Abstract
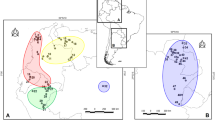
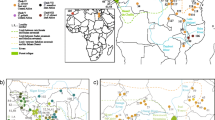
Many peninsulas in the temperate zone played an important role as refugia of various flora and fauna, and the southern Korean Peninsula also served as a refugium for many small mammals in East Asia during the Pleistocene. The Asian lesser white-toothed shrew, Crocidura shantungensis, is a widely distributed species in East Asia, and is an appropriate model organism for exploring the role of the Korean Peninsula as a refugium of small mammals. Here, we investigated phylogenetic relationships and genetic diversity based on the entire sequence of the mitochondrial cytochrome b gene (1140 bp). A Bayesian tree for 98 haplotypes detected in 228 C. shantungensis specimens from East Asia revealed the presence of three major groups with at least 5 subgroups. Most haplotypes were distributed according to their geographic proximity. Pairwise FST’s and analysis of molecular variance (AMOVA) revealed a high degree of genetic differentiation and variance among regions as well as among populations within region, implying little gene flow among local populations. Genetic evidence from South Korean islands, Jeju-do Island of South Korea, and Taiwan leads us to reject the hypothesis of recent population expansion. We observed unique island-type genetic characteristics consistent with geographic isolation and resultant genetic drift. Phylogeographic inference, together with estimates of genetic differentiation and diversity, suggest that the southern most part the Korean Peninsula, including offshore islands, played an important role as a refugium for C. shantungensis during the Pleistocene. However, the presence of several refugia on the mainland of northeast Asia is also proposed.





Similar content being viewed by others
References
Abe H (2005) A guide to the mammals of Japan. Tokai University Press, Hadano
Avise JC (2000) Phylogeography: the history and formation of species. Harvard University Press, Cambridge
Bandelt H-J, Forster P, Rohl A (1999) Median_Joining networks for inferring intraspecific phylogenies. Mol Biol Evol 16:37–48
Bannikova AA, Sheftel BI, Lebedev VS et al (2009) Crocidura shantungensis, a new species for Mongolia and Buryatia. Doklady Biol Sci 424:68–71
Butler PM (1998) Fossil history of shrews in Africa. In: Wójcik JM, Wolsan M (eds) Evolution of Shrews. Mammal Research Institute, Polish Academy of Sciences, Bialowieza, pp 121–132
Canestrelli D, Cimmaruta R, Nascetti G (2007) Phylogeography and historical demography of the Italian treefrog, Hyla intermedia, reveals multiple refugia, population expansions and secondary contacts within peninsular Italy. Mol Ecol 16:4808–4821
Chen D, Zhang X, Kang H et al (2012) Phylogeography of Quercus variabilis based on chloroplast DNA sequence in East Asia: multiple glacial refugia and mainland-migrated island populations. PLoS One 7:e47268
Chung K-H, Yang H-J (1999) A study on the fauna and speciation of the Cheju Island and Ulrung Dageletin Korea. J Kyonggi Basic Sci 12:189–200
Churchfield S (1990) The natural history of shrews. Christopher Helm, A&C Black, London
Corbet GB, Hill JE (1991) A world list of mammalian species, 3rd edn. Natural History Museum Publications & Oxford University Press, London
Creer S, Malhotra A, Thorpe RS, Chou WH (2002) Multiple causation of phylogeographical pattern as revealed by nested clade analysis of the bamboo viper (Trimeresurus stejnegeri) within Taiwan. Mol Ecol 10:1967–1981
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and high- performance computing. Nat Methods 9:772
Drummond AJ, Rambaut A (2007) BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol 7:214. https://doi.org/10.1186/1471-2148-7-214
Dubey S, Zaitsev M, Cosson J-F et al (2006) Pliocene and Pleistocene diversification and multiple refugia in a Eurasian shrew (Crocidura suaveolens group). Mol Phylogenet Evol 38:635–647. https://doi.org/10.1016/j.ympev.2005.11.005
