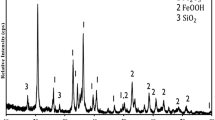
A two-step technological scheme for removing iron from porcelain stone is presented: a combination of biological and chemical reduction of iron in stagnant and washing water regimes. The whiteness of porcelain stone increases after aging as well as after subsequent iron-removal operations with differing contributions: washing with ammonia oxalate > magnetic separation > washing with water.


Similar content being viewed by others
References
R. A. Platova, G. N. Maslennikova, and Yu. T. Platov, “Biochemical method of removing iron from Zhuravlinyi Log kaolin,” Steklo Keram., No. 2, 15 – 22 (2013); R. A. Platova, G. N. Maslennikova, and Yu. T. Platov, “Biochemical method of removing iron from Zhuravlinyi Log kaolin,” Glass Ceram., 70(1 – 2), 51 – 56 (2013).
T. C. Eisele and K. L. Gabby, “Review of Reductive Leaching of iron by Anaerobic Bacteria,” Mineral Proc. Extractive Metall. Review: Int. J., 35(2), 75 – 105 (2014); Placed on site June 11, 2012, published on site July 22, 2013.
J. A. Gonzalez and M. del C. Ruiz, “Bleaching of kaolins and clays by chlorination of iron and titanium,” Applied Clay Sci., 33(3 – 4), 219 – 229 (2006).
F. Veglio, “Factorial experiments in the development of a kaolin bleaching process using thiourea in sulphuric acid solutions,” Hydrometallurgy, 45(1 – 2), 181 – 197 (1997).
V. R. Ambikadevi and M. Lalithambika, “Åffect of organic acid on ferric iron removal from iron–stained kaolinite,” Applied Clay Sci., 16(3 – 4), 133 – 145 (2000).
A. Tuncuk, S. Ciftlik, and A. Akcil, “Factorial experiments for iron removal from kaolin by using single and two-step leaching with sulfuric acid,” Hydrometallurgy, 134 – 135, 80 – 86 (2013).
S. Oh Lee, T. Tran, B. Hi Juhg, et al., “Dissolution of iron oxide using oxalic acid,” Hydrometallurgy, 87(3 – 4), 91 – 99 (2007).
U. Schwertmann, “Solubility and dissolution of iron oxides,” Plant and Soil, 130(1 – 2), 1 – 25 (1991).
Yu. N. Vodyanitskii, Chemistry, Mineralogy and Color of Gleified Soils [in Russian], GNU Pochvennyi Institut im. V. V. Dokuchaeva, Russian Academy of Agricultural Sciences, Moscow (2006).
R. A. Platova and G. N. Maslennikova, “Biochemical nature of gleification during aging of clayey materials (review),” Steklo Keram., No. 9, 14 – 20 (2009); R. A. Platova and G. N. Maslennikova, “Biochemical nature of gley formation during aging of clayey materials (review),” Glass Ceram., 66(9 – 10), 318 – 323 (2009).
R. A. Platova, A. N. Chernyshov, and G. N. Maslennikova, “Biotreatment of clayey materials and ceramic pastes: directions, methods and experience (review),” Steklo Keram., No. 7, 15 – 22 (2012); R. A. Platova, A. N. Chernyshov, and G. N. Maslennikova, “Biotreatment of clayey materials and ceramic pastes: directions, methods and experience (review),” Glass Ceram., 69(7 – 8), 229 – 235 (2009).
Y-Su Luu and J. A. Ramsay, “Review: microbial mechanisms of accessing insoluble Fe(III) as an energy source,” World Journal of Microbiology and Biotechnology, 19(2), 215 – 225 (2003).
D. R. Lovley, “Dissimilatory Fe(III) and Mn(IV) reduction,” Microbiological Rev., 55(2), 259 – 287 (1991).
D. R. Lovley, “Extracellular electron transfer: wires, capacitors, iron lungs, and more,” Geobiology, No. 6, 225 – 231 (2008).
E. E. Roden and M. M. Urrutia, “Influence of biogenic Fe(II) on bacterial crystalline Fe(III) oxide reduction,” Geomicrobiol. J., 19(2), 209 – 251 (2002).
G. N. Maslennikova, R. A. Khalilullova, and Yu. T. Platov, “Identification of iron compounds in clay-containing materials,” Steklo Keram., No. 2, 12 – 15 (1999); G. N. Maslennikova, R. A. Khalilullova, and Yu. T. Platov, “Identification of iron compounds in clay-containing materials,” Glass Ceram., 56(1 – 2), 48 – 51 (1999).
J. K. Fredrickson, J. M. Zachara, D. W. Kennedy, et al., “Biogenic iron mineralization accompanying the dissimilatory reduction of hydrous ferric oxide by a groundwater bacterium,” Geochim. Cosmochim. Acta, 62(19 – 20), 3239 – 3257 (1998).
M. A. Nakhamkin and G. I. Zhuravlev, “On ferromagnetic filtration of ceramic suspensions,” Steklo Keram., No. 7, 25 – 27 (1977); M. A. Nakhamkin and G. I. Zhuravlev, “Ferromagnetic filtration of ceramic suspensions,” Glass Ceram., 34(8), 508 – 511 (1977).
C. Liu, S. Kota, J. M. Zachara, et al., “Kinetic analysis of the bacterial reduction of goethite,” Environ. Sci. Technol., 35, 2482 – 2490 (2001).
Q.-X. He, X.-Ch. Huang and Z.-L. Chen, “Influence of organic acids, complexing agents and heavy metals on the bioleaching of iron from kaolin using Fe(III)-reducing bacteria,” Applied Clay Sci., 51(4), 478 – 483 (2011).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Steklo i Keramika, No. 1, pp. 30 – 37, January, 2014.
Rights and permissions
About this article
Cite this article
Platova, R.A., Platov, Y.T. Biochemical Method of Iron Removal and its Effect on the Color Characteristics of Porcelain Stone. Glass Ceram 71, 28–34 (2014). https://doi.org/10.1007/s10717-014-9609-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10717-014-9609-y