Abstract
Targeted use of animal manures as a nitrogen (N) fertilizer is challenging because of their poorly predictable N fertilizer value. An enhanced understanding of their N transformation processes in soil under field conditions is necessary to better synchronize N availability and crop N demand. 15N labeled cattle slurry, produced by feeding a heifer with 15N labeled ryegrass hay, was used in an on-farm trial on two neighboring fields, cropped with maize or grass-clover, in order to assess crop N uptake and N dynamics in the topsoil. Recovery of applied total N in plant biomass was higher for mineral fertilizer (Min) (45–48%) than for slurry (Slu) (17–22%) when applied at the same rate of mineral N. Also, N derived from fertilizer in plant biomass was higher for Min than for Slu, due to both greater NH3 emissions and greater initial immobilization of slurry N. Despite initial differences between the two in the relative distribution of residual fertilizer N in soil N pools, already in the following spring the majority (77–89%) of residual N from both fertilizers was found in the non-microbial organic N pool. Of the applied total N, 18–26% remained in the topsoil after the first winter for Min, compared to 32–52% for Slu. Thus, the proportion of fertilizer N not taken up by the first crop after application, enters the soil organic N pool and must be re-mineralized to become plant available.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Targeted use of animal manures as a nitrogen (N) fertilizer is difficult due to their variable composition. A high proportion of total N in animal manures is present as organic N compounds (Pagliari et al. 2020) which must be mineralized before becoming plant available. Ammonium-N within manure is available for uptake by plants, but it is also prone to NH3 volatilization. In soil, ammonium gets rapidly immobilized by microbes or can be nitrified. If not taken up by plants, there is a high potential for losses via nitrate leaching, especially under high rainfall, and gaseous losses via N2O or N2 (Gutser and Dosch 1996). These losses cause adverse effects on the environment (Erisman et al. 2013; Galloway et al. 2003) and human health (Ward et al. 2018). At the same time, in Europe bovine manure could replace about 4.3 million tons of mineral N fertilizer (Zavattaro et al. 2017), but to this end, an improved understanding of N transformation in the soil in relation to plant N needs under field conditions is necessary.
Under current farming practices, fertilizer N use efficiency (NUE) of both mineral fertilizer and animal manure is low, with N recoveries in crops averaging 42 ± 13% for mineral fertilizer and 26 ± 10% for animal manure in the year of fertilizer application (Smith and Chalk 2018). This leaves a large share of fertilizer N in the soil, with a consistently low residual fertilizer value in the following years (Smith and Chalk 2018). At the same time, plants take up more than half of their N demand from other sources than fertilizer applied that year, with major shares assumed to originate from soil organic N mineralization (Yan et al. 2020; Gardner and Drinkwater 2009). It must be noted that NUE tends to be overestimated at research stations and is not necessarily representative of achievable NUE values on farmers’ fields (Ladha et al. 2005; Cassman et al. 2002). This is due to a scale effect as researchers’ fields are commonly small, and attentively managed, often over several years, which is not feasible in practice. Therefore, on-farm data obtained from farmers’ fields is needed.
15N labeling represents a sensitive method for assessing fertilizer NUE, including direct assessment of its residual effect. However, it requires that N in the tested fertilizer is homogeneously labeled (Hauck and Bremner 1976). Homogeneous 15N labeling of both inorganic and organic N compounds in animal manures is challenging to achieve (e.g., Bosshard et al. 2011; Chalk et al. 2020; Hoekstra et al. 2011). Nevertheless, Powell and Wu (1999) suggested that mixing excreta portions around peak 15N enrichment after feeding a ruminant with 15N labeled feed over several days would allow for quantitative and accurate evaluation of NUE. The variation in the results due to inhomogeneous labeling can be assessed by characterizing the 15N distribution in different slurry N fractions (Langmeier et al. 2002; Bosshard et al. 2011).
Tracing N from animal manure into different soil N pools could facilitate the prediction of its NUE, but such field data is scarce. Sørensen (2004) and Jensen et al. (2000) found a rapid decline in the recovery of 15N from slurry in both microbial N (Nmic) and mineral N (Nmin), and an increasing share of N recovered in non-microbial organic N (Norg) under field conditions. However, their labeling approach could trace only the mineral N but not the organic N in the slurry. Others only looked into 15N recovery in Nmin, but not Nmic (Bosshard et al. 2009), or did not provide a direct comparison with mineral fertilizer (Hoekstra et al. 2011). This leaves a knowledge gap regarding the distribution of either 15N labeled mineral fertilizer or 15N labeled cattle slurry, produced by mixing 15N labeled urine and faeces, into different soil N pools under arable field conditions. Furthermore, to our knowledge, none of the previous studies investigated the effect of repeated field applications of labeled fertilizers on the 15N recovery in Nmic and Nmin. However, repeated application of slurry is a common agricultural practice.
We applied 15N labeled cattle slurry under on-farm conditions to gain a more realistic view of the NUE of cattle slurry under field conditions in Switzerland. While we acknowledge that our microplot design to some extent contradicts the on-farm setting, placing our experiment on fields managed by a farmer still allowed more representative results than experiments on research stations. The Gäu region, where this study was conducted, is located on the Swiss Central Plateau, and is characterized by the agricultural production of vegetables and arable crops. Common arable rotations of mixed crop-livestock farms include silage maize, winter cereals, canola, and grass-clover leys. The groundwater in this region is specifically vulnerable to nitrate leaching, not only due to agricultural production, but also because it is barely diluted with water from non-agricultural land (Gerber et al. 2018). In the Gäu region, authorities are searching for ways to improve NUE of both organic and inorganic fertilizers in order to reduce nitrate leaching. We chose silage maize and grass-clover as model crops since these crops are most commonly fertilized with animal manure. Following the official Swiss fertilization recommendations (Richner and Sinaj 2017), we took the NH4-share of the slurry as a proxy for its N availability and established a 15N mineral fertilizer treatment with the same rate of mineral N for comparison.
