Abstract
Ammonia (NH3) constitutes the single largest loss of manure nitrogen (N), making measures targeted at reducing its emissions meaningful for the environment and the overall efficiency of manure N use. In this study, the performance of two emission-reducing techniques, acidification and injection, were studied in a field experiment with grass ley over two growing seasons. Emissions of NH3, crop growth, and N use efficiency were determined from plots fertilized with cattle slurry either band spread, injected or band spread after acidification. The approximate cumulative NH3 losses from the plots with band-spread untreated slurry amounted to about 22 kg NH3-N ha−1 over the observation periods in 2017 and 10 kg NH3-N ha−1 in 2018. The injection and acidification reduced the estimated cumulative NH3 emissions by 43 and 95% respectively in 2018, and both by 97% in 2017. In 2017, the emission-reducing techniques had no impacts on crop growth, but in 2018, acidification increased dry matter yield by 29% and apparent N recovery by 65% compared with band-spread untreated slurry. According to the current results, acidification consistently produced the lowest NH3 emissions and a discernible positive yield effect. It can therefore be recommended instead of injection for reducing NH3 emissions in boreal grass cultivation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite decades of efforts to improve nitrogen (N) use efficiency in agriculture, a major reservoir of N excreted in livestock manures remains underutilized (Bouwman et al. 2009; Leip et al. 2011; Liu et al. 2017). At best, roughly half of the manure N supply is used by crops, while the rest is lost to the environment in different forms (Oenema et al. 2007; McCrakin et al. 2018). Leached nitrate (NO3−) causes eutrophication of surface waters and pollution of groundwaters, volatilized ammonia (NH3) induces the acidification and eutrophication of wider ecosystems, whereas nitrous oxide (N2O) acts as a greenhouse gas (Webb et al. 2013). In addition to the environmental hazards, leakages of reactive N are not economically sensible. Reducing the losses of manure N requires systemic action comprised of ways to ensure sufficient field area for manure application in relation to stock density, to identify alternative management strategies for direct land spreading, and to optimize animal diets, housing, manure storage, and application technologies (Rotz 2004; Sims et al. 2005; Liu et al. 2017).
Losses of manure N over storage and during and directly after soil application are dominated by NH3 volatilization (Webb et al. 2013). According to the modeling of Oenema et al. (2007), an average of 19% of the N excreted in animal housing volatilized as NH3 during storage, and another 19% following soil application. The relative proportions of NH3 and its non-volatile conjugate acid ammonium (NH4+) depend on pH and temperature, so that the presence of NH3 increases with an increase in these variables (Hartung and Phillips 1994; Martinelle and Häggström 1997). In fresh animal excreta, N occurs as mainly organically bound (Bristow et al. 1992; Kirchmann and Witter 1992). The organic N compounds are mineralized at various rates to an inorganic form as NH4+, the urea in urine being a major rapidly degradable source of N (Van Kessel et al. 2000). Marked NH3 release can be expected at pH levels above 7, which are typically reached in manures (Sommer and Hutchings 2001). Due to the dominance of NH3 in N losses, measures targeted at reducing its emissions are meaningful in increasing the overall efficiency of manure N use.
Lowering the slurry pH to 4.5–6.5 by the addition of acid can be used to increase the ratio of NH4+ to NH3 and thus minimize NH3 volatilization (Ndegwa et al. 2008). This acidification practice with strong sulfuric acid (H2SO4) has already become established in Denmark (e.g. Fangueiro et al. 2015). Its effectiveness varies, but in field applications, decreases of up to 80% in NH3 emissions and several dozen kg of conserved N ha−1 have been reported (e.g. Pain et al. 1994; Kai et al. 2008; Fangueiro et al. 2015). Although there are safety risks in handling H2SO4, its high efficacy in comparison to weaker acids and high accessory sulfur (S) content can be considered advantages, especially in conditions where there is a concern of sufficient S supply due to decreased atmospheric deposition and low S fertilization (Keskinen et al. 2016; Michalovicz et al. 2021).
