Abstract
Binary mixtures of plastic fines and a non-cohesive host material are used in geotechnical barriers to obtain high shear strength and low permeability. To achieve such properties, the sand grains must form the force chain and the clay must fill the voids of the host material. However, clays are known to affect the grain to grain contacts by forming cohesive membranes. Since engineered barriers are usually compacted at water contents below saturation (Proctor conditions), it is still unclear whether such membranes will form. The aim of the current research is to shed light on the compaction characteristics of such mixtures. Proctor datasets were analysed in terms of skeleton and fines void ratio and optimal water content. Oedometric compression behaviour and limiting void ratios were studied to outline the characteristics of mixtures in a saturated or dry state, respectively. The results show that depending on the mixing ratio, compaction properties can be divided into three different ranges, where either the properties of one of the input materials dominate the behaviour or a transitional range exists that is characterised by a strong mutual influence of both materials. Furthermore, even a small amount of the highly plastic clay was found to influence the properties of the host sand.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The hydro-mechanical behaviour of binary mixtures of sands containing clay has been the subject of research for decades (Carraro 2004; Agus et al. 2010; Fei 2016). From a geotechnical perspective clays are fine grained mineral mixtures able to bind a significant number of water molecules due to high surface area and hydration of exchangeable cations. They are therefore typically found with a high liquid limit and low to very low values of shear strength and hydraulic conductivity. Sand, in contrast, typically has a higher friction angle and a higher permeability than clay. These materials are therefore often used in barriers to combine low permeability of fine grained clayey soils and high mechanical strength of the sand (Kenney et al. 1992; Agus et al. 2010; Shaker and Elkady 2015). In addition to the aforementioned beneficial hydro-mechanical properties, such mixtures are less susceptible to drying induced shrinkage and frost damage (Studds et al. 1998).
The ideal composition of sand and clay in a compacted barrier is achieved by combining two soil conditions: (I) the sand forms the main matrix and thus determines the mechanical properties of the soil, (II) the clay located in the voids causes an appropriate reduction of the permeability (Kenney et al. 1992).
However, combining sand and cohesive material is accompanied with complex soil mechanical characteristics (Kenney et al. 1992; Goudarzy et al. 2021). It is generally believed that clays are able to form a cohesive membrane around coarser particles and therefore affect the grain contacts (Pandian et al. 1995). Thus, even small amounts of clay (i.e. plastic fines) play an important role for the grain to grain contacts. The mechanical response of mixtures containing clay under static (Carraro 2004) and dynamic loads (Ochoa-Cornejo et al. 2020) has therefore been found to be significantly altered far below the point where the volumetric fraction of the fines is dominant in the soil matrix or fill the entire pore space.
However, it is still not proven up to now, whether this effect is relevant under proctor boundary conditions, as plasticity requires the presence of a certain amount of water. By contrast, the optimal moisture content (w\(_{opt}\)) at proctor maximum density (\(\rho _{pr}\)) is generally below that of the saturated state. Here, the dependence of the effective stresses on the water content and effects resulting from partial saturation (e.g. capillary cohesion) are relevant. Consequently, the soil parameters determined in the fully saturated state (i.e. liquid limit) are only of limited value in reflecting the behaviour of a fine-grained soil in a Proctor test (Gurtug and Sridharan 2004).
In this paper, the influence of plastic fines on the compactability of sand under proctor boundary conditions is investigated. Therefore, different sand-fines-mixtures were proctor compacted and results are presented and analysed in terms of skeleton and fines void ratio. In order to represent a wide range of different soils, a kaolin, a bentonite and a mica were used as fine fractions differing greatly in their plasticity properties. The investigations of the proctor samples compacted under partially saturated conditions are supplemented by the minimum and maximum void ratio (determined at hygroscopic water content) and oedometer tests (saturated conditions). In contrast to previous studies on this subject, more emphasis was placed on the influence of mineralogy and the resulting different plasticities. Therefore, the binary mixtures were not only considered as host material influenced by fines content, but also discussed with regard to the characterstics of the plastic fine components.
2 Skeleton and Fines Void Ratio
Several studies have investigated the limit void ratios of granular soils, and isolated various parameters responsible for the magnitudes of e\(_{max}\) and e\(_{min}\). Most studies acknowledge the grain shape and mean grain size or uniformity coefficient as the major influencing factors, e.g. Youd (1973), Cho et al. (2006), Zheng and Hryciw (2016) and Sarkar et al. (2019). In recent years, the variation of e\(_{max}\) and e\(_{min}\) for sand mixed with non-plastic fines has gained attention. Thevanayagam (1998) proposed a micromechanical interpretation for the variation of void ratio based on the concept of binary mixtures of two materials. Further studies (Arab and Belkhatir 2012; Belkhatir et al. 2013; Cubrinovski and Ishihara 2002; Dash et al. 2010; Hsiao et al. 2015; Lade et al. 1998; Papadopoulou and Tika 2008; Phan et al. 2016; Polito and Martin II 2001; Rahman et al. 2008; Yang et al. 2006; Goudarzy et al. 2016a) have generally found that both limit void ratios decrease with an increase in non-plastic fines till a certain threshold after which the trend reverses. However, further discussion on this topic lies beyond the scope of this paper.