Overall, this study aimed at (a) assessing the NUE of 15N labeled cattle slurry in comparison to 15N labeled synthetic mineral fertilizer in an on-farm field trial under recommended agricultural practice conditions, and (b) investigating their N dynamics in topsoil during the season of application. We expected N availability from cattle slurry during the year of application to be equal to its NH4-content. Thereby, we presumed that mineralization of organic N in slurry would compensate for immobilization of slurry mineral N, and that plant N derived from fertilizer would be the same for slurry and mineral fertilizer when applied at the same dose of mineral N. Finally, we assumed fertilizer N recovery in crop biomass to be higher for mineral fertilizer than for slurry, but to be equal for both fertilizers when considering the whole plant-soil system.
Material and methods
Field site and experimental design
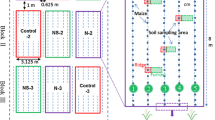
The field experiment was conducted as an on-farm trial on two neighboring fields in the Canton Solothurn, Switzerland, during 2018/19. While Field A was cropped with silage maize followed by winter wheat during 2018, Field B was cropped with grass-clover (Fig. 1). Both fields had been cultivated with sown grass-clover for at least three years before the experiment began and regularly received animal manure according to common agricultural practice. Fields differed slightly in bulk density and texture but were overall similar in basic soil properties in the uppermost 0.15 m (Table 1). Climatic conditions at the field site are temperate, with a mean annual temperature of 9.0 °C and yearly precipitation of 1129 mm (1981–2010). However, weather conditions in 2018 were exceptionally hot and dry, especially during the summer, with temperatures between April and September about 2.4 °C above average and about 30% less precipitation (BAFU 2019) (Fig. 2 and SI Fig. 1).
Daily average temperature and daily precipitation at Wynau (closest meteorological station, 422 m a.s.l., 47.255025 / 7.787475) during the timeframe of the experiment. Vertical dashed lines indicate time points for application of 15N labeled fertilizers. (GC = fertilizer application to grass-clover at Field B, SM = fertilizer application to silage maize at Field A)
Three fertilizer treatments were implemented: 15N labeled mineral fertilizer as 15NH415NO3 (Min, 8.00 atom% 15N abundance), 15N labeled cattle slurry (Slu, 7.89 atom% 15N abundance), and a control (Con) treatment not receiving any 15N labeled fertilizers. Each fertilizer treatment was replicated four times, resulting in 12 microplots per field. On both fields, microplots were arranged in a complete randomized block design on a 3 m wide strip (SI Fig. 2). Following the design proposed by Jokela and Randall (1987), non-confined microplots were located in a way that two maize rows formed the edges of each microplot and one maize row formed the centerline of the plot (SI Fig. 3). Microplots had a size of 1.5 m × 2 m, while row spacing was 0.75 m. Although Field B was not cropped with maize in 2018, the same dimensions for the microplots were used.
Production and characterization of 15N labeled cattle slurry
15N labeled cattle slurry was produced by feeding a female heifer (240 kg live weight) with 15N labeled ryegrass (Lolium multiflorum var. Westerwoldicum) hay with a 15N abundance of 12.6 atom% (for hay production see SI. 3). After a preceding adaptation phase of seven days, the heifer was fed with the 15N labeled ryegrass hay for eight days (daily ratio: 5.6 kg dry weight). Both in the adaptation phase and the three days after feeding with 15N labeled hay, feed consisted of non-labeled hay produced under the same conditions as the 15N labeled hay (Sørensen et al. 1994; Bosshard et al. 2011). From Day 8 (start of feeding with 15N labeled hay) until the end of the feeding period, faeces and urine were collected quantitatively and separately using a urinal which was attached to the hindquarter of the animal (Langmeier et al. 2002; Hoekstra et al. 2011). Faeces were collected directly from the rubber mat on which the heifer was bedded. Both portions were frozen in 24 h-intervals at −20 °C until later use. This experiment was approved by the Cantonal Veterinary Office Zurich, Switzerland (license ZH195/17).
Subsamples of the individual faeces portions from each feeding day were freeze-dried, pulverized with a ball mill, and analyzed for 15N. The highest 15N enrichment in both urine and faeces was reached between three to eight days after starting to feed with 15N labeled feed (i.e., from Day 11 until Day 16 of the feeding period) (SI Fig. 5). The total amounts of faeces (48 kg) and urine (56 kg) from these days were then mixed and diluted 1:1 (w/v) with demineralized water, since cattle slurry under common husbandry conditions usually gets diluted with water at about this ratio. The slurry had a total N content of 67.8 g N kg−1 DM, an ammonium-N content of 42.2 g N kg−1 DM and a 15N enrichment of 7.89 atom% 15N (Table 2, SI Table 1).
The homogeneity in the 15N labeling of the slurry was assessed using the fractionation method described by Mason (1969) and simplified by Kreuzer and Kirchgessner (1985). The fractionation revealed that of total slurry N, about 75% was water-soluble N (WSN), 4.6% was undigested dietary N (UDN), and the remainder consisted of bacterial and endogenous debris N (BEDN) (SI Table 1). The 15N enrichment in these fractions ranged from 7.39 to 8.78 atom% 15N, following the order of WSN < total slurry N < UDN < BEDN (SI Table 1). We assumed that within the time scale of one year considered in this experiment, only BEDN and WSN would become plant available, due to the stable nature of UDN (Kreuzer and Kirchgessner 1985; Sørensen et al. 1994; Bosshard et al. 2011). Thus, only the enrichment of BEDN and WSN might be relevant when calculating plant N uptake. Their weighted mean label is 7.72 atom% 15N, slightly lower than the 7.89 atom% 15N label of total slurry N (SI Table 1). Therefore, the label of total slurry N can be used as a source signature for quantitative tracing of slurry N transformation and uptake processes.
Fertilizer application and microplot management
In 2018, 15N labeled fertilizers were applied once to maize at the three to four leaf stage on Field A and four times (once after each cut of the grass-clover ley) on Field B (Fig. 1). 15N slurry was further diluted with demineralized water upon application, in order to apply slurry at a volume and N content that is representative for cattle slurry in Switzerland. Thus, the slurry was applied at a rate of 30 m3 ha−1 containing 60 kg N ha−1, equivalent to 36.8 kg NH4-N ha−1, while the N rate of the mineral fertilizer treatment was equal to the NH4-N content of the slurry (i.e., 36.8 kg N ha−1). The slurry was applied on the microplot surface using canisters, imitating drag hose application. For the Min treatment, 15NH415NO3 was dissolved in demineralized water and the same volume was applied as for slurry, and in the same way. The same amount of water as applied with slurry or mineral fertilizer was distributed on the control plots. Min and Con plots were additionally fertilized with 75 kg K ha−1 (as potassium sulfate) and 6.7 kg P ha−1 (as triple super phosphate) per fertilizer application. Due to hot and dry weather conditions during summer 2018 (Fig. 2), fertilizer application was primarily performed in the evenings to avoid excessive N losses due to NH3 volatilization.