An alternative to acidification with acids in preventing NH3 volatilization is to quickly cover the manure with soil following application to minimize the surface area of slurry exposed to the atmosphere (Sommer and Hutchings 2001; Webb et al. 2013). In comparison to the conventional surface broadcasting method, emissions of NH3 can be roughly halved by band spreading, and reduced by up to ca. 90% by slurry injection or incorporation below the soil surface (Webb et al. 2005). To make the most of the injection technique in NH3 emission reduction, application rates need to be adjusted to the slot volume so that it can hold the amount of slurry applied (Webb et al. 2013). Increasing the volume of the slots decreases NH3 losses but increases the demand for energy due to the soil’s penetration resistance (Hansen et al. 2003).
In this study, the effects of applying cattle slurry with acidification and injection on NH3 emissions, crop growth, and N use efficiency were studied in boreal grass cultivation during two growing seasons. It was hypothesized that the N conserved from volatilization increased the amount of N available for crop uptake and was thus reflected as increased yields. However, a successful reduction of emissions has not always resulted in significant increases in crop N uptake (Webb et al. 2010). This may be related to difficulties in measuring small changes against large background variations caused by the heterogeneity in manure composition, soil fertility, and/or weather conditions or that N has not been the growth limiting factor. On the other hand, the tested methods may have other than N-related influences on crop growth. The injection technique may mechanically damage the crops and thus impair growth (Rodhe et al. 2006). Acidification permits gentler surface application, but pH manipulation affects the composition and processes in the slurry, the impacts of which are not yet thoroughly understood (Fangueiro et al. 2015). This field scale comparison of the two slurry NH3 reduction techniques aims to deepen the understanding of their overall performance in northern growing conditions and serves to encourage their adoption among farmers.
Material and methods
Study site and experimental design
The two-year field experiment was conducted in 2017–2018 in a grass ley field on fine sandy loam (Humic Dystric Regosol, WRB) on the premises of the Natural Resources Institute Finland (Luke) in Maaninka, Kuopio, in east-central Finland (63°8’N, 27°18’E). In the plough layer, the soil pH (H2O) was 6.2, and the content of total carbon (C) and N was 1.6 and 0.15% respectively (Supplementary Table 1). According to the Finnish agronomic soil test, the status of soil phosphorus (P), potassium (K), and S was classified as satisfactory, and calcium (Ca) and magnesium (Mg) as fair. In terms of acid-extractable nutrient reserves in a five-grade classification system ranging from poor to good, the reserves of P, K, and Mg were classified as good.
During the field experiment, meteorological data were obtained from Finnish Meteorological Institute’s observation station located at Luke Maaninka, Kuopio (Finnish Meteorological Institute 2019), less than 200 m from the experimental site, and long-term averages for the climatological normal period 1981–2010 from Pirinen et al. (2012). The mean annual precipitations in 2017 and 2018 were 623 and 512 mm respectively, and the respective mean annual temperatures were 3.8 and 4.5 °C, exceeding the long-term average by 0.7 and 1.3 °C respectively (Supplementary Fig. 1). The Growing degree day (GDD) sum (the daily mean temperature > + 5 °C) ranged from 1073 in 2017 to 1617 °C days in 2018. In 2017, the mean annual precipitation was nearly equal to the long-term average of 612 mm, whereas it was clearly lower in 2018. In the summer season, the monthly precipitation sum varied from 38 in June to 93 mm in July in 2017, the respective values being 99 and 21 mm in 2018. In comparison with the long-term averages of 66 mm in June and 77 mm in July, June and July 2017 were characterized by distinctly drier and wetter conditions respectively, the situation being the opposite in 2018.
On August 10 in 2016, an experimental field comprising grass ley plots of 15 m2 (1.5 m × 10 m) was seeded with a mixture of timothy (Phleum pratense L., cv. Nuutti, seed rate 16 kg ha−1) and meadow fescue (Festuca pratensis Huds., cv. Valtteri, seed rate 9 kg ha−1), and fertilized with NPK compound fertilizer (31 kg N ha−1, 11 kg P ha−1, 10 kg K ha−1). Slurry application treatments implemented after the first cuts in 2017 and 2018 were arranged on the field in a randomized complete block design with four replicates, including non-experimental plots between each experimental plot. The set-up included three cattle slurry treatments with an application rate of 42.5 t ha−1: (1) surface application of untreated slurry (US); (2) (shallow) injection of untreated