While a vast majority of studies can be found on sand-silt mixtures, relatively fewer studies can be encountered on sand-clay mixtures owing to the difficulty in estimating the interactions between sand grains and the cohesive material. While the study of Lade et al. (1998) extensively investigates a theoretical model to predict the e\(_{max}\) and e\(_{min}\) values for sand mixed with non-cohesive soils, the same is lacking for cohesive mixtures. Cubrinovski and Ishihara (2002) used a large database of sand fines mixtures, some of which included clay particles (kaolinite), however, with relatively low plasticity indices (I\(_P\) = 5–20%). An increase in e\(_{max}\) and e\(_{min}\) with increasing fines was reported throughout. Certain previous studies (e.g. Vallejo and Mawby 2000; Elkady et al. 2013; Zuo and Baudet 2015) have also stated that the behavior of the binary mixture changes from sand to clay with increasing clay content. In the last few years, Othman and Marto (2018) performed tests on mixtures using kaolin ranging between 0–40%. An increase in e\(_{max}\) and e\(_{min}\) was found after 30% kaolin content. Shan-shan et al. (2018) reported a decrease of the limit void ratios till 30% kaolin content for sands having various grain size distribution curves. The maximum void ratio of sand was found to decrease with increasing silty clay and clay content, and increase with increasing content of kaolin clay, thereby partially contradicting the study of Othman and Marto (2018) and some others. It may be concluded that the variation of limit void ratios of sand-clay mixtures with fines content has been scarcely studied and is thus not well understood yet, which emphasises the need of more studies on this aspect.
General approaches to idealise the arrangement of fines in a coarse grained material (host material) are based on three different conceptual regimes where (I) coarse grains determine the grain to grain contacts and fines are located within the voids (II) fines affect the soil skeleton of the host material till reaching the loosest state, followed by a transition where (III) fines become the host material and coarse grains are floating in the fines matrix (Thevanayagam 1998; Cubrinovski and Ishihara 2002; Park and Santamarina 2017). It is vital to consider the void ratio of the two individual materials (i.e. sand and fines) in order to identify the governing concept for a given global void ratio.
The void ratios of the respective materials in the mixture can be expressed in terms of skeleton void ratio and interfines void ratio. The skeleton void ratio \(e_s\) [Eq. (1)] is defined as the ratio of air (\(V_a\)) or water filled voids (\(V_w\)) as well as the volume of fines (\(V_{fc}\)), which is also considered as voids, to the volume of the sand (\(V_{sa}\)).
The interfine void ratio \(e_f\) is defined as the ratio between air or water filled voids and the volume of the fines (\(V_{fc}\)).
Both void ratios can be calculated based on the global void ratio (e) and the fines content (fc) i.e. the amount of fines, assuming a similar specific gravity of the solids. Adapted equations can be found in the literature that allow the calculation of \(e_s\), \(e_f\) and the respective dry density of the fines \(\rho _f\) based on the density of the mixture and the specific gravity of sand and fines (Agus et al. 2010; Shaker and Elkady 2015).
Due to the higher ability to adsorb water, the available water in a mixture of sand and clay will be mainly present in the clay (Agus et al. 2010). This has experimentally been proven by Kenney et al. (1992), who reported that irrespective of whether water was added to the sand or bentonite clay before mixing, the final image was always of a wet bentonite mixed with a dry sand. The global water content (w) can therefore be considered as the water content of the clay (\(w_{clay}\)) according to Eq. (3), where \(w_m\) is the water content of the mixture.
3 Materials and Methods
3.1 Sand
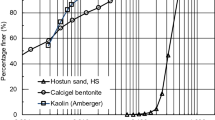
Two different sands were used to prepare different mixtures to investigate the influence of potential variations in grain size distribution. The first sand, Hostun sand (abbreviation HS), which has been widely studied in the literature (e.g. Goudarzy et al. 2016a, b, 2017, 2018), is a quartz sand with grain sizes ranging from 0.1 to 1.0 mm in diameter. According to the USCS classification, the material is a poorly graded medium sand with a uniformity coefficient \(C_u\) of 1.8. Several other studies that also tested mixtures of sands with similar \(C_u\) to that of the Hostun sand with kaolinite or bentonite are included in the study to complement the data set. As the second host sand, a commercially available quartz sand (Rhine sand, abbreviation RS) with \(C_u\) of 2.3 was employed to prepare mixtures containing kaolin. The grain size distribution curves of the different sands are given in Fig. 1 and an overview of the different mixtures including the characteristics of the fines is provided in Tables 1 and 2.