Under conventional farming, it is common practice to apply slurry once during the early growth stages of the maize and use mineral fertilizer for the second fertilizer application. As we aimed to investigate the fate of N from slurry under conditions representative for agricultural practice, five weeks after the “experimental” N dose, non-labeled urea (68 kg N ha−1) was applied to all microplots at Field A. For grass-clover (Field B), farmers usually apply slurry after each cut, resulting in four to five applications per year. Thus, we performed four repeated 15N labeled fertilizer applications to the same microplots throughout the year. Since the residual fertilizer values of 15N labeled fertilizers applied during 2018 were to be assessed during a subsequent experimental phase (Frick 2022), in spring 2019, all microplots at Field B received unlabeled cattle slurry applied by the farmer (95 kg N ha−1).
According to common agricultural practice, the farmer performed crop protection measures in silage maize (Field A) for the whole field including microplots. Cultivation measures that involved soil movement, such as ploughing after harvesting the maize, were conducted manually on the microplots.
Measurement of ammonia volatilization
NH3 emissions from the microplots were measured according to the Standard Comparison Method (SCM) described by Vandré and Kaupenjohann (1998). In short, passive samplers filled with 20 mL 0.05 M sulfuric acid were installed at about 0.1 m above the soil surface. The acid was exchanged regularly during a 60-h period after applying the fertilizers, followed by colorimetric determination of ammonium. Two reference outgassing systems, emitting NH3 at known rates, were used to calculate a transfer factor and calibrate the measured ammonium concentration in the solution. Details can be found in SI. 4.
Biomass and soil sample collection and preparation
Aboveground biomass was harvested from the central area of the microplots, at least 0.375 m away from the plot edges (Jokela and Randall 1987). Upon maturity, maize plants at Field A were harvested about 0.1 m above ground. Only the center row on a length of 1.25 m (i.e., 0.375 m away from the plots edge) (SI Fig. 3, Sample 5) was used for 15N analysis, while the edge rows of the microplot (SI Fig. 3, Samples 3 and 4) were used for getting a more representative estimate of the dry matter yield. Samples from outside the microplot (SI Fig. 3, Samples 1 & 2) did not have any enrichment in 15N over the control. This indicates that the 15N values from the central row—having the same distance from the plot edge as the outside row—can be considered undiluted from the outside and thus representative for N uptake solely from the area on which 15N labeled fertilizers were applied. The plants were split into stems, leaves, grain, and husk + cobs, dried and weighed.
For Field B (grass-clover), aboveground biomass was harvested four times throughout the season from a 0.5 m × 0.5 m frame placed in the middle of each microplot (“inner frame”) (SI Fig. 4). Biomass was sorted into grass, legumes and other herbs and dried. To get a representative estimate of the yield, the harvesting area was increased to the whole central area of the microplot (1.25 m × 0.75 m, “outer frame”) for which the same relative share of grass, legumes and herbs as in the inner frame was assumed.
Dried biomass samples of maize and grass-clover were homogenized in a cutting mill. A subsample was pulverized in a ball mill (MM200 Retsch, Haan, Germany) to analyze N content and 15N enrichment.
The soil was sampled once before fertilizer application on both fields (t0) and, after the treatments, five times at Field A, and eight times at Field B (Fig. 1). Composite samples were made from eight cores (0.02 m diameter) per plot to a depth of 0.15 m, with a distance of at least 0.15 m and at most 0.375 m from the edge of the microplot, in order not to disturb the central part of the microplot (SI Fig. 3 and SI Fig. 4). Samples were stored in cooling boxes on the field and at 4 °C after reaching the lab. Within 24 h, soil was sieved at 5 mm and extracted by the chloroform fumigation method in order to determine microbial N (Nmic) (Brookes et al. 1985; Vance et al. 1987). In short, from each sample, two subsamples of 20 g dry weight equivalent each were weighed. One subsample was immediately extracted with 80 ml 0.5 M K2SO4, while the other subsample was fumigated with chloroform for 20–24 h and extracted thereafter. Extracts were filtered through folded paper filters (Macherey Nagel type 615, Ø 185 mm) and stored at −20 °C until analysis on the TOC/TNb-analyzer. The non-fumigated extracts were analyzed for both total dissolved N and mineral N (Nmin). Upon each extraction, a reference soil sample (stored at 4 °C) was included in triplicate and extracted the same way to correct for deviations between extraction series.
The 15N enrichment in different soil N pools was assessed for sampling t1, t3, and t5 (Field A) and for sampling t6, t7, and t8 (Field B) (Fig. 1). For analysis of 15N in Nmic, both fumigated and non-fumigated extracts were oxidized by autoclaving extracts with K2S2O8 (Cabrera and Beare 1993), and total N afterwards was diffused on acidified filter traps (Whatman QM/A) by adding Devarda’s alloy (0.4 g per sample), 4 mL of 5 M NaCl, and 0.75 mL of 5 M NaOH per 10 mL of extract (Goerges and Dittert 1998; Mayer et al. 2003). Ammonium and nitrate contained in non-fumigated extracts were diffused in order to determine 15N in Nmin following a similar procedure on non-oxidized extracts, but adding only 0.2 g MgO and 0.4 g Devarda’s alloy.
Laboratory analysis of slurry, soil and biomass samples
Total N, NH4-N, P and K content of the fresh slurry were analyzed at the laboratory for soil and environmental analysis (LBU, Eric Schweizer AG, Steffisburg, Switzerland).
Total N in fumigated and non-fumigated soil extracts was measured with a TOC/TNb-analyzer (Shimadzu, TOC-L (Model CPH), Japan). Microbial N (Nmic) was calculated as the difference between fumigated and non-fumigated extracts using a conversion factor of kEN = 0.54 (Joergensen and Mueller 1996). Non-fumigated extracts were additionally analyzed colorimetrically for nitrate and ammonium (Keeney and Nelson 1982; Krom 1980).
