaccines and immunological reagents. Vaccine 14(3), 190–198 (1996)
Lee, C.H., Kuo, W.C., Beri, S., Kapre, S., Joshi, J.S., Bouveret, N., LaForce, F.M., Frasch, C.E.: Preparation and characterization of an immunogenic meningococcal group A conjugate vaccine for use in Africa. Vaccine 27(5), 726–732 (2009)
Guerry, P., Szymanski, C.M.: Campylobacter sugars sticking out. Trends Microbiol. 16(9), 428–435 (2008)
Feldman, M.F., Wacker, M., Hernandez, M., Hitchen, P.G., Marolda, C.L., Kowarik, M., Morris, H.R., Dell, A., Valvano, M.A., Aebi, M.: Engineering N-linked protein glycosylation with diverse O antigen lipopolysaccharide structures in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 102(8), 3016–3021 (2005)
Schwarz, F., Huang, W., Li, C., Schulz, B.L., Lizak, C., Palumbo, A., Numao, S., Neri, D., Aebi, M., Wang, L.X.: A combined method for producing homogeneous glycoproteins with eukaryotic N-glycosylation. Nat. Chem. Biol. 6(4), 264–266 (2010)
Wacker, M., Linton, D., Hitchen, P.G., Nita-Lazar, M., Haslam, S.M., North, S.J., Panico, M., Morris, H.R., Dell, A., Wren, B.W., Aebi, M.: N-linked glycosylation in Campylobacter jejuni and its functional transfer into E. coli. Science 298(5599), 1790–1793 (2002)
Wetter, M., Kowarik, M., Steffen, M., Carranza, P., Corradin, G., Wacker, M.: Engineering, conjugation, and immunogenicity assessment of Escherichia coli O121 O antigen for its potential use as a typhoid vaccine component. Glycoconj. J. 30, 511–522 (2013)
Mosley, S.L., Rancy, P.C., Peterson, D.C., Vionnet, J., Saksena, R., Vann, W.F.: Chemoenzymatic synthesis of conjugatable oligosialic acids. Biocatal. Biotransform. 28(1), 41–50 (2010)
Chiu, C.P., Watts, A.G., Lairson, L.L., Gilbert, M., Lim, D., Wakarchuk, W.W., Withers, S.G., Strynadka, N.C.: Structural analysis of the sialyltransferase CstII from Campylobacter jejuni in complex with a substrate analog. Nat. Struct. Mol. Biol. 11(2), 163–170 (2004)
Peterson, D.C., Arakere, G., Vionnet, J., McCarthy, P.C., Vann, W.F.: Characterization and acceptor preference of a soluble meningococcal group C polysialyltransferase. J. Bacteriol. 193(7), 1576–1582 (2011)
Kolb, H.C., Finn, M.G., Sharpless, K.B.: Click chemistry: diverse chemical function from a few good reactions. Angew Chem. Int. Ed. Engl. 40(11), 2004–2021 (2001)
Bongat, A.F., Saksena, R., Adamo, R., Fujimoto, Y., Shiokawa, Z., Peterson, D.C., Fukase, K., Vann, W.F., Kovac, P.: Multimeric bivalent immunogens from recombinant tetanus toxin HC fragment, synthetic hexasaccharides, and a glycopeptide adjuvant. Glycoconj. J. 27(1), 69–77 (2010)
Helting, T.B., Zwisler, O.: Structure of tetanus toxin. I. Breakdown of the toxin molecule and discrimination between polypeptide fragments. J. Biol. Chem. 252(1), 187–193 (1977)
Thaysen-Andersen, M., Jorgensen, S.B., Wilhelmsen, E.S., Petersen, J.W., Hojrup, P.: Investigation of the detoxification mechanism of formaldehyde-treated tetanus toxin. Vaccine 25(12), 2213–2227 (2007)
Umland, T.C., Wingert, L.M., Swaminathan, S., Furey, W.F., Schmidt, J.J., Sax, M.: Structure of the receptor binding fragment HC of tetanus neurotoxin. Nat. Struct. Biol. 4(10), 788–792 (1997)
Fairweather, N.F., Chatfield, S.N., Makoff, A.J., Strugnell, R.A., Bester, J., Maskell, D.J., Dougan, G.: Oral vaccination of mice against tetanus by use of a live attenuated Salmonella carrier. Infect. Immun. 58(5), 1323–1326 (1990)
Tregoning, J.S., Nixon, P., Kuroda, H., Svab, Z., Clare, S., Bowe, F., Fairweather, N., Ytterberg, J., van Wijk, K.J., Dougan, G., Maliga, P.: Expression of tetanus toxin fragment C in tobacco chloroplasts. Nucleic Acids Res. 31(4), 1174–1179 (2003)
Makoff, A.J., Oxer, M.D., Romanos, M.A., Fairweather, N.F., Ballantine, S.: Expression of tetanus toxin fragment C in E. coli: high level expression by removing rare codons. Nucleic Acids Res. 17(24), 10191–10202 (1989)
Anderson, R., Gao, X.M., Papakonstantinopoulou, A., Roberts, M., Dougan, G.: Immune response in mice following immunization with DNA encoding fragment C of tetanus toxin. Infect. Immun. 64(8), 3168–3173 (1996)