3.2 Fines
Three different fines were used in the current study to represent a broad variety of different plasticity properties. All materials were initially present as powder. Calcigel is a highly plastic calcium bentonite from Bavaria in Germany intensively studied in literature (Agus et al. 2010; Baille et al. 2014). The cation exchange capacity of the calcigel is 64 meq/100 g (Baille et al. 2014) which indicates the high ability to interact with water. Amberger kaolin is a medium plastic clay with a cation exchange capacity of 5 meq/100 g. HLM7 is a commercially available mica found with d\(_{98}\) \(\le\) 20\(\mu\)m and d\(_{50}\) of 7 \(\mu\)m. During preparation for the tests on the liquid limit the mica showed a partially cohesive and adhesive behaviour, while it was completely liquid in other adjacent areas. It was therefore not possible to determine the plasticity properties. Mica itself is not considered a clay mineral. The low cation exchange capacity of the mica (1.5 meq/100 g) indicates that the plasticity of the mica is associated to the fine-grained nature rather than to its mineralogy. An overview of the material properties is summarised in Table 1.
3.3 Microscopic Photographs
In order to investigate the adsorption properties and the effects of the fines on the host sand, two different investigations were carried out on the micro fabric. Microscopic photographs are used to assess the possible adsorption of fines even in the dry state. For this purpose, a grain of sand was glued to the head of a pin and, after the glue had dried, immersed in a bed of fine material previously dried at 60 \(^{\circ }\)C. The photographs were taken with a digital light microscope. Figure 2 shows the sand grains before (left) and after contact with the dry fine material. All the three different fines are able to adsorb on the sand surface. This observation is consistent with that of Ochoa-Cornejo et al. (2020), who observed adsorption of laponite, a synthetic clay mineral with an I\(_P\) = 1000%, on a sand grain in a similar test.
Scanning electron microscope (SEM) examinations were carried out to investigate the effect and location of the fines in the host sand. For the investigation, mixtures with 20% fines were mixed and compacted to proctor optimum. This value has been chosen as it was found to be sufficiently high to have a significant impact on the host sand without becoming the host material. Following compaction, a block of approx. 5 \(\times\) 5 mm was frozen in liquid nitrogen and then freeze-dried. After freeze-drying, the sample was glued onto carbon pads and sputtered with gold. The sample containing mica was unstable, which is why no examination could be carried out on the intact microstructure. In addition, the mica components became charged during the investigation. The results of these investigations, shown in Fig. 3, are discussed in more detail in Sect. 4.
3.4 Limiting Void Ratios
To prepare the sand fines mixtures, the materials were mixed in corresponding proportions by weight. The mixing was initially performed by hand until a homogenous distribution was visible. The mixtures were then kept in a sealed container for at least 12 h before performing tests to determine e\(_{max}\) and e\(_{min}\). According to various standards (DIN 18126:1996-11 2012; ASTM D4254-16 2016), it is not recommended to perform index void ratio tests on mixtures containing more than 15% fines. However, no specifications for higher fines contents are given, which is why many researchers have applied the methods recommended for clean sands or sands with low fines contents (e.g. Tao et al. 2004; Goudarzy et al. 2016b). For the limiting void ratios, in addition to the mixtures also tested in the Proctor and Oedometer tests (fc = 0, 10 and 20%), the fines contents fc = 5 and 25% were also tested. The void ratios in the loosest and densest state were determined according to DIN 18126:1996-11 (2012).
3.5 Oedometric Compression Test
To investigate the influence of fines on the mechanical behaviour of Hostun sand, oedometer tests were carried out on the mixtures of Hostun sand with 10 and 20% of bentonite, kaolin and mica, respectively. The tests were performed in a standard oedometer according to DIN EN ISO 17892-5 (2017). The tested samples measured 2 cm in height and 7 cm in diameter. Stress was applied by a load frame using dead loads. Drainage during the test was facilitated through porous stones on the top and bottom of the sample.
All mixtures were tested under two initial conditions in order to represent different possible boundary conditions during proctor compaction. The first was to install samples as loosely as possible. In this initial condition, there is a large pore space and fines potentially attached at grain to grain contacts are not subjected to any mechanical preloading before oedometer testing. For this purpose, the hygroscopic material was filled homogenously into the oedometer ring in small quantities using a spoon. In the other initial condition, the sample was installed as dense as possible. This was to prestress the grain to grain contacts and potential attachments of fines by compaction energy. For this, hygroscopic material was compacted in two layers by tamping in the specimen holder. However, it was not possible to achieve the proctor density in that way. It should therefore be noted that the compaction energy was lower than proctor compaction energy, which could practically not be applied in the used equipment. This affects the initial void ratio whereas the overall fabric and therefore mechanical response can be considered similar. After installation, a small vertical stress of \(\sigma _v' =\) 1.5 kPa was applied. The sample was hydrated and then gradually loaded to 400 kPa by doubling the load followed by a stepwise unloading.
3.6 Proctor Compaction Tests
The proctor samples were prepared with a standard proctor equipment, following the procedure described in DIN 18127:2012-09 (2012). The material was mixed at hygroscopic water content at the desired mixing ratio to produce the mixtures. Different water contents were then adjusted by adding the desired amount of water and the material was left to rest for homogenisation. The mixtures containing swellable clay (kaolin and bentonite) were homogenised overnight in a closed bucket, while the pure sand and mica were only homogenised for a short time (\(\le\) 1 h) due to the low ability to adsorb water.