Emery KO, Nino H, Sullivan B (1971) Post-pleistocene levels of the East China Sea. Woods Hole Oceanographic Institute Press, Massachusetts
Esselstyn JA, Brown RM (2009) The role of repeated sea-level fluctuations in the generation of shrew (Soricidae: Crocidura) diversity in the Philippine Archipelago. Mol Phylogenet Evol 53:171–181. https://doi.org/10.1016/j.ympev.2009.05.034
Excoffier L, Lischer HEL (2010) Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resour 10:564–567
Frantz AC, McDevitt AD, Pope LC et al (2014) Revisiting the phylogeography and demography of European badgers (Meles meles) based on broad sampling, multiple markers and simulations. Heredity 113:443–453. https://doi.org/10.1038/hdy.2014.45
Fu YX (1997) Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 147:915–925
Gómez A, Lunt DH (2006) Refugia within refugia: patterns of phylogeographic concordance in the Iberian Peninsula. In: Phylogeography of Southern European Refugia. Springer, London
Graur D, Li W-H (2000) Fundamentals of molecular evolution, 2nd edn. Sinauer Associates, Inc., Sunderland
Han S, Iwasa MA, Ohdachi SD et al (2002) Molecular phylogeny of Crocidura shrews in northeastern Asia: a special reference to specimens on Cheju Island, South Korea. Acta Theriol 47:369–379
Harpending HC (1994) Signature of ancient population growth in a low-resolution mitochondrial DNA mismatch distribution. Hum Biol 66:591–600
Harpending HC, Sherry ST, Rogers AR, Stoneking M (1993) The genetic structure of ancient human populations. Curr Anthropol 34:483–496
Harrison SP, Yu G, Takahara H, Prentice IC (2001) Diversity of temperate plants in east Asia. Nature 413:129–130
Head MJ, Gibbard PL (2015) Formal subdivision of the quaternary system/period: past, present, and future. Quatern Int 383:4–35
Hewitt GM (1996) Some genetic consequences of ice ages, and their role, in divergence and speciation. Biol J Lin Soc 58:247–276
Hewitt GM (2000) The genetic legacy of the quaternary ice ages. Nature 405:907–913
Hewitt GM (2003) Ice ages: their impact on species distributions and evolution. In: Evolution on planet Earth. Academic press, New York
Hewitt GM (2004) Genetic consequences of climatic oscillations in the Quaternary. R Soc 359:183–195. https://doi.org/10.1098/rstb.2003.1388
Hutterer R (2005) Mammal species of the world: a taxonomic and geographic reference, 3rd edn. Johns Hopkins University Press, Baltimore
Irwin DM, Kocher TD, Wilson AC (1991) Evolution of the cytochrome b gene of mammals. J Mol Evol 32:128–144
Iwasa MA, Ohdachi S, Han S-H et al (2001) Karyotype and RFLP of the nuclear rDNA of Crocidura sp. on Cheju Island, South Korea (Mammalia, Insectivora). Mammalia 65:451–460. https://doi.org/10.1515/mamm.2001.65.4.451
Jang-Liaw N-H, Chou W-H (2011) Phylogeography of the fanged dicroglossine frog, Limnonectes fujianensis (Anura, Ranidae), in Taiwan. Zoolog Sci 28:254–263. https://doi.org/10.2108/zsj.28.254
Jang-Liaw N-H, Lee T-H, Chou W-H (2008) Phylogeography of Sylvirana latouchii (Anura, Ranidae) in Taiwan. Zool Sci 25:68–79. https://doi.org/10.2108/zsj.25.68
Jiang X, Hoffmann RS (2001) A revision of the white-toothed shrews (Crocidura) of Southern China. J Mammal 82:1059–1079
Kartavtseva IV, Park I-S (2010) Y chromosome peculiarities and chromosomal G- and C-staining in Crocidura shantungensis Miller, 1901 (Soricomorpha: Soricidae). Comp Cytogenet 4:67–71
Kearse M, Moir R, Wilson A et al (2012) Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649. https://doi.org/10.1093/bioinformatics/bts199
Kim S-I, Park S-K, Lee H et al (2013) Phylogeography of Korean raccoon dogs: implications of peripheral isolation of a forest mammal in East Asia. J Zool 290:225–235. https://doi.org/10.1111/jzo.12031