All Ntot and 15N analyses (cattle slurry, soil, biomass, diffusion filters) were performed on an elemental analyzer coupled with a continuous flow isotope ratio mass spectrometer (Pyro cube + isoprime100, Elementar, Germany). Urine samples were analyzed for total N and 15N enrichment using a liquid autosampler mounted on the elemental analyzer.
Calculations
For all 15N data, isotopic excess was calculated by subtracting the mean 15N abundance (i.e., proportion of 15N relative to total N) of non-labeled reference samples from the measured 15N abundance. For the mineral fertilizer, the natural abundance of 15N in air was subtracted as a reference (i.e. 0.3663 atom%), while for slurry the weighted mean 15N abundance of the non-labeled faeces and urine samples was used as non-labeled reference (0.386 atom%). For plant biomass, soil or soil extracts, the mean of the control treatment (Con) at the corresponding sampling time in the corresponding sample type (soil, plant, extracts) was used as a reference.
The 15N excess was used to calculate the share of N derived from fertilizer (Ndff) in the corresponding compartment (Hauck and Bremner 1976):
where atom% 15Nexcess sample is the 15N enrichment of the considered compartment (i.e., soil, extracts or different plant groups or parts) and atom% 15Nexcess fertilizer refers to N enrichment of either mineral fertilizer or slurry.
The amount of N derived from the fertilizer was calculated as:
where TNi is the total amount of N in the considered compartment expressed in kg N ha−1.
For Field B (cropped with grass-clover) also biological nitrogen fixation (BNF) was determined. For the fertilized treatments (Min and Slu), N from BNF of clover (Nfix) was derived from the 15N enriched dilution method (McAuliffe et al. 1958), while for the control treatment, the natural abundance method (Shearer and Kohl 1986) was used (see SI. 5 for details).
The remaining part of N uptake by crops, i.e. N derived from other sources (Ndfo) such as soil, deposition or unlabeled fertilizer N, was calculated as the difference between total N uptake, Ndff and Nfix (where applicable).
The 15N enrichment in the Nmic pool was calculated according to Mayer et al. (2003):
where “fum” indicates fumigated samples while “nonfum” indicates non-fumigated samples. Total N concentrations determined by TOC/TNb were used for both total Nfum and total Nnonfum.
The recovery of the applied fertilizer in the different compartments was then calculated as:
where N applied is the total amount of N applied with the labeled fertilizer. For the repeated fertilizer applications and cuts of grass-clover at Field B, recovery was calculated cumulatively over the applications and cuts.
Additionally, recovery of mineral N was calculated relative to the amount of mineral N applied with the fertilizers, which was the same for Min and Slu. This calculation neglects that also organic N within Slu was labeled with 15N. However, it allows for a normalized comparison between Min and Slu, which had been added at the same amount of mineral N but different rates of total N.
Statistical analysis
Data preparation and statistical analysis were performed using R (Version 3.5.3) (R Core Team 2019). Throughout, a significance level of p < 0.05 was applied. Data for Field A and Field B were analyzed separately.
Analysis of variance (ANOVA) was used for analyzing differences between treatments in terms of dry matter yield, total N uptake, Ndff and recovery (Field A). In case of repeated sampling events (several biomass cuts at Field B; repeated soil sampling), linear mixed-effects models (lmer within lme4-package) were used including treatment, block and sampling as well as the interaction between treatment:sampling as fixed effects and microplots as a random effect. Model validation was performed by qq-plotting and Shapiro–Wilk normality test. In case of non-normal distribution of residuals, statistical analysis was performed on square-root- or log-transformed data. Contrast-function within the emmeans-package was used for deriving p-values for pairwise comparisons. p-value adjustment for multiple comparisons was performed according to the Holm-Bonferroni-method (Holm 1979).
Spearman`s rank correlation coefficient was used for analyzing the relationship between soil moisture content and both Nmic and Nmin.
Results
Yield and source of N uptake in the crop
For silage maize on Field A, dry matter yield was 16.4 to 17.9 t ha−1, while N uptake ranged between 137 and 150 kg N ha−1 (Table 3). Dry matter yield was similar in all treatments, while N uptake was slightly higher for Slu than for both Min and Con (by 7 to 9%). For Field B, significant treatment differences were found only for Cut 3 and Cut 4, with grass-clover dry matter yield between 26 and 34% greater for the fertilized treatments than for the non-fertilized control (Table 4). On average, N uptake did not differ between treatments (p = 0.13), but for Cut 4 it was 28 to 31% lower for Con than for Min or Slu.
The amount of N derived from the labeled fertilizers, Ndff (kg N ha−1), was markedly higher for Min than for Slu on both Field A (p < 0.001) and Field B (p < 0.01 for cumulated values over the four cuts). For the last cut considered in this study, which was taken in spring 2019 on Field B, the trend was reversed, with a higher Ndff value for Slu than for Min, although this was not statistically significant (p = 0.33).
In the grass-clover mixture on Field B, cumulated Nfix over all four cuts reached 41 kg N ha−1 for Con, while it was 19 kg N ha−1 for Slu and 14 kg N ha−1 for Min (Table 4). However, treatment effects were not significant (p = 0.24) due to the high data variability. Nfix strongly decreased for the later cuts (p < 0.001), especially in Min and Slu.
On average, less than 25% of N taken up by grass-clover on Field B derived from mineral fertilizer compared to less than 20% for slurry. For silage maize on Field A, less than 12% of N uptake derived from mineral fertilizer, while less than 8% derived from slurry. On Field B, Nfix added about 18% of total N uptake for Con and about 7% for Min and 5% for Slu. Thus, for both fields, 69 to 92% of N uptake originated from other sources than 15N labeled fertilizer application or BNF, probably mainly from soil N and non-labeled mineral fertilizer (Field A) or slurry N (Field B). Noteworthy, for the latter non-labeled slurry was only applied before the last cut, thus, for the first three cuts, Ndfo presumably equals N derived from soil.
