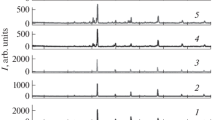
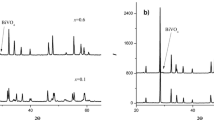
The structure and thermophysical properties of materials formed in the system Dy2O3–HfO2 (molar ratio 1 : 3 to 3 : 1) as a result of isothermal firing of x-ray amorphous mixed hydroxides at temperature to 1600°C are investigated. It is shown that for ratios 1 : 3 to 1 : 1 the crystallization process results in the formation of single-phase solid solutions with the structure of defective fluorite and marked nonequivalence of the parameters of the local environment of the Dy and Hf atoms. It is determined that the ceramic based on dysprosium hafnate (Dy2O3: HfO2 = 1 : 1) possesses low, practically temperature independent (to 800°C), thermal conductivity about 1.4 W/(m · K).






Similar content being viewed by others
References
M. A. Subramanian, G. Aravamudan, and G. V. Subba Rao, “Oxide pyrochlores – A review,” Prog. Solid State Chem., 15(2), 55 – 143 (1983).
P. A. Arsen’ev, V. B. Glushkova, A. A. Evdokimov, et al., Compounds of Rare Earth Elements: Zirconates, Hafnates, Niobates, Tantalates, Antimonides [in Russian], Nauka, Moscow (1985).
V. N. Vladimirov, E. S. Lukin, N. A. Popova, et al., “New types of refractory heat-insulation materials for long-term use at extremely high temperature,” Steklo Keram., No. 4, 14 – 21 (2011); V. S. Vladimirov, E. S. Lukin, N. A. Popova, et al., “New types of light-weight refractory and heat-insulation materials for long-term use at extremely high temperatures,” Glass Ceram., 68(3 – 4), 116 – 122 (2011).
A. V. Shlyakhtina and L. G. Shcherbakova, “New solid electrolytes of the pyrochlore family,” Russ. J. Electrochem., 48(1), 1 – 25 (2012).
V. D. Risovany, A. V. Zakharov, E. M. Muraleva, et al., “Dysprosium hafnate as absorbing material for control rods,” J. Nucl. Mater., 355(1), 163 – 170 (2006).
R. C. Ewing, W. J. Weber, and J. Lian, “Nuclear waste disposal–pyrochlore A2B2O7: Nuclear waste form for the immobilization of plutonium and ‘minor’ actinides,” J. Appl. Phys., 95(11), 5949 – 5971 (2004).
J. S. Gardner, M. J. P. Gingras, and J. E. Greedan, “Magnetic pyrochlore oxides,” Rev. Modern Phys., 82(1), 53 – 107 (2010).
V. V. Popov, A. P. Menushenkov, Ya. V. Zubavichus, et al., “Characteristic features of the nanocrystalline structure formation in Ln2Hf2O7 (Ln = Gd, Dy) compounds,” Russ. J. Inorg. Chem., 58(12), 1400 – 1407 (2013).
J. Emsley, The Elements [Russian translation], Mir, Moscow (1993).
E. R. Andrievskaya, “Phase equilibria in the refractory oxide systems of zirconia, hafnia and yttria with the rare-earth oxides,” J. Eur. Ceram. Soc., 28(12), 2363 – 2388 (2008).
C. R. Stanek and R. W. Grimes, “Prediction of rare-earth A2Hf2O7 pyrochlore phases,” J. Am. Ceram. Soc., 85(8), 2139 – 2141 (2002).
X. T. Zu, N. Li, and F. Gao, “First-principles study of structural and energetic properties of A2Hf2O7 (A = Dy, Ho, Er) compounds,” J. Appl. Phys., 104, 043517(4) (2008).
B. P. Mandal, N. Garg, and S. M. Sarma, “Preparation, XRD and Raman spectroscopic studies on new compounds E2Hf2O7 (RE = Dy, Ho, Er, Tm, Lu, Y): Pyrochlores or defect-fluorite?,” J. Solid State Chem., 179(7), 1990 – 1994 (2006).
V. V. Popov, Ya. V. Zubavichus, A. P. Menushenkov, et al., “Lanthanide effect on the formation and evolution of nanocrystalline structures in Ln2Hf2O7 compounds (Ln = Sm–Dy),” Russ. J. Inorg. Chem., 60(1), 16 – 22 (2015).
A. P. Hammersley, S. O. Svensson, M. Hanfland, et al., “Two-dimensional detector software: From real detector to idealized image or two-theta scan,” High Press. Res., 14(4 – 6), 235 – 248 (1996).
V. Petricek, M. Dusek, L. Palatinus, Jana: The Crystallographic Computing System, Inst. Physics, Praha, Czech. Republic (2006).
V. V. Popov, A. P. Menushenkov, Ya. V. Zubavichus, et al., “Trends in formation of the nanocrystalline structure and cationic ordering in the Dy2O3–HfO2 (1 : 1) system,” Russ. J. Inorg. Chem., 58(3), 331 – 337 (2013).
V. V. Popov, V. F. Petrunin, and S. A. Korovin, Method of Obtaining Nanocrystalline Powders and Ceramic Materials Based on Mixed Oxides of Rare-Earth Elements and Methods of Subgroup IVB, RF Patent 2467983, IPC C04B 35/46; published Nov. 27, 2012.
A. V. Belyakov and E. B. Bendovskii, “Fabrication of single-phase dense ceramic from high-sintering complex oxides,” Steklo Keram., No. 6, 23 – 28 (2015); A. V. Belyakov and E. B. Bendovskii, “Fabrication of single-phase dense ceramic from high-sintering complex oxides new types of light-weight refractory and heat-insulation materials for long-term use at extremely high temperatures,” Glass Ceram., 72(5 – 6), 206 – 211 (2015).
G. Panneerselvam, R. Venkata Krishnan, N. Nagarajan, et al., “Thermal expansion and heat capacity of dysprosium hafnate,” J. Therm. Anal. Calorim., 101(1), 169 – 173 (2010).
V. G. Toporova, V. V. Pimenov, V. D. Risovanyi, et al., “Results of SM reactor tests of dysprosium hafnate,” Atomic Energy, 110(4), 259 – 264 (2011).
The Russian Scientific Foundation provided partial support for this work under grant No. 14-22-00098.
We thank Doctor of Chemical Sciences Profession A. V. Belyakov who was deeply familiar with the material presented here and made a number of valuable remarks.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Steklo i Keramika, No. 2, pp. 11 – 17, February, 2016.
Rights and permissions
About this article
Cite this article
Popov, V.V., Menushenkov, A.P., Zubavichus, Y.V. et al. Structural Characteristics and Thermophysical Properties of Complex Ceramic Oxides in the System Dy2O3–HfO2 . Glass Ceram 73, 47–52 (2016). https://doi.org/10.1007/s10717-016-9823-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10717-016-9823-x