Kim T, Park S, Kim Y et al (2015) Characteristics of external and cranial morphological characters of asian lesser white-toothed shrew (Crocidura shantungensis). Korean Soc Environ Biol 33:441–449
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120. https://doi.org/10.1007/BF01731581
Koh HS, Kartavtseva IV, Lee BK et al (2013) A preliminary study on genetic divergence of the Asian lesser white-toothed shrew Crocidura shantungensis (mammalia: Soricomorpha) in mainland Korea, adjacent islands and continental east Asia: cytochrome b sequence analysis. Russ J Theriol 12:71–78
Krystufek B, Buzan EV, Hutchinson WF, Hänfling B (2007) Phylogeography of the rare Balkan endemic Martino’s vole, Dinaromys bogdanovi, reveals strong differentiation within the western Balkan Peninsula. Mol Ecol 16:1221–1232
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. https://doi.org/10.1093/molbev/msw054
Lee M-Y, Lissovsky AA, Park S et al (2008) Mitochondrial cytochrome B sequence variations and population structure of Siberian Chipmunk (Tamias Sibiricus) in Northeastern Asia and population substructure in South Korea. Mol Cells 26:566–575
Lee YS, Markov N, Argunov A et al (2016) Genetic diversity and phylogeography of Siberian roe deer, Caproulus pygargus, in central and peripheral populations. Ecol Evol. https://doi.org/10.1002/ece3.2458
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452
Lin T-T, You E-M, Lin YK (2009) Social and genetic mating systems of the Asian lesser white-toothed shrew, Crocidura shantungensis, in Taiwan. J Mammal 90:1370–1380. https://doi.org/10.1644/08-MAMM-A-346R1.1
Lin HD, Chen YR, Lin SM (2012) Strict consistency between genetic and topographic landscapes of the brown tree frog (Buergeria robusta) in Taiwan. Mol Phylogenet Evol 62:251–262. https://doi.org/10.1016/j.ympev.2011.09.022
Miller SG (1901) Descriptions of three new Asiatic shrews. Biol Soc Wash 14:157–159
Miraldo A, Hewitt GM, Paulo OS, Emerson BC (2011) Phylogeography and demographic history of Lacerta lepida in the Iberian Peninsula: multiple refugia, range expansions and secondary contact zones. BMC Evol Biol. https://doi.org/10.1186/1471-2148-11-170
Motokawa M, Lin L-K, Harada M, Hattori S (2003) Morphometric geographic variation in the Asian lesser white-toothed shrew Crocidura shantungensis (Mammalia, Insectivora) in East Asia. Zoolog Sci 20:789–795
Motokawa M, Yu H-T, Harada M (2005) Diversification of the white-toothed shrews of the genus Crocidura (Insectivora: Soricidae) in East and Southeast Asia. Mammal Study 30:S53–S64
Nowak RM (1999) Walker’s mammals of the world, 6th edn. Johns Hopkins University Press, Baltimore
Ohdachi SD, Iwasa MA, Nesterenko VA et al (2004) Molecular phylogenetics of crocidura shrews (insectivora) in East and Central Asia. J Mammal 85:396–403
Ohdachi SD, Ishibashi Y, Iwasa MA et al (2009) The wild mammals of Japan. Shoukadoh Book Sellers and Mammalogical Society of Japan, Kyoto
Qiu Y, Fu C, Peter H (2011) molecular phylogenetics and evolution plant molecular phylogeography in China and adjacent regions: tracing the genetic imprints of quaternary climate and environmental change in the world’s most diverse temperate flora. Mol Phylogenet Evol 59:225–244
Rambaut A, Suchard MA, Xie D, Drummond AJ (2014) Tracer v 1.6. http://beast.bio.ed.ac.uk/Tracer. Accessed 1 May 2018
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574. https://doi.org/10.1093/bioinformatics/btg180
Sakka H, QUÉRÉ JP, Kartavtseva I et al (2010) Comparative phylogeography of four Apodemus species (Mammalia: Rodentia) in the Asian Far East: evidence of Quaternary climatic changes in their genetic structure. Biol J Lin Soc 100:797–821
Schmitt T (2007) Molecular biogeography of Europe: pleistocene cycles and postglacial trends. Front Zool 4:11. https://doi.org/10.1186/1742-9994-4-11