4 Results and Discussion
4.1 Micro Fabric
Figure 3a shows the micro fabric of the kaolin-Hostun sand mixture at the mixing ratio of 20% of fines and 80% of sand. The figure reveals a sand dominated fabric with clay covering the sand grains. Figure 3b shows a zoom-in to a grain to grain contact. The kaolin forms a bridge between the sand grains by covering the grain to grain contact. In contrast to former investigations, where the kaolin was believed to be adsorbed in an unregular and aggregated form (Beroya et al. 2009), Figs. 3c, d show a rather regular coating of the sand particles. Closer examination of the sand surface in Fig. 3c shows that the kaolin is not perfectly in contact with the surface due to its irregularly shaped and relatively large minerals.
The bentonite-sand matrix shown in Fig. 3e is characterised by the fabric of the host sand, similar to the case of kaolin. However, in the close-up given in Fig. 3f, the grain to grain contact can only be guessed due to the pronounced accumulation of the bentonite, which seems to merge with the grains. The bentonite coats the sand surface in a gel-like film (Fig. 3g, h). The direct comparison between the kaolin and bentonite mixtures, however, shows distinct different kinds of interparticle connectors which could differ in their shape and functionality (Collins and McGown 1974). In case of kaolin, the connectors appear as a film of kaolin covering the grain to grain contacts of the sand, whereas the overall appearance of the connectors in case of the bentonite is much more bulky, so that the spatial extent of the connector goes far beyond the grain to grain contact (see Fig. 3f).
Figure 3i presents the mica-sand mixture. Although the structure is not intact, some features of the mica-sand mixture can be identified. Figure 3j, k show a connector that is not an attachment to but an aggregate between the grain to grain contacts. The adsorption of mica to the sand surface is highly irregular. Some areas of the sand are left blank while others are covered by mica. The unevenness caused by this also explains the strong electrostatic charges of the grains during the SEM examination seen as bright spots in the image. The close-up in Fig. 3l shows the irregular accumulation of mica on the surface of the sand. It is noticeable that the heterogeneous size of the mica leads to small components being deposited between larger ones. This produces a rough and loose attachment to the sand surface with only a few points of contact.
4.2 Limiting Void Ratios
The minimum and maximum void ratios of the different mixtures are shown as function of clay content in the mixture in Fig. 4 and plotted as \(e_s\) in Fig. 5. The presence of kaolin in the mixture causes a continuous increase of both minimum and maximum void ratio within the range of tested fines contents. The observed increase is less pronounced up to the addition of 10% kaolin compared to the increase after exceeding 10%. The increase of both limiting void ratios by the addition of kaolin shows the ability of this clay to connect different sand grains to larger conglomerates. The ability of the kaolin to be adsorbed on the sand surface has already been demonstrated by the push-pin experiments described in Sect. 3.3. The bonds of adsorbed kaolin are strong enough to resist the mechanical stresses associated with the determination of the minimum void ratio. The trends e\(_{max}\) over fc show that the mixtures with Hostun Sand and Rhine Sand have a very similar behaviour, i.e. the influence of the grain size distribution is not significant for the studied materials.
Both limiting void ratios of the sand-bentonite mixtures slightly decrease with the amount of added bentonite. After exceeding 20% bentonite this trend reverses. This behaviour, even if the trend is rather moderate, corresponds to the characteristic behaviour of gap graded mixtures containing non-plastic fines. However, a closer look at the development of the \(e_s\) shows that the addition of 5 and 10% fines already leads to a moderate increase in the maximum void ratio of the sand. It can therefore not be concluded that the bentonite is deposited primarily in the voids of the host sand till exceeding the threshold value where clay becomes the dominant matrix, as is described for classical models of non-plastic materials. Still, in the case of very fine bentonite, more material seems to be needed than in the case of kaolin, in order to attach itself stably to the sand.
The limiting void ratios of the sand-mica mixtures do not follow a common trend. The maximum void ratio strongly increases with growing amount of mica, while the minimum void ratio decreases following a rather non-linear trend. According to literature, the influence of mica on a host sand at a mica/sand size ratio \(\le\) 1 is mainly related to pore filling effects (Lee et al. 2007). This mechanism can explain both the increase in maximum void ratio due to void filling caused by an irregular arrangement of the plate-like mica and a compact parallel arrangement after the application of compacting energy in the case of minimum void ratio. The increase in maximum void ratio even at low amounts of mica (5%) in the mixture indicates an effect beyond merely filling voids of the host sand. As shown in Figs. 2 and 3i–l, mica can stick to the sand surface similar to other fines. The adhesion of mica is related to the high specific surface area and, thus, the fine-grained nature of the mica used in this study, while such an effect may not be relevant for larger mica. The results of the minimum void ratio indicate that the binding of the mica to the sand surface and the resulting effects are weak and even small mechanical stresses are able to remove them.