Liu, M.-Z., Lee, Y.C.: Comparison of chemical and enzymatic synthesis of 2-acetamido-2-deoxy-d-mannose 6-phosphate: a new approach. Carbohydr. Res. 330(3), 413–419 (2001)
Sundaram, A.K., Pitts, L., Muhammad, K., Wu, J., Betenbaugh, M., Woodard, R.W., Vann, W.F.: Characterization of N-acetylneuraminic acid synthase isoenzyme 1 from Campylobacter jejuni. Biochem. J. 383(Pt 1), 83–89 (2004)
Blacklow, R.S., Warren, L.: Biosynthesis of sialic acids by Neisseria meningitidis. J. Biol. Chem. 237, 3520–3526 (1962)
Karwaski, M.-F., Wakarchuk, W.W., Gilbert, M.: High-level expression of recombinant Neisseria CMP-sialic acid synthetase in Escherichia coli. Protein Expr. Purif. 25(2), 237–240 (2002)
Mei, X., Heng, L., Fu, M., Li, Z., Ning, J.: Synthesis of beta-D-Glcp-(1– > 3)-[beta-D-Glcp-(1– > 6)]-beta-D-Glcp-(1– > 3)-beta-D-Glcp- (1– > 6)-[beta-D-Galp-(1– > 4)-beta-D-Glcp-(1– > 3)]-beta-D-GlcpOLauryl, an oligosaccharide with anti-tumor activity. Carbohydr. Res. 340(15), 2345–2351 (2005)
Sawa, M., Hsu, T.L., Itoh, T., Sugiyama, M., Hanson, S.R., Vogt, P.K., Wong, C.H.: Glycoproteomic probes for fluorescent imaging of fucosylated glycans in vivo. Proc. Natl. Acad. Sci. U. S. A. 103(33), 12371–12376 (2006)
Inoue, S., Inoue, Y.: Ultrasensitive analysis of sialic acids and oligo/polysialic acids by fluorometric high-performance liquid chromatography. Methods Enzymol. 362, 543–560 (2003)
Inoue, S., Lin, S.L., Lee, Y.C., Inoue, Y.: An ultrasensitive chemical method for polysialic acid analysis. Glycobiology 11(9), 759–767 (2001)
Hou, S.J., Saksena, R., Kovac, P.: Preparation of glycoconjugates by dialkyl squarate chemistry revisited. Carbohydr. Res. 343(2), 196–210 (2008)
Vionnet, J., Vann, W.F.: Successive glycosyltransfer of sialic acid by Escherichia coli K92 polysialyltransferase in elongation of oligosialic acceptors. Glycobiology 17(7), 735–743 (2007)
Garcia-Ojeda, P.A., Monser, M.E., Rubinstein, L.J., Jennings, H.J., Stein, K.E.: Murine immune response to Neisseria meningitidis group C capsular polysaccharide: analysis of monoclonal antibodies generated in response to a thymus-independent antigen and a thymus-dependent toxoid conjugate vaccine. Infect. Immun. 68(1), 239–246 (2000)
Willis, L.M., Gilbert, M., Karwaski, M.F., Blanchard, M.C., Wakarchuk, W.W.: Characterization of the alpha-2,8-polysialyltransferase from Neisseria meningitidis with synthetic acceptors, and the development of a self-priming polysialyltransferase fusion enzyme. Glycobiology 18(2), 177–186 (2008)
Jahouh, F., Hou, S.J., Kovac, P., Banoub, J.H.: Determination of glycation sites by tandem mass spectrometry in a synthetic lactose-bovine serum albumin conjugate, a vaccine model prepared by dialkyl squarate chemistry. Rapid Commun. Mass Spectrom. 26(7), 749–758 (2012)
Pozsgay, V., Chu, C., Pannell, L., Wolfe, J., Robbins, J.B., Schneerson, R.: Protein conjugates of synthetic saccharides elicit higher levels of serum IgG lipopolysaccharide antibodies in mice than do those of the O-specific polysaccharide from Shigella dysenteriae type 1. Proc. Natl. Acad. Sci. U. S. A. 96(9), 5194–5197 (1999)
Acknowledgments
P.C.M was supported by postdoctoral fellowships from the Oak Ridge Institute for Science and Education and the National Institutes of Health Pharmacology Research Associate Training (PRAT) Program. The authors acknowledge Justine Vionnet and Dr. Karen Muindi for technical assistance. The authors also acknowledge Drs. Daron Freedberg and Marcos Battistel for HSQC NMR spectra.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supp. Table 1
Peptides detected by LC/MS-MSE analysis containing squarate modification sites (PDF 34 kb)
Supp. Fig. 1
Comparison of reaction rates of aromatic acceptors and aliphatic acceptors (PDF 159 kb)
Supp. Fig. 2
HSQC NMR data of 9-azidononanyllactoside with assignments (PDF 111 kb)
Rights and permissions
About this article
Cite this article
McCarthy, P.C., Saksena, R., Peterson, D.C. et al. Chemoenzymatic synthesis of immunogenic meningococcal group C polysialic acid-tetanus Hc fragment glycoconjugates. Glycoconj J 30, 857–870 (2013). https://doi.org/10.1007/s10719-013-9490-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-013-9490-x