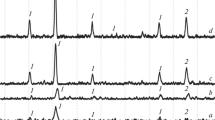
The effect of mechanochemical activation on the formation of a cobalt ferrite phase from iron and cobalt oxalates was studied. X-ray phase, x-ray diffraction, and simultaneous thermal analysis, scanning electron microscopy, and the low-temperature nitrogen adsorption-desorption method were used to study the composition and properties of the resulting product. It was established that on solid-phase interaction of the initial components cobalt ferrite is formed at 1100°C. Preliminary mechanochemical activation of the initial components in a roll-ring vibratory mill makes it possible to reduce the temperature of cobalt ferrite synthesis to 400°C. The properties of cobalt ferrite can be improved by lowering its synthesis temperature. It is shown that CoFe2O4 obtained by the mechanochemical method of synthesis has a more developed specific surface area and porous structure compared to cobalt ferrite obtained by solid-phase interaction of the initial components without pretreatment.





Similar content being viewed by others
References
C. Kaçar, B. Dalkiran, P. E. Erden, and E. Kiliç, “An amperometric hydrogen peroxide biosensor based on Co3O4 nanoparticles and multiwalled carbon nanotube modified glassy carbon electrode,” J. Appl. Surf. Sci., 311, 139 – 146 (2014). DOI: https://doi.org/10.1016/j.msec.2011.10.028
F. A. Tourinho, R. Franck, and R. Massart, “Aqueous ferrofluids based on manganese and cobalt ferrites,” J. Mater. Sci., 25(7), 3249 – 3254 (1990).
V. Cabuil, V. Dupuis, D. Talbot, and S. Neveu, “Ionic magnetic fluid based on cobalt ferrite nanoparticles: influence of hydrothermal treatment on the nanoparticle size,” J. Magn. Magn. Mater., 323(10), 1238 – 1241 (2011). DOI: https://doi.org/10.1016/j.jmmm.2010.11.013
J. B. Silva, C. F. Diniz, R. M. Lago, and N. D. Mohallem, “Catalytic properties of nanocomposites based on cobalt ferrites dispersed in sol-gel silica,” J. Non-Cryst. Solids, 348, 201 – 204 (2004).
A. A. Thomas, A. Pietro, M. Emanuela, et al., “Synthesis and characterization of copper ferrite magnetic nanoparticles by hydrothermal route,” J. Chem. Eng. Trans., 47, 151 – 156 (2016). DOI: https://doi.org/10.3303/CET1647026
Z. Zi, Y. Sun, X. Zhu, and W. Song, “Synthesis and magnetic properties of CoFe2O4 ferrite nanoparticles,” J. Magn. Magn. Mater., 321, 1251 – 1256 (2009).
V. Gopalan, P. A. Joy, I. A. Al-Omari, et al., “On the structural, magnetic and electrical properties of sol–gel derived nanosized cobalt ferrite,” J. Alloys Compd., 485 711 – 718 (2009).
T. P. Braga, B. M. C. Sales, A. N. Pinheiro, et al., “Catalytic properties of cobalt and nickel ferrites dispersed in mesoporous silicon oxide for ethylbenzene dehydrogenation with CO2,” J. Catal. Sci. Technol., 1(8), 1383 – 1392 (2011). DOI: https://doi.org/10.1039/C1CY00176K
A. A. Magaeva, E. P. Naiden, O. G. Terekhova, et al., “Mechanochemical synthesis, phase composition, structural parameters, and magnetic properties of manganese ferrospinels,” J. Nanotechnologies in Russia, 8(7 – 8), 495 – 501 (2013). DOI: https://doi.org/10.1134/S1995078013040083
L. J. Berchmans, R. Karthikeyan, M. Helan, et al., “Mechanochemical synthesis and electrochemical characterization of nano crystalline calcium ferrite,” J. Catal. Lett., 141(10), 1451 – 1457 (2011). DOI: https://doi.org/10.1007/s10562-011-0636-9
E. Manova, D. Paneva, B. Kunev, et al., “Mechanochemical synthesis and characterization of nanodimensional iron-cobalt spinel oxides,” J. Alloys Compd., 485(1 – 2), 356 – 361 (2009). DOI: https://doi.org/10.1016/j.jallcom.2009.05.107
K. O. Denisova, A. A. Ilyin, R. N. Rumyantsev, et al., “Low-temperature synthesis and catalytic activity of cobalt ferrite in nitrous oxide (N2O) decomposition reaction,” J. Catalysts, 11(8), 889 (2021). DOI: https://doi.org/10.3390/catal11080889
T. Ekström, C. Chatfield, W. Wruss, M. Maly-Schreiber, “The use of x-ray diffraction peak-broadening analysis to characterize ground Al2O3 powders,” J. Mater. Sci., 20(4), 1266 – 1274 (1985).
H. Heegn, “On the connection between ultrafine grinding and mechanical activation of minerals,” J. Aufbereitungs-technik, 30(10), 635 – 642 (1989).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Steklo i Keramika, No. 1, pp. 21 – 30, January, 2022.
Rights and permissions
About this article
Cite this article
Ptitsyna, K.O., Il’in, A.A., Rumyantsev, R.N. et al. Mechanochemical and Ceramic Synthesis of Cobalt Ferrite. Glass Ceram 79, 15–21 (2022). https://doi.org/10.1007/s10717-022-00446-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10717-022-00446-9