The results for the mixtures containing more than 15 % fines must be seen with the restriction that they do not correspond to the recommendation which limits the maximum amount of fines in the testing procedure. However, the evaluation of e\(_s\) shown in Fig. 5, which reflects the influence of the fines on the void ratio of the host material, shows a clear trend that does not change after exceeding 15 % fines. Therefore the results of the material containing more than 15 % fines can be considered useful to support the overall observations and trends described above.
4.3 Oedometric Compression Tests
The oedometric compression curves of the different mixtures are given in Fig. 6a–c, respectively, shown in terms of skeleton void ratio versus vertical effective stress. A general trend can be seen for all mixtures. All compression curves are sorted according to the amount of fines. At the same stress, the materials have the highest skeleton void ratio for the pure sand and the lowest for the material with 20% fines. To investigate the influence of fines on the compressibility of sand, the compression index (C\(_{c,es}\)) of the oedometer tests related to the skeleton void ratio was calculated according to Eq. (4). Here, \(\Delta e_{s}\) is the change in skeleton void ratio due to the corresponding load increment \(\Delta \sigma '\).
The relation between \(e_{s}\) and \(C_{c,es}\) is plotted in Fig. 7a–c for the different fines respectively. The dashed line indicates the compression index \(C_c\) of pure Hostun sand reported in Krim et al. (2019). The compression behaviour of the sand in the loose state approximately follows this line whereas the sand starting from the dense state approaches this line due to overconsolidation effects.
Figure 7a shows the \(e_{s}\) over \(C_{c,es}\) for the kaolin-sand-mixtures. The mixture with 10% kaolin starting from dense conditions shows a slighter softer behaviour than the dense sand sample without fines. However, samples starting from a loose state and the mixture containing 20% kaolin starting from the dense state show similar \(C_{c,es}\) as the loose Hostun sand without fines even though some of the mixtures are found with higher \(e_{s}\). The reduction of the number of grain to grain contacts as indicated by the higher \(e_{s}\) shows the ability of the kaolin to stabilise the grain to grain contacts and therefore support an open porous network (Gratchev et al. 2006). The kaolin connectors counteract the compaction energy during installation, so that overconsolidation effects are less pronounced and initial compaction by hand was unsuccessful in the case of the 20% kaolin mixture. It must be taken into account that the compaction during installation in the oedometer was done by hand without using a proctor compaction device. However, as shown by the unchanged \(C_{c,es}\), the grain to grain contacts of the sand remain unaffected under saturated conditions, which could be justified by the microstructural observation that the clay is mainly located at the side of the grain to grain contacts and not between them.
The plot of \(e_{s}\) versus \(C_{c,es}\) for the benonite-sand mixtures in Fig. 7b shows a different behaviour when compared to the mixtures containing kaolin. The mixtures with 10% bentonite show a slightly higher \(C_{c,es}\) than the pure sand, starting from both loose and dense conditions. Similarly, both mixtures with 20% bentonite show a higher \(C_{c,es}\) than the pure sand. Due to its small mineral size and large specific surface area, bentonite forms a cohesive membrane around the sand grains (Beroya et al. 2009) and hence directly affects the grain to grain contacts (Gratchev et al. 2006). The microstructural investigations on the bentonite-sand mixtures revealed an accumulation of clay at the grain to grain contacts, and the higher \(C_{c,es}\) value suggests that clay is also present between the grain to grain contacts. Therefore, the bentonite could be considered a separator rather than a connector. Like kaolin, bentonite can also counteract the energy of compaction during installation given that the amount of bentonite in the mixture is high enough to form a stable membrane around the sand grains.
The \(e_{s}\) over \(C_{c,es}\) plot for the sand-mica-mixtures is given in Fig. 7c. The presence of mica does not cause a general change in the material behaviour. The test started from loose conditions containing 10% mica is found with a lower \(e_{s}\) and similar \(C_{c,es}\) than the pure sand whereas the material with 20% mica is found with higher \(e_{s}\) and \(C_{c,es}\) in comparison to the mixture containing 10% mica.
The increase of compressibility, as observed for the later mixture, is typically associated to an addition of mica (Lee et al. 2007). Although an increase in compressibility was also observed for the mixtures with bentonite, the effect can be attributed to different mechanisms in view of the extreme differences in mineral size but also the different surface characteristics and thus interactions with water. The influence of mica can be attributed to the ability of the platy mica to fill all the voids even at a small amount and therefore become the host material (Cabalar 2010).
Similar to the minimum void ratio, the mixtures containing 10% mica are found with much lower \(e_{s}\) indicating a strong influence of the mica on the compaction characteristics without strong effects on the grain to grain contacts during compression.
4.4 Proctor Compaction Tests
The results of the proctor compaction curves of the kaolin-sand-mixtures, the bentonite-sand-mixtures, and the mica-sand-mixtures are given in Fig. 8a–d, respectively. The overall trend of \(\rho _{pr}\) and w\(_{opt}\) of the mixtures with increasing clay content is similar. Both \(\rho _{pr}\) and w\(_{opt}\) are initially increasing with the amount of fines in the mixture. After exceeding a specific amount of fines in the mixture, a further increase is associated with a progressive decrease of \(\rho _{pr}\) till reaching that of the pure clay. Beyond that transition the proctor maxima are in the range S\(_r\) =80–90% and thus display a typical clay behaviour.