15N fertilizer recovery in the soil–plant system
Fertilizer recovery in aboveground biomass was on both fields significantly higher for Min than for Slu (p < 0.001), ranging from 45 to 46% for Min and 19 to 22% for Slu (for Field B cumulated over all four cuts of the grass-clover and all 15N labeled fertilizer applications) (Tables 3 and 4). Although the same amount of mineral N was applied, also recovery of mineral N was significantly higher for Min than for Slu (p < 0.01), for which it reached up to 35%.
Fertilizer recoveries in topsoil were markedly higher for Slu than for Min at all sampling times, except for the first sampling at one week after fertilizer application to maize at Field A (Fig. 3). At this time point, fertilizer recovery for both Min and Slu was still around 100%. In contrast, in grass-clover at Field B only 29 ± 4% of 15N applied with Min and 61 ± 3% of 15N applied with Slu were recovered in topsoil one week after fertilizer application.
Adding the recovery in topsoil three weeks after harvest to the recovery in the harvested maize biomass, total recovery in the topsoil-plant system of Field A summed up to 75% for Min and 69% for Slu. On Field B, topsoil was sampled at Cut 1, Cut 2 and Cut 3. At these three cuts, cumulative recovery in the harvested aboveground biomass and recovery in topsoil summed up to 66%, 81% and 75% for Min and to 77%, 100% and 89% for Slu. The last soil sampling took place in February 2019 in order to sample before the spring application of non-labeled cattle slurry by the farmer. Thus, this last sampling point of topsoil cannot be directly linked to fertilizer recovery in aboveground biomass at Cut 4, which took place in April 2019.
Cumulated NH3-emissions at Field B were markedly higher for Slu (24 kg N ha−1), than for Min (5 kg N ha−1) (SI Fig. 6). For the single fertilizer application at Field A, about 4 kg N ha−1 were lost via NH3 volatilization from Slu, while for Min no emissions were detected. Noteworthy, to determine NH3 emissions only absolute N amounts could be measured, since ammonium concentrations in the acid traps were too low for reliable 15N measurements. However, it can be assumed that emitted ammonia originated only from the applied 15N labeled fertilizers.
N dynamics in topsoil and 15N recovery in different soil N pools
Generally, Nmin and especially NO3-N responded strongly to fertilizer addition, except for the first and second fertilizer application on Field B (Fig. 4). NH4-N followed a similar pattern, but differences were less clear than for NO3-N, mostly due to the high data variability for Slu.
Temporal development of (a, b) NO3-N, (c, d) NH4-N, (e, f) Nmic-N in topsoil (0–0.15 m) (mean ± standard deviation, n = 4, except Field B Nmic sampling t1 control n = 2). Arrows indicate application of 15N labeled fertilizers, dashed lines indicate aboveground biomass harvest. Sampling time points for which 15N abundance in the Nmic and Nmin pool were determined (see Fig. 5), are marked with black bars at the bottom
Nmic was less directly affected by fertilizer addition than Nmin (Fig. 4). Across both fields, Nmic, NH4-N and NO3-N were significantly correlated with the gravimetric moisture content of the soil upon sampling (Spearman’s rank correlation coefficient r = 0.24 for Nmic, r = −0.40 for NH4-N, and r = −0.37 for NO3-N) (SI Table 2). With grass-clover on Field B, differences between treatments increased over time (p = 0.002), resulting in Nmic in the order Slu > Con > Min (Fig. 4).
To trace the fertilizer into different soil N pools, extracts from selected time points were analyzed for their 15N abundance. Shortly after fertilizer application on Field A, most N from Min was found in the mineral N pool, but some of it was already assimilated into Nmic (Fig. 5). At the same time, the biggest share of slurry N was found in the Norg pool. With time, the share of Ndff in Nmin decreased drastically in both treatments, while the share in the Norg pool increased.
(a, b) Absolute amounts of N derived from fertilizer (Ndff) in different soil N pools (kg N ha−1); (c, d) distribution of fertilizer N in different soil N pools relative to the residual amount of fertilizer N (given in % of the absolute amount of residual fertilizer N [Ndff (kg N ha−1)], see (a, b) in soil); (e, f) 15N recovery in different soil N pools relative to applied amounts of 15N; all numbers refer to topsoil (0–0.15 m); for time points of fertilizer application compare Fig. 1; Nmic = microbial N, Nmin = mineral N, Norg = non-microbial organic N; (mean ± standard deviation, n = 4)
For Field B, a clear effect of the repeated applications of 15N labeled fertilizer to grass-clover was observed. One week after the fourth fertilizer application (t7), the share of fertilizer N in the Nmin pool increased, especially for Min (Fig. 5). By that time point, however, there was already quite a big share of fertilizer N from both fertilizers found in the Norg pool, originating from previous 15N labeled fertilizer applications.
With time, differences in the distribution of fertilizer N in soil between treatments and between fields declined, reaching a similar distribution upon spring. More than 77% of residual fertilizer N in soil, defined here as the amount of fertilizer N not taken up by the crop by a certain time point, were found in the Norg pool and only minor shares were found as Nmin. Differences persisted for the share of fertilizer N found in Nmic, which was higher for Field B (20–21%) than for Field A (10–12%). Since total recovery of fertilizers in the total soil N pool was higher for Slu than for Min, this translated into a greater amount of slurry N recovered in the Nmic pool. Thus, in the following spring after fertilizer application, more slurry N was still in the relatively dynamic pool of Nmic as compared to N from mineral fertilizer (Fig. 5).
Discussion
Lower fertilizer value of cattle slurry than mineral fertilizer under on-farm conditions
In accordance with the assumptions underlying the Swiss fertilization guidelines, and since equal rates of mineral N were applied with slurry and mineral fertilizer, we expected no differences in dry matter yield, N uptake or Ndff between Min and Slu. The underlying assumption is that organic slurry N mineralization will compensate for an expected higher immobilization of inorganic slurry N compared to Min, induced by the additional C input with Slu (Table 2).
Indeed, there were no differences in dry matter yield or total N uptake between Min and Slu on either of the fields (Tables 3 and 4). Even the Con treatment reached the same yield level, except for the last two grass-clover cuts at Field B. Yield levels on both fields stayed on the lower end of yields expected for Switzerland (Richner and Sinaj 2017), likely due to the dry and hot weather conditions during summer 2018. Weather conditions also impeded the ability to see clear differences in N uptake between Min and Slu, as water limitation was probably a more limiting factor than N. Besides, both fields had been under grass-clover previously and had received regular N inputs with three to four applications of cattle slurry per year by the farmer. Therefore, mineral N release from accumulated soil N probably further overlaid the effect of the applied 15N labeled fertilizers.