Shafer ABA, Cullingham CI, Côté SD, Coltman DW (2010) Of glaciers and refugia: a decade of study sheds new light on the phylogeography of northwestern North America. Mol Ecol 19:4589–4621
Shi YF (2006) The quaternary glaciations and environmental change in China (Hardbac ks). Hebei Science Technology Press, Shijiazhuang, pp 65–101
Taberlet P, Fumagalli L, Wust-Saucy A-G, Cosson J-F (1998) Comparative phylogeography and postglacial colonization routes in Europe. Mol Ecol 7:453–464
Tajima F (1989) The effect of change in population size on DNA polymorphism. Genetics 123:597–601
Tomasik E, Cook J (2005) Mitochondrial phylogeography and conservation genetics of wolverine (Gulo Gulo) of Northwestern North America. J Mammal 86:386–396. https://doi.org/10.1644/BER-121.1
Tzeng C (1986) Distribution of the freshwater fishes of Taiwan. Taiwan Museum 39:127–146
Wang JP, Lin HD, Huang S et al (2004) Phylogeography of Varicorhinus barbatulus (Cyprinidae) in Taiwan based on nucleotide variation of mtDNA and allozymes. Mol Phylogenet Evol 31:1143–1156
Weiss S, Ferrand N (2007) Phylogeography of southern european refugia: evolutionary perspectives on the origins and conservation of European biodiversity. Springer, Dordrecht
Wójcik JM, Wolsan M (1998) Evolution of shrews. Mammal Research Institute, Polish Academy of Sciences, Bialowieza
Won C, Smith KG (1999) History and current status of mammals of the Korean Peninsula. Mammal Review 29:3–33
Yang Z (1991) Evolution of eastern shelf of China in quaternary and its environmental consequences. In: Liang M, Zhang J (eds) Correlation of onshore and offshore quaternary in China. Science Press, Beijing, pp 1–22
Yoon M-H, Han S-H, Oh H-S, Kim JG (2004) The mammals of Korea. Dongbangmedia, Seoul
Yu G, Chen X, N i J, Cheddadi R et al (2000) Palaeovegetation of China: a pollen data-based synthesis for the mid-Holocene and last glacial maximum. J Biogeogr 27:635–664
Yuan SL, Lin LK, Oshida T (2006) Phylogeography of the mole-shrew (Anourosorex yamashinai) in Taiwan: implications of interglacial refugia in a high-elevation small mammal. Mol Ecol 15:2119–2130. https://doi.org/10.1111/j.1365-294X.2006.02875.x
Zhang H, Yan J, Zhang G, Zhou K (2008) Phylogeography and demographic history of chinese black-spotted frog populations (Pelophylax nigromaculata): evidence for Independent refugia expansion and secondary contact. BMC Evol Biol 8:21. https://doi.org/10.1186/1471-2148-8-21
Zheng YQ, Yu G, Wang SM et al (2003) Simulations of LGM climate of East Asia by regional climate model. Sci China 46:753–764
Zhou SZ, Jijun L, Zhang SQ et al (2004) Quaternary glaciations in China. Dev Quat Sci 2:105–113. https://doi.org/10.1016/S1571-0866(04)80116-7
Acknowledgements
We gratefully acknowledge Dr. Thomas W. Sappington, USDA-ARS, for his critical comments and edits on this manuscript. We would like to express our deep gratitude to all cooperators who donated the Asian lesser white-toothed shrew samples for this study including Conservation Genome Resource Bank for Korean Wildlife, National Institute of Biological Resources, Korea National Institute of Health, Tunghai University, National Taiwan University, and Shandong University at Weihai. This study was supported by a Grant (2010–0025751) from the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology, and partially supported by the Research Institute for Veterinary Science, Seoul National University. Also, this study was supported by the Major International (Regional) Joint Research Project of National Sciences Foundation of China (NSFC, 31110103910).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Lee, SJ., Lee, MY., Lin, LK. et al. Phylogeography of the Asian lesser white-toothed shrew, Crocidura shantungensis, in East Asia: role of the Korean Peninsula as refugium for small mammals. Genetica 146, 211–226 (2018). https://doi.org/10.1007/s10709-018-0014-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10709-018-0014-2