Abstract
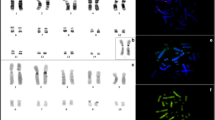
The genera Leptodactylus and Adenomera comprise 92 species distributed throughout the Neotropical region. These species have a modal diploid chromosome number 2n = 22. However, chromosome rearrangements are evident in the differentiation of five intra-generic groups in the genus Leptodactylus (L. fuscus, L. latrans, L. marmoratus (formally composed by the species of the genus Adenomera), L. melanonotus, L. pentadactylus), yet it is not clear if there is a karyotype pattern for each group. Aiming to understand the intra-generic and interspecific karyotype patterns of Leptodactylus and Adenomera, cytogenetic analyses were performed in A. andreae, L. macrosternum, L. pentadactylus, L. petersii, and L. riveroi using conventional staining, C-banding, nucleolus organizer region (NOR) and hybridization in situ fluorescent (FISH). The karyotype of Leptodactylus riveroi was described for the first time. Adenomera andreae had 2n = 26, while the remaining species 2n = 22. The NOR was found on pair No. 8 of A. andreae, L. macrosternum, L. pentadactylus, and L. riveroi, whereas L. petersii had it on pairs Nos. 6 and 10. These locations were confirmed by the FISH with 18S rDNA probe, except for pair No. 10 of L. petersii. The C-banding pattern was evident at the centromeres of chromosomes of all species and some interspecific variations were also observed. 2n = 22 was observed in the species of the L. latrans group, as well as in the intra-generic groups L. fuscus and L. pentadactylus; in the L. melanonotus group there were three diploid chromosome numbers 2n = 20, 22 and 24; and a larger variation in 2n was also evident in the L. marmoratus group.

Similar content being viewed by others
References
Amaro-Ghilardi RC, Rodrigues MT, Yonenaga-Yassuda Y (2004) Chromosomal studies after differential staining and fluorescence in situ hybridization using telomeric probe in three Leptodactylus species (Leptodactylidae, Anura). Caryologia 57:53–65
Amaro-Ghilardi RC, Skuk G, de Sá RO, Rodrigues MT, Yonenaga-Yassuda Y (2006) Karyotypes of eight species of Leptodactylus (Anura, Leptodactylidae) with a description of a new karyotype for the genus. Phyllomedusa 5:119–133
Andrades-Miranda J, Zanchin NIT, Oliveira LFB, Langguth AR, Mattevi MS (2002) (TTAGGG) n telomeric sequence hybridization indicating centric fusion rearrangements in karyotype of the rodent Oryzomys subflavus. Genetica 144:11–16
Angulo A, Icochea J (2010) Cryptic species complexes, widespread species and conservation: lessons from Amazonian frogs of the Leptodactylus marmoratus group (Anura: Leptodactylidae). Syst Biodivers 8:357–370
Angulo A, Cocroft RB, Reichle S (2003) Species identity in the genus Adenomera (Anura: Leptodactylidae) in southeastern Peru. Herpetologica 59:490–504
Arruda MP, Morielle-Versute E (2008) Cytogenetic and random amplified polymorphic DNA analysis of Leptodactylus species from rural and urban environments (Anura, Amphibia). Genet Mol Res 7:161–176
Baldissera FA Jr, Oliveira PSL, Kasahara S (1993) Cytogenetics of four Brazilian Hyla species (Amphibia-Anura) and description of a case with a supernumerary chromosome. Rev Bras Genet 16:335–345
Beçak ML (1968) Chromosomal analysis of eighteen species of Anura. Caryologia 21:191–208
Bogart JP (1973) Evolution of anuran karyotypes. In: Vial JL (ed) Evolutionary biology of the anurans: contemporary research on major problems. University of Missouri Press, Columbia, pp 337–349
Bogart JP (1974) A karyosystematic study of frogs in the genus Leptodactylus (Anura: Leptodactylidae). Copeia 3:728–737
Bohne A, Brunet F, Galiana-Arnoux D, Schulthesis C, Volff JN (2008) Transposable elements as drivers of genomic and biological diversity in vertebrates. Chromosome Res 16:203–215
Busin CS, Vinciprova G, Recco-Pimentel SM (2001) Chromosomal rearrangements as the source of variation in the number of chromosomes in Pseudis (Amphibia, Anura). Genetica 110:131–141
Campos JRC (2010) Constituição cariotípica em leptodactilídeos do gênero Leptodactylus e em espécies de famílias relacionadas à Leptodactylidae (Amphibia: Anura). Thesis, Universidade Estadual Paulista “Júlio de Mesquita Filho”