Contrary to our expectations, and in spite that WSN of slurry was even higher than the ammonium-N content of the slurry (SI Table 1), both Ndff and recovery of mineral N in aboveground biomass were higher for Min than for Slu on both fields and all sampling points, except for the last cut of Field B (Tables 3 and 4). Lower Ndff and recoveries of mineral N for Slu than for Min were also observed by others (Paul and Beauchamp 1995). In their field study, Paul and Beauchamp (1995) recovered 15% of mineral N from cattle slurry, but 29% of mineral fertilizer N in maize biomass, which is lower than the values found in our study (Table 3). However, others found higher recoveries of mineral N from cattle slurry (e.g. Jensen et al. 2000), and overall our values fall well within the range summarized by Chalk et al. (2020). Lower Ndff and recovery of mineral N for Slu than for Min could possibly be explained by either high immobilization or volatilization losses of inorganic slurry N, or low mineralization of organic slurry N, or a combination of all. Mineralization of organic slurry N could not be directly assessed within our study. However, it can be speculated that mineralization of the added slurry N might have been lowered by the hot, dry weather conditions, leading to an underestimation of slurry N recovery in plant biomass in our study. Higher N immobilization in soils from the Slu treatment compared to Min seems likely and is supported by greater amounts of residual fertilizer N in Nmic for Slu than for Min (see Fig. 5) and was also found in several other studies (Griffin et al. 2005; Paul and Beauchamp 1995; Gutser and Dosch 1996). NH3-emissions were lower for Min (3.4% of applied N) than for Slu, where losses reached 6.6 and 10% of total applied N for Field A and Field B, respectively, which is equivalent to 10.8 and 16.4% of applied mineral N (SI Fig. 6), resulting in less mineral N being deposited on the soil. Higher Ndff values for Slu than for Min upon the last cut of the grass-clover could be indicative of a higher residual fertilizer value for Slu than for Min (Webb et al. 2013).
Recovery of total fertilizer N in plant biomass was markedly lower for Slu than for Min, which fits the expectations, as applied amounts of total N were higher with Slu than with Min (about 1.6 times). Overall, values compare well with Smith and Chalk (2018) who reported average 15N recoveries in crops in the year of fertilizer application in the order of 40% for mineral fertilizer and 25% for animal manure.
At the same time, we presumed that the additional organic N applied with slurry would be preserved and recovered in soil, thus, the sum of fertilizer N recovered in soil and biomass should be equal for Slu and Min. For Field A, upon harvest of the maize, total recovery in aboveground biomass and topsoil reached similar levels for Min and Slu and, thus, confirmed our predictions (Table 3 and Fig. 3). This was not the case for Field B, where from Cut 1 to Cut 3, higher total recoveries in the topsoil-biomass-system were reached for Slu compared to Min (Table 4 and Fig. 3). It must be noted, though, that N recovered in stubble and roots was not accounted for, as no destructive sampling took place. Since recovery in aboveground biomass was higher in Min than in Slu throughout these cuts, we assumed the recovery in roots and stubble to follow the same pattern, thus, to be higher for Min than Slu. This could explain the observed differences in total recoveries in the topsoil-biomass-system, in other words the higher proportion of N that remained unaccounted for in Min.
Similar fate of residual N from cattle slurry or mineral fertilizer in topsoil
15N recovery dynamics in topsoil differed between a single fertilizer application under silage maize (Field A) and repeated fertilizer applications under grass-clover (Field B) (Fig. 3). In maize, initially almost all of the applied fertilizer N was recovered in topsoil, both for Slu and Min, with recovery decreasing thereafter due to increasing N uptake of the maize plants and/or translocation into deeper soil layers over time (Hoekstra et al. 2011). The much lower recovery of fertilizer N in topsoil one week after fertilizer application on Field B is likely due to the rapid and more efficient N uptake by grass-clover compared to young maize plants. Indeed, 17% and 45% of Slu and Min, respectively, were recovered in the biomass of the first cut which took place only 24 days after the fertilizer application. With repeated fertilizer applications at Field B, cumulative 15N recovery in total soil N increased. Besides increasing amounts of residual fertilizer N in soil, also internal re-cycling and mineralization of roots could explain the observed temporal pattern. Furthermore, with time more organic residues from the slurry applied on the soil surface might have gotten incorporated into the soil, for example, by earthworms (Hoekstra et al. 2011). Overall, dry weather conditions during summer 2018 might have reduced nitrate movement into deeper soil layers from both Min and Slu. Thus, the observed 15N recoveries in topsoil are likely higher than they would have been in a wetter year.
While the temporal development of the amount of Nmin seemed to be driven mostly by fertilizer addition and the counteracting uptake by plants and microbes (Fig. 4), for the amount of Nmic, no clear effect of fertilizers was observed. However, the amount of Nmic was positively correlated with soil water content (SI Table 2), indicating that besides fertilizer addition, also dry and hot weather conditions during summer 2018 (Fig. 2, SI Fig. 1) likely influenced soil N transformation processes. Our measured values for 15N recovery in Nmic ranged between 2 and 14%, in agreement with values reported by others (Jensen et al. 2000; Hoekstra et al. 2011). Both fertilizer recovery in Nmic and absolute Ndff in Nmic were always markedly higher for Slu than for Min, except for sampling t6 at Field B (Fig. 5), supporting our hypothesis of an increased microbial immobilization of slurry N.
Despite initial differences, the relative distribution of fertilizer N recovered in different soil N pools was similar between Min and Slu in spring of the next year (Fig. 5). Averaged over both treatments, 88.0, 10.9, and 1.1% of residual fertilizer N in soil of Field A were found in Norg, Nmic and Nmin, respectively, while corresponding values at Field B were 77.9, 20.4 and 1.7%. The distribution of residual N in soil N pools is in agreement with other studies (Jensen et al. 2000; Sørensen 2004; Douxchamps et al. 2011). Since total recovery in soil was much higher for Slu than for Min (Fig. 3), these results indicate that in absolute terms, more N derived from fertilizer was still in the rather dynamic Nmic and Nmin pools for Slu compared to Min (Fig. 5). This could explain the higher Ndff values in biomass upon Cut 4 and indicate a higher residual fertilizer value of Slu compared to Min (Schröder et al. 2013; Webb et al. 2013). Norg from fertilizers was previously found to re-mineralize only very slowly and remain in soil for a long time (up to decades) (Sebilo et al. 2013; Sørensen 2004).