The optimum water content of the materials shown in Fig. 9 decreases with the addition of fines till reaching a point where this trend reverses followed by an increase till reaching the w\(_{opt}\) of the pure fines. This turning point follows the order kaolin < mica < calcigel. This trend demonstrates that the clay has a clear influence on the optimum water content of all mixture in all cases, even when only small amounts are added. In addition to the proctor tests conducted as part of the study, the diagram also includes data from the literature for kaolin and bentonite. It is evident that the data from the literature confirm the trends of the experiments.
Figure 10 shows the dry density of the clay (\(\rho _{d,clay}\)) obtained by conversion of Eq. (2) plotted over \(w_{clay}\), the water content normalized to the clay content according to Eq. (3). The points for water contents up to 100% are mainly located in the range of degrees of saturation \(S_r\) = 80–90%, which is typical for fine-grained materials. For water contents above 100%, the points tend to lie in the range of 60–80%. It should be noted here that the calculation of the clay dry density was based on the total available pore space whereas the clay preferably accumulates on the sand surface and at the grain to grain contacts. Therefore, the actual density of the clay and the degree of saturation can be considered underestimated. However, this plot shows that the clay in the mixtures has a similar degree of saturation at the proctor optimum regardless of the amount added. The initial decrease in optimal water content visible in Fig. 9 can be attributed to the fact that both water and clay attach to the grain to grain contacts of the sand matrix. Since the water bound by the clay at \(S_r\)=80–90% is less than that which can attach to the grain to grain contacts in the form of water menisci, the optimum water content decreases in the presence of clay.
Figures 11, 12 and 13 show the \(e_s\) and \(e_f\) values obtained from proctor optimum of the different mixtures plotted as a function of the amount of clay. The evolution of \(e_s\) and \(e_f\) with the amount of added clay follows similar trends for the different mixtures, even though the qualitative values differ for the different mineral types. In general, four different regimes can be separated: (I) at low clay contents \(e_s\) remains unaffected by the addition of clay revealing that there is a modest effect of the added clay on the compaction characteristics of the sand. This observation is remarkable, as by contrast, the water content has been found to be affected by the presence of even of small amounts of clay. (IIa) By further increasing the amount of clay in the mixture \(e_s\) starts to increase till reaching the \(e_{max}\) of the pure sand, where contact points between the sand grains diminish and the clay becomes the host material. (IIb) A transitional range can be observed where the sand is not the host material anymore, but the clay has not reached its proctor density (\(e_f\) \(\le\) \(e_{pr,clay}\)). The areas (IIa) and (IIb) demonstrate that clay affects the compactability of sand and, vice versa, sand affects the compactability of clay given a sufficiently high proportion. (III) The clay is found with proctor conditions (\(e_f\) = \(e_{pr,clay}\)) revealing a picture of sand floating in a clay matrix without a pronounced effect on its compactability.
The plots of the mixtures containing kaolin and those containing mica shown in Figs. 11 and 13 indicate an unaffected \(e_s\) up to 20% kaolin, whereas the bentonite already has an influence on the \(e_s\) from about 15%. The transition range at which \(e_s\) \(\ge\) \(e_{max}\) but the clay has not yet reached Proctor density is approximately between 25% and 45% for the bentonite and between 30% and 55% for the kaolin. In the investigated range, the mica mixtures show a similar trend to those containing kaolin. It should be noted, however, that the mica data set is the smallest and further research is needed to test the generality of the observations. This has to be considered particularly in view of the complex soil-mechanical properties of mica.
5 Conclusions
In this article, the influence of fines on the compactability of sand was investigated. In addition to the partially saturated condition prevailing in the Proctor test, the fully saturated condition (oedometer test) and the hygroscopic condition (minimum and maximum void ratio) were also studied.
Different types of clays have different effects on the intrinsic and mechanical properties of sand-clay mixtures. When combined together, the kaolin typically forms a bridge between the sand grains along with a relatively smooth coating, although kaolin particles are not perfectly in contact with the surface owing to their irregular shape and relatively large size. For the bentonite, a smooth gel-like film coating is seen with a thicker spatial extent as compared to the kaolin. For mica, this is more like an aggregate with a highly irregular pattern showing smaller aggregates depositing between larger ones.
From the limit void ratio tests, it can be concluded that the bentonite deposits mostly in the voids till a threshold value beyond which the bentonite becomes the dominant matrix. This behavior is absent in the case of the sand-kaolin mixtures. In the case of mica, an increase in the maximum void ratio can be explained by void filling due to irregular arrangement, while the observed reduction in minimum void ratio with increasing amount of fines can be attributed to a compact arrangement after application of energy. Even though adhesion of mica is evident in the present case, small mechanical stresses can remove them from sticking to the sand surfaces.
