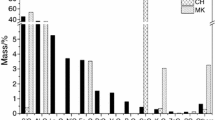
It is established by calculating the equilibrium partial pressure of carbon dioxide gas \({p}_{{\mathrm{CO}}_{2}}\) or the equilibrium activity of carbonation \(\left[{\mathrm{CO}}_{3}^{2-}\right]\) in the carbonation reactions of various mineral compounds that practically all investigated compounds are capable of carbonation under the influence of dry or wet carbon dioxide with the formation of calcium or magnesium carbonates. The comparative thermodynamic activity of certain mineral compounds in the carbonation reactions is much higher than that of the minerals wollastonite CS and rankinite C3S2, which are present in clinker for the production of carbonate hardening cement Solidia.


Similar content being viewed by others
References
K. Xia, Gu Yue, Linhua Jiang, et al. “Carbonation resistance of surface protective materials modified with hybrid NanoSiO2,” Coatings, 11(3), 269 (2021).
B. J. Zhan, D. Xuan, C. Poon, and C. Shi, “Mechanism for rapid hardening of cement pastes under coupled CO2-water curing regime,” Cement and Concrete Composites, 97, 78 – 88 (2019).
Y. Fang and J. Chang, “Rapid hardening â-C2S mineral and microstructure changes activated by accelerated carbonation curing,” J. Thermal Anal. Calorim., 129(2), 681 – 689 (2017).
I. V. Korchunov and E. N. Potapova, “Phase composition of CO2-hardened cement in the presence of chloride ions,” Mater. Today: Proc., 38, 1963 – 1967 (2021).
V. Meyer, N. de Cristofaro, J. Bryant, and S. Sahu, “Solidia cement an example of carbon capture and utilization,” Key Engi. Mater., 761, 197 – 203 (2018).
X. Xian, D. Zhang, and Y. Shao, “Flue gas carbonation curing of cement paste and concrete at ambient pressure,” J. Cleaner Prod., 313, 127943 (2021).
L.Wang, L. Chen, JL Provis, et al., “Accelerated carbonation of reactive MgO and Portland cement blends under flowing CO2 gas,” Cement and Concrete Composites, 106, 103489 (2020).
L. Wang, L. Chen, D. C. W. Tsang, et al., “Biochar as green additives in cement-based composites with carbon dioxide curing,” J. Cleaner Prod., 258, 120678 (2020).
R. S. Lin, Wang Xiao-Yong, and Yi-han, “Effects of cement types and addition of quartz and limestone on the normal and carbonation curing of cement paste,” Constr. Building Mater., 305, 124799 (2021).
D. Zhang, V. C. Li, and B. R. Ellis, “Optimal pre-hydration age for CO2 sequestration through Portland cement carbonation,” ACS Sustainable Chem. Eng., 6(12), 15976 – 15981 (2018).
N. Lippiatt, T. C. Ling, and S. Eggermont, “Combining hydration and carbonation of cement using super-saturated aqueous CO2 solution,” Constr. Building Mater., 229, 116825 (2019).
A. N. Junior, R. D. T. Filho, E. M. R. Fairbairn, and J. Dweck, “CO2 sequestration by high initial strength Portland cement pastes,” J. Thermal Anal. Calorim., 113(3), 1577 – 1584 (2013).
S. Siddique, A. Naqi, and Naqi J. G. Naqi, “Influence of water to cement ratio on CO2 uptake capacity of belite-rich cement upon exposure to carbonation curing,” Cement and Concrete Composites, 111, 103616 (2020).
V. Rostami, Y. Shao, and A. J. Boyd, “Microstructure of cement paste subject to early carbonation curing,” Cement Concrete Res., 42(1), 186 – 193 (2012).
J. G. Jang and H. K. Lee, “Microstructural densification and CO2 uptake promoted by the carbonation curing of belite-rich Portland cement,” Cement Concrete Res., 82, 50 – 57 (2016).
J. Ibáñez, Lluís Artús, Ramon Cuscó, et al., “Hydration and carbonation of monoclinic C2S and C3S studied by Raman spectroscopy,” J. Raman Spectrosc.: An International Journal for Original Work in all Aspects of Raman Spectroscopy, Including Higher Order Processes, and also Brillouin and Rayleigh Scattering, 38(1), 61 – 67 (2007).
Solidia. Solutions. URL: https://www.solidiatech.com/solutions.html (accessed 12/13/2021).
Solidia Life Project. URL: https://www.solidlife.eu/ (date of access: 12/13/2021).
V. I. Babushkin, G. M. Matveev, O. P. Mchedlov-Petrosyan, Thermodynamics of Silicates [in Russian], Stroiizdat, Moscow (1986).
B. Lottenbach, DA Kulik, T. Matschei, et al. “Cemdata18: A chemical thermodynamic database for hydrated Portland cements and alkali-activated materials,” Cement Concrete Res., 115, 472 – 506 (2018).
T. Matschei, B. Lothenbach, and F. P. Glasser, “Thermodynamic properties of Portland cement hydrates in the system CaO–Al2O3–SiO2–CaSO4–CaCO3–H2O,” Cement Concrete Res., 37(10), 1379 – 1410 (2007).
M. E. Parron-Rubio, F. Perez-Garcia, A. Gonzalez-Herrera, et al., “Slag substitution as a cementing material in concrete: Mechanical, physical and environmental properties,” Materials, 12(18), 2845 (2019).
I. V. Korchunov, E. A. Dmitrieva, and E. N. Potapova, “Structural features of a cement matrix modified with additives of sedimentary origin,” Mater. Sci. Eng., 1083(1), 012033 (2021).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Steklo i Keramika, No. 9, pp. 34 – 43, September, 2022.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sivkov, S.P., Korchunov, I.V., Potapova, E.N. et al. Activity Thermodynamics of Compounds in Carbonation-Hydration Hardening Cements. Glass Ceram 79, 371–377 (2023). https://doi.org/10.1007/s10717-023-00516-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10717-023-00516-6