Conclusion
In this study, 15N labeling was successfully used under on-farm conditions to assess fertilizer value of both cattle slurry and mineral fertilizer in the year of application, and to trace fertilizers into different soil N pools. Contrary to the assumptions of the Swiss fertilizer guidelines, the fertilizer value of slurry in the year of application was lower than its ammonium content, due to increased NH3 emissions, increased immobilization, and likely lower than expected mineralization of organic slurry N. Despite differences in the year of application, residual N of both fertilizers was found mostly in the non-microbial organic N pool in the following spring, potentially providing N for plant uptake over a very long timeframe, but at a slow rate. In absolute terms, however, more slurry N than mineral fertilizer N remained in the soil, indicating a higher residual fertilizer value of Slu. Further research should focus on the dynamics and driving factors of (re-)mineralization of this organically bound fertilizer N. A better understanding of these processes would facilitate prediction of both the residual fertilizer effect of cattle slurry as well as potential losses via nitrate leaching.
Data availability
The datasets generated during the current study are available from the corresponding author on reasoned request.
References
Bafu (2019) Hitze und Trockenheit im Sommer 2018. In: Umwelt BF (ed). Auswirkungen auf Mensch und Umwelt. Bern
Bosshard C, Oberson A, Leinweber P, Jandl G, Knicker H, Wettstein HR, Kreuzer M, Frossard E (2011) Characterization of fecal nitrogen forms produced by a sheep fed with N-15 labeled ryegrass. Nutr Cycl Agroecosyst 90:355–368
Bosshard C, Sørensen P, Frossard E, Dubois D, Maeder P, Nanzer S, Oberson A (2009) Nitrogen use efficiency of N-15-labelled sheep manure and mineral fertiliser applied to microplots in long-term organic and conventional cropping systems. Nutr Cycl Agroecosyst 83:271–287
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Cabrera M, Beare M (1993) Alkaline persulfate oxidation for determining total nitrogen in microbial biomass extracts. Soil Sci Soc Am J 57:1007–1012
Cassman KG, Dobermann A, Walters DT (2002) Agroecosystems, nitrogen-use efficiency, and nitrogen management. AMBIO J Hum Environ 31:132–140
Chalk PM, Inácio CT, Chen D (2020) Tracing the dynamics of animal excreta N in the soil-plant-atmosphere continuum using 15N enrichment. Adv Agron 61:1
Douxchamps S, Frossard E, Bernasconi SM, van der Hoek R, Schmidt A, Rao IM, Oberson A (2011) Nitrogen recoveries from organic amendments in crop and soil assessed by isotope techniques under tropical field conditions. Plant Soil 341:179–192
Erisman JW, Galloway JN, Seitzinger S, Bleeker A, Dise NB, Petrescu AR, Leach AM, de Vries W (2013) Consequences of human modification of the global nitrogen cycle. Philos Trans Royal Soc B: Biol Sci 368:20130116
Frick H (2022) Nitrate leaching from animal manure—Insights from on-farm and greenhouse studies using 15N labelled cattle slurry. PhD, ETH Zurich
Galloway JN, Aber JD, Erisman JW, Seitzinger SP, Howarth RW, Cowling EB, Cosby BJ (2003) The nitrogen cascade. AIBS Bull 53:341–356
Gardner JB, Drinkwater LE (2009) The fate of nitrogen in grain cropping systems: a meta-analysis of N-15 field experiments. Ecol Appl 19:2167–2184
Gerber C, Purtschert R, Hunkeler D, Hug R, Sültenfuss J (2018) Using environmental tracers to determine the relative importance of travel times in the unsaturated and saturated zones for the delay of nitrate reduction measures. J Hydrol 561:250–266
Goerges T, Dittert K (1998) Improved diffusion technique for 15N: 14N analysis of ammonium and nitrate from aqueous samples by stable isotope spectrometry. Commun Soil Sci Plant Anal 29:361–368
Griffin T, He Z, Honeycutt C (2005) Manure composition affects net transformation of nitrogen from dairy manures. Plant Soil 273:29–38
Gutser R, Dosch P (1996) Cattle-slurry—15N turnover in a long-term lysimeter trial. Springer, Fertilizers and Environment
Hauck RD, Bremner JM (1976) Use of tracers for soil and fertilizer nitrogen research. In: Brady NC (ed) Advances in agronomy. Academic Press.