In terms of the oedometric compression tests, the similar values of the compression index \(C_{c,es}\) for sand-kaolin mixtures with different amounts of fines points out to the fact that kaolin does not actively affect the skeleton contacts. The kaolin particles are rather located at the side of the grain to grain contacts, while bentonite forms a cohesive membrane around sand grains thereby preventing active contacts between individual grains and acting as a separator. The higher compressibility of sand-mica mixtures can be attributed to the ability of mica to fill all the voids and therefore, dominate the mixture behavior even at smaller amounts.
In terms of Proctor tests, at lower clay contents, the skeleton void ratio \(e_s\) remains unaffected while the optimum water content changes in the presence of small amounts of clay. Upon further increase in the amount of clay, \(e_s\) increases till reaching the e\(_{max}\) of the clean host sand signifying a threshold clay content, above which any further addition of clay results in clay being the dominant material with the sand floating in it. The transition water content at which \(e_s\) be greater than e\(_{max}\) is different for different clays, with kaolin and mica having larger values than bentonite.
Availability of data and materials
Enquiries about data availability should be directed to the authors.
References
Agus SS, Schanz T, Fredlund DG (2010) Measurements of suction versus water content for bentonite-sand mixtures. Can Geotech J 47(5):583–594
Alabdullah J (2010) Testing unsaturated soil for plane strain conditions: a new double-wall biaxial device. Dissertation, Ruhr-Universität Bochum, Germany
Arab A, Belkhatir M (2012) Fines content and cyclic preloading effect on liquefaction potential of silty sand: a laboratory study. Acta Polytech Hungar 9(4):47–64
ASTM D4254-16 (2016) Standard test methods for minimum index density and unit weight of soils and calculation of relative density, ASTM International (West Conshohocken, PA,). https://www.astm.org.
Baille W, Tripathy S, Schanz T (2014) Effective stress in clays of various mineralogy. Vadose Zone J 13(5):1–10
Belkhatir M, Schanz T, Arab A (2013) Effect of fines content and void ratio on the saturated hydraulic conductivity and undrained shear strength of sand-silt mixtures. Environ Earth Sci 70(6):2469–2479
Beroya M, Aydin A, Katzenbach R (2009) Insight into the effects of clay mineralogy on the cyclic behavior of silt-clay mixtures. Eng Geol 106(3):154–162. https://www.sciencedirect.com/science/article/pii/S001379520900057X
Cabalar AF (2010) Applications of the oedometer, triaxial and resonant column tests to the study of micaceous sands. Eng Geol 112(1):21–28. https://www.sciencedirect.com/science/article/pii/S0013795210000050
Carraro JAH (2004) Mechanical behavior of silty and clayey sands. Dissertation, Purdue University, USA
Cho GC, Dodds J, Santamarina JC (2006) Particle shape effects on packing density, stiffness, and strength: natural and crushed sands. J Geotech Geoenviron Eng 132(5):591–602
Collins K, McGown A (1974) The form and function of microfabric features in a variety of natural soils. Géotechnique 24(2):223–254. https://doi.org/10.1680/geot.1974.24.2.223
Cubrinovski M, Ishihara K (2002) Maximum and minimum viod ratio characteristics of sands. Soils Found 42(6):65–78
Dash HK, Sitharam TG, Baudet BA (2010) Influence of non-plastic fines on the response of a silty sand to cyclic loading. Soils Found 50(5):695–704
DIN 18126:1996-11 (2012) Soil, investigation and testing—determination of density of non-cohesive soils for maximum and minimum compactness, Deutsches Institut für Normung (Germany)
DIN 18127:2012-09 (2012) Soil, investigation and testing—proctor-test, Deutsches Institut für Normung (Germany)
DIN EN ISO 17892-5 (2017) Geotechnical investigation and testing—laboratory testing of soil—part 5: incremental loading oedometer test, Deutsches Institut für Normung (Germany)
Elkady T, Al-Mahbashi AM, Alrefeai TO (2013) Stress-dependent soil-water characteristic curves of lime-treated expansive clay. J Mater Civ Eng 27(3):04014127
Fei K (2016) Experimental study of the mechanical behavior of clay-aggregate mixtures. Eng Geol 210:1–9. http://www.sciencedirect.com/science/article/pii/S001379521630148X
Goudarzy M, König D, Schanz T (2016) Small strain stiffness of granular materials containing fines. Soils Found 56(5):756–764
Goudarzy M, Rahman MM, König D, Schanz T (2016) Influence of non-plastic fines content on maximum shear modulus of granular materials. Soils Found 56(6):973–983
Goudarzy M, Rahemi N, Rahman MM, Schanz T (2017) Predicting the maximum shear modulus of sands containing nonplastic fines. J Geotech Geoenviron Eng 143(9):06017013
Goudarzy M, König D, Santamarina J, Schanz T (2018) The influence of the anisotropic stress state on the intermediate strain properties of granular material. Géotechnique 68(3):221–232