Campos JRC, Ananias F, Brasileiro CA, Yamamoto M, Haddad CFB, Kasahara S (2009) Chromosome evolution in three Brazilian Leptodactylus species (Anura, Leptodactylidae), with phylogenetic considerations. Hereditas 146:104–111
Coelho AC (2013) Citogenética comparativa de seis espécies de anuros do gênero Leptodactylus (Leptodactylidae) coletadas no estado do Amazonas, Brasil. Dissertation, Instituto Nacional de Pesquisas da Amazônia
Cole CJ, Leavens CR (1971) Chromosome preparations of amphibians and reptiles: improved technique. Herpetol Rev 3:102
De Lucca E, Jim J (1974) Os cromossomos de alguns Leptodactylidae (Amphibia, Anura). Rev Bras Biol 34:407–410
de Sá RO, Grant T, Camargo A, Heyer WR, Ponssa ML, Stanley E (2014) Systematics of the Neotropical genus Leptodactylus Fitzinger, 1826 (Anura: Leptodactylidae): phylogeny, the relevance of non-molecular evidence, and species accounts. S Am J Herpetol 9:S1–S128
Denaro L (1972) Karyotypes of Leptodactylidae anurans. J Herpetol 6:71–74
Ford C, Hamerton J (1956) A colchicine hypothonic citrate squash sequence for mammalian chromosomes. Stain Technol 31:247–251
Frost DR (2015) Amphibian species of the world: an online reference. Version 6.0. American Museum of Natural History, New York. http://research.amnh.org/vz/herpetology/amphibia/index.html. Accessed 24 April 2015
Frost DR, Grant T, Faivovich J, Bain RH, Haas A, Haddad CFB, de Sá RO, Channing A, Wilkinson M, Donnellan SC et al (2006) The amphibian tree of life. Bull Am Mus Nat Hist 297:1–370
Gazoni T, Gruber SL, Silva APZ, Araújo OGS, Narimatsu H, Strüssmann C, Haddad CFB, Kasahara S (2012) Cytogenetic analyses of eight species in the genus Leptodactylus Fitzinger, 1843 (Amphibia, Anura, Leptodactylidae), including a new diploid number and a karyotype with multiple translocations. BMC Genet 13:109
Grant T, Frost DR, Caldwell JP, Gagliardo R, Haddad CFB, Kok PJR, Means DB, Noonan BP, Schargel WE, Wheeler WC (2006) Phylogenetic systematic of dart-poison frogs and their relatives (Amphibia: Athesphatanura: Dendrobatidae). Bull Am Mus Nat Hist 269:1–262
Green DM, Sessions SK (1991) Nomenclature for chromosomes. In: Green DM, Sessions SK (eds) Amphibian cytogenetics and evolution. Academic Press, New York, pp 431–432
Green DM, Sessions SK (2007) Karyology and cytogenetics. In: Heatwole H, Tyler M (eds) Amphibian biology, vol 7. Surrey Beatty and Sons, Chipping Norton, pp 2756–2841
Gross MC, Schneider CH, Valente GT, Martins C, Feldberg E (2010) Variability of 18S rDNA locus among Symphysodon fishes: chromosomal rearrangements. J Fish Biol 76:1117–1127
Haddad CFB, Prado CPA (2005) Reproductive modes in frogs and their unexpected diversity in the Atlantic forest of Brazil. Bioscience 55:207–217
Heyer WR (1969) The adaptive ecology of the species groups of the frog genus Leptodactylus (Amphibia, Leptodactylidae). Evolution 23:421–428
Heyer WR (1974) Relationships of the marmoratus species group (Amphibia, Leptodactylidae) within the subfamily Leptodactylinae. Nat Hist Mus Los Angeles Co Contr Sci 256:1–46
Howell WM, Black DA (1980) Controlled silver-staining of nucleolus organizer regions with a protective colloidal developer: a 1-step method. Experientia 36:1014–1015
King M (1991) The evolution of heterochromatin in the Amphibian genome. In: Green DM, Session SK (eds) Amphibian cytogenetics and evolution. Academic Press, San Diego, pp 359–391
Kuramoto M, Allison A (1989) Karyotypes of michrohylid frogs of Papua New Guinea and their systematic implication. Herpetologica 45:250–259
Lima AP, Magnusson WE, Menin M, Erdtmann LK, Rodrigues DJ, Keller C, Hödl W (2012) Guia de Sapos da Reserva Adolpho Ducke, Amazônia Central = Guide to the Frogs to Reserva Adolpho Ducke, Central Amazonia, 2nd edn. Instituto Nacional de Pesquisas da Amazônia, Manaus
López-Flores I, Garrido-Ramos MA (2012) The repetitive DNA content of eukaryotic genomes. In: Garrido-Ramos MA (ed) Repetitive DNA, vol 7. Karger, Basel, pp 1–28
Mattos TL, Coelho AC, Schneider CH, Telles OC, Menin M, Gross MC (2014) Karyotypic diversity in seven Amazonian anurans in the genus Hypsiboas (family Hylidae). BMC Genet 15:43