Hoekstra NJ, Lalor STJ, Richards KG, O’hea, N., Dungait, J. A. J., Schulte, R. P. O. & Schmidt, O. (2011) The fate of slurry-N fractions in herbage and soil during two growing seasons following application. Plant Soil 342:83–96
Holm S (1979) A simple sequentially rejective multiple test procedure. Scandin J Stat 1:65–70
Jensen L, Pedersen I, Hansen T, Nielsen N (2000) Turnover and fate of 15N-labelled cattle slurry ammonium-N applied in the autumn to winter wheat. Eur J Agron 12:23–35
Joergensen RG, Mueller T (1996) The fumigation-extraction method to estimate soil microbial biomass: calibration of the kEN value. Soil Biol Biochem 28:33–37
Jokela W, Randall G (1987) A nitrogen-15 microplot design for measuring plant and soil recovery of fertilizer nitrogen applied to corn. Agron J 79:322–325
Keeney DR, Nelson DW (1982) Nitrogen—inorganic forms 1. Methods of soil analysis. Part 2. Chemical and microbiological properties, pp 643–698
Kreuzer M, Kirchgessner M (1985) Zum Einfluss von Stärkeart und-menge in der Ration auf scheinbare und wahre Verdaulichkeit des Stickstoffs und auf die N-Bilanz beim Schaf. Arch Tierernahr 35:723–731
Krom MD (1980) Spectrophotometric determination of ammonia: a study of a modified Berthelot reaction using salicylate and dichloroisocyanurate. Analyst 105:305–316
Ladha JK, Pathak H, Krupnik TJ, Six J, van Kessel C (2005) Efficiency of fertilizer nitrogen in cereal production: retrospects and prospects. Adv Agron 87:85–156
Langmeier M, Frossard E, Kreuzer M, Mäder P, Dubois D, Oberson A (2002) Nitrogen fertilizer value of cattle manure applied on soils originating from organic and conventional farming systems. Agronomie 22:789–800
Mason VC (1969) Some observations on the distribution and origin of nitrogen in sheep faeces. J Agric Sci 73:99–111
Mayer J, Buegger F, Jensen ES, Schloter M, Heß J (2003) Estimating N rhizodeposition of grain legumes using a 15N in situ stem labelling method. Soil Biol Biochem 35:21–28
Mcauliffe C, Chamblee D, Uribe-Arango H, Woodhouse JR, W. (1958) Influence of Inorganic Nitrogen on Nitrogen Fixation by Legumes as Revealed by N15 1. Agron J 50:334–337
Pagliari PH, Wilson M, Waldrip HM, He Z (2020) Nitrogen and phosphorus characteristics of beef and dairy manure. Anim Manure: Prod Char Environ Concerns Manag 67:45–62
Paul JW, Beauchamp EG (1995) Availability of manure slurry ammonium for corn using 15N-labelled (NH4)2SO4. Can J Soil Sci 75:35–42
Powell JM, Wu Z (1999) Nitrogen-15 labeling of dairy feces and urine for nutrient cycling studies. Agron J 91:814–818
R Core Team (2019) R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing
Richner W, Sinaj S (2017) Grundlagen für die Düngung landwirtschaftlicher Kulturen in der Schweiz (GRUD 2017). Agrarforschung Schweiz 8:1
Schröder J, Bechini L, Bittman S, Brito M, Delin S, Lalor S, Morvan T, Chambers B, Sakrabani R, Sørensen P (2013) Residual N effects from livestock manure inputs to soils. Ramiran International Conference, 2013. Recycling Agricultural, Municipal and Industrial Residues in Agriculture Network (RAMIRAN), S10. 04.1-S10. 04.4
Sebilo M, Mayer B, Nicolardot B, Pinay G, Mariotti A (2013) Long-term fate of nitrate fertilizer in agricultural soils. Proc Natl Acad Sci 110:18185–18189
Shearer G, Kohl DH (1986) N2-fixation in field settings: estimations based on natural 15N abundance. Funct Plant Biol 13:699–756
Smith CJ, Chalk PM (2018) The residual value of fertiliser N in crop sequences: an appraisal of 60 years of research using 15N tracer. Field Crop Res 217:66–74
Sørensen P (2004) Immobilisation, remineralisation and residual effects in subsequent crops of dairy cattle slurry nitrogen compared to mineral fertiliser nitrogen. Plant Soil 267:285–296
Sørensen P, Jensen ES, Nielsen N (1994) Labelling of animal manure nitrogen with 15 N. Plant Soil 162:31–37
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Vandré R, Kaupenjohann M (1998) In situ measurement of ammonia emissions from organic fertilizers in plot experiments. Soil Sci Soc Am J 62:467–473
Ward MH, Jones RR, Brender JD, de Kok TM, Weyer PJ, Nolan BT, Villanueva CM, van Breda SG (2018) Drinking water nitrate and human health: an updated review. Int J Environ Res Public Health 15:1557
Webb J, Sørensen P, Velthof G, Amon B, Pinto M, Rodhe L, Salomon E, Hutchings N, Burczyk P, Reid J (2013) An assessment of the variation of manure nitrogen efficiency throughout Europe and an appraisal of means to increase manure-N efficiency. Adv Agron 119:371–442
Yan M, Pan G, Lavallee JM, Conant RT (2020) Rethinking sources of nitrogen to cereal crops. Glob Change Biol 26:191–199
Zavattaro L, Bechini L, Grignani C, van Evert FK, Mallast J, Spiegel H, Sandén T, Pecio A, Cervera JVG, Guzmán G (2017) Agronomic effects of bovine manure: A review of long-term European field experiments. Eur J Agron 90:127–138
Acknowledgements
The authors would like to thank: A. Kuhn, M. Macsai, A. Munyangabe, L. Schönholzer, and F. Tamburini for analyses and assistance with lab work; F. Perrochet, M. Sauter, B. Stehle, and further technical staff at the Research Institute of Organic Agriculture FiBL for help with the production of 15N labeled hay, field work, and sample preparation; B. Reidy and M. Leuenberger for providing the field site and the greenhouse for production of 15N labeled hay; T. Denninger, C. Kunz, and M. Kreuzer for support with the production and analyses of 15N labeled cattle slurry; N. Bodenhausen for statistical support; H. Wey for meteorological data and valuable discussions; the farmer for providing the two fields and conducting management operations; and L. Dietemann for English proof-reading. Three anonymous reviewers are acknowledged for their valuable comments that helped to improve the manuscript.
Funding
Open access funding provided by Swiss Federal Institute of Technology Zurich. This study was conducted in the framework of the research project “NitroGäu”. The project received funding and was scientifically assisted by the Swiss Federal Office for Agriculture (FOAG) and the Environmental Office of the Canton Solothurn.
Author information
Authors and Affiliations
Contributions
H.F., A.O., E.F. and E.B. designed the experiment. H.R.W., A.O. and H.F. conducted the animal experiment for the production of 15N labeled cattle slurry. H.F. conducted the field experiment. M.C. measured and modelled ammonia emissions. H.F. and M.C. performed lab work. H.F. wrote the manuscript, and A.O., E.B. and E.F. supported internal revisions. All co-authors have commented on a draft of the manuscript and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Animal Testing
The animal experiment for the production of 15N labeled cattle slurry was approved by the Cantonal Veterinary Office Zurich, Switzerland, 31st of January 2018 (license ZH195/17).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Frick, H., Oberson, A., Cormann, M. et al. Similar distribution of 15N labeled cattle slurry and mineral fertilizer in soil after one year. Nutr Cycl Agroecosyst 125, 153–169 (2023). https://doi.org/10.1007/s10705-022-10205-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10705-022-10205-5