Goudarzy M, Sarkar D, Lieske W, Wichtmann T (2021) Influence of plastic fines content on the liquefaction susceptibility of sands: monotonic loading. Acta Geotechnica 17:1719–1737. https://doi.org/10.1007/s11440-021-01283-w
Gratchev IB, Sassa K, Osipov VI, Sokolov VN (2006) The liquefaction of clayey soils under cyclic loading. Eng Geol 86(1):70–84
Gurtug Y, Sridharan A (2004) Compaction behaviour and prediction of its characteristics of fine grained soils with particular reference to compaction energy. Soils Found 44(5):27–36
Hsiao D, Phan VT, Hsieh Y, Kuo H (2015) Engineering behavior and correlated parameters from obtained results of sand-silt mixtures. Soil Dyn Earthq Eng 77:137–151
Kenney T, Veen WV, Swallow MA, Sungaila M (1992) Hydraulic conductivity of compacted bentonite-sand mixtures. Can Geotech J 29(3):364–374
Krim A, Arab A, Chemam M, Brahim A, Sadek M, Shahrour I (2019) Experimental study on the liquefaction resistance of sand-clay mixtures: effect of clay content and grading characteristics. Marine Georesour Geotechnol 37(2):129–141
Lade PV, Liggio CD Jr, Yamamuro JA (1998) Effects of non-plastic fines on minimum and maximum void ratios of sand. Geotech Test J (ASTM) 21(4):336–347
Lee J-S, Guimaraes M, Santamarina JC (2007) Micaceous sands: Microscale mechanisms and macroscale response. J Geotech Geoenviron Eng 133(9):1136–1143
Ochoa-Cornejo F, Bobet A, Johnston C, Santagata M, Sinfield JV (2020) Dynamic properties of a sand-nanoclay composite. Géotechnique 70(3):210–225
Othman BA, Marto A (2018) Laboratory test on maximum and minimum void ratio of tropical sand matrix soils. IOP Confer Ser: Earth Environ Sci 140:012084
Pandian N, Nagaraj T, Raju PN (1995) Permeability and compressibility behavior of bentonite-sand/soil mixes. Geotech Test J 18(1):86–93
Papadopoulou A, Tika T (2008) The effect of fines on critical state and liquefaction resistance characteristics of non-plastic silty sands. Soils Found 48(5):713–725
Park J, Santamarina JC (2017) Revised soil classification system for coarse-fine mixtures. J Geotech Geoenviron Eng 143(8):04017039
Phan VT, Hsiao D, Nguyen PT (2016) Effects of fine contents on engineering properties of sand-fines mixtures. Procedia Eng—Sustain Dev Civil, Urban Transp Eng Confer 142:213–220
Polito CP, Martin JR II (2001) Effects of non-plastic fines on the liquefaction resistance of sands. J Geotech Geoenviron Eng 127(5):408–415
Pourzargar A (2017) Application of suction stress concept to partially saturated compacted soils. Dissertation, Ruhr-Universität Bochum, Germany
Rahman MM, Lo SR, Gnanendran CT (2008) On equivalent granular void ratio and steady state behavior of loose sand with fines. Can Geotech J 45:1439–1456
Röchter L (2011) Systeme paralleler scherbänder unter extension im ebenen verformungszustand. Dissertation, Ruhr-Universität Bochum, Germany
Sarkar D, König D, Goudarzy M (2019) The influence of particle characteristics on the index void ratios in granular materials. Particuology 46:1–13
Shaker A, Elkady T (2015) Hydraulic performance of sand-clay mixtures: soil fabric perspective. Géotech Lett 5(3):198–204
Shan-shan L, Da-yong L, Yu-feng G (2018) Determination of maximum and minimum void ratios of sands and their influence factors. Chin J Geotech Eng 40(3):554–561
Studds P, Stewart D, Cousens T (1998) The effects of salt solutions on the properties of bentonite-sand mixtures. Clay Miner 33:651–660
Tao M, Figueroa JL, Saada AS (2004) Influence of non-plastic fines content on the liquefaction resistance of soil in terms of the unit energy. In: Proceedings of the cyclic behaviour of soils and liquefaction phenemena. A.A. Balkema Publishers, Bochum, pp 223–231
Thevanayagam S (1998) Effect of fines and confining stress on undrained shear strength of silty sands. J Geotech Geoenviron Eng 124(6):479–491
Vallejo LE, Mawby R (2000) Porosity influence on the shear strength of granular material-clay mixtures. Eng Geol 58(2):125–136
Yang S, Lacasse S, Sandven R (2006) Determination of the transitional fines content of mixtures of sand and non-plastic fines. Geotech Test J (ASTM) 29(2):102–107
Youd TL (1973) Factors controlling maximum and minimum density of sands. ASTM STP 523:98–112
Zheng J, Hryciw RD (2016) Index void ratios of sands from their intrinsic properties. J Geotech Geoenviron Eng 142(12):06016019
Zuo L, Baudet BA (2015) Determination of the transitional fines content of sand-non plastic fines mixtures. Soils Found 55(1):213–219
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lieske, W., Sarkar, D., Goudarzy, M. et al. On the Impact of Plastic Fines on the Compaction Characteristics of Sand. Geotech Geol Eng 42, 3451–3469 (2024). https://doi.org/10.1007/s10706-023-02738-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10706-023-02738-5