Meyne J, Baker RJ, Hobart HH, Hsu TC, Ryder OA, Ward OG, Wiley JE, Wurster-Hill DH, Yates TL, Moyziz RK (1990) Distribution of non-telomeric sites of the (TTAGGG)n telomeric sequence in vertebrate chromosomes. Chromosoma 99:3–10
Miura I, Niishioka M, Bordikin LJ, Wu Z (1995) The origin of the brown frogs with 2n = 24 chromosomes. Experientia 51:79–188
Morescalchi A (1973) Amphibia. In: Chiarelli AB, Capana E (eds) Cytotaxonomy and vertebrate evolution. Academic Press, New York, pp 233–348
Oliveira HHP, Souza CCN, Ribeiro CL, da Cruz AD, Bastos RP, Melo e Silva D (2010) Citogenética comparativa das famílias Leptodactylidae e Hylidae do cerrado Goiano. Estudos 37:725–735
Pinkel D, Straume T, Gray JW (1986) Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc Natl Acad Sci USA 83:2934–2938
Ponssa ML (2008) Cladistic analysis and osteological descriptions of the frog species in the Leptodactylus fuscus species group (Anura, Leptodactylidae). J Zool Syst Evol Res 46:249–266
Pyron A, Wiens JJ (2011) A large-scale phylogeny of Amphibia including over 2800 species, and a revised classification of extant frogs, salamanders, and caecilians. Mol Phylogenet Evol 61:543–583
Rocchi M, Archidiacono N, Schempp W, Capozzi O, Stanyon R (2012) Centromere repositioning in mammals. Heredity 108:59–67
Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, Mullis KB, Erlich HA (1988) Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 239:487–491
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, vol 1. Cold Spring Harbor Press, New York
Silva APZ, Haddad CFB, Kasahara S (2000) Chromosomal studies on five species of the genus Leptodactylus Fitzinger, 1826 (Amphibia, Anura) using differential staining. Cytobios 103:25–38
Silva APZ, Garcia PCA, Martins VG, Bacci M, Kasahara S (2004) Chromosomal and molecular analyses of Leptodactylus gracilis gracilis, L. gracilis delattini, and L. plaumanni (Anura, Leptodactylidae): taxonomic implications. Amphib-Reptil 25:185–196
Silva APZ, Haddad CFB, Galassi G, Kasahara S (2006) Multiple nucleolus organizer regions in Leptodactylus mystacinus (Amphibia, Anura) and comments on its systematic position in the L. fuscus group based on cytogenetic and molecular analyses. Genetica 127:35–44
Suárez P (2010) Estudos cromossômicos em anuros das famílias Hylidae Rafinesque, 1815 e Leptodactylidae Werner, 1896 (Amphibia: Anura). Thesis, Museu Paraense Emílio Goeldi/Universidade Federal do Pará
Sumner AT (1972) A simple technique for demonstrating centromeric heterochromatin. Exp Cell Res 74:304–306
Sumner AT (1990) Chromosome banding. Unwin Hyman Ltd., London
Vittorazzi SE, Lourenço LB, Recco-Pimentel SM (2014) Long-time evolution and highly dynamic satellite DNA in leptodactylid and hylodid frogs. BMC Genet 15:111
Wiley JE, Meyne J, Little MN, Stout JC (1992) Interstitial hybridization sites of the (TTAGGG)ntelomeric sequence on the chromosomes of some North American hylid frogs. Cytogenet Cell Genet 51:55–57
Zaracho VH, Hernando AB (2011) The karyotype of Adenomera diptyx (Boettger 1885) (Anura, Leptodactylidae) from northeastern Argentina. Genet Mol Biol 34:84–87
Acknowledgments
We thank Anne Baldisseri for the English review; Alexandre Almeida and Karla da Silva for granting use of facilities at the INPA and UFAM Zoological Collection; the Herpetology group from the SISBIOTA project for collection and identification of some specimens. Financial support was provided by Fundação de Amparo à Pesquisa do Estado do Amazonas (FAPEAM) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (CNPq # 573976/2008-2, 558318/2009-6, 563348/2010-0; FAPEAM # 062.00383/2013; FAPEAM # 20/2013; FAPEAM/CNPq 062.02317/2011). The current study was supported by graduate fellowships from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) to ACC and TLM and a Research Productivity grant from CNPq to MM and FAPEAM to MCG.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Coelho, A.C., de Mattos, T.L., Viana, P. et al. Intra-generic and interspecific karyotype patterns of Leptodactylus and Adenomera (Anura, Leptodactylidae) with inclusion of five species from Central Amazonia. Genetica 144, 37–46 (2016). https://doi.org/10.1007/s10709-015-9876-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10709-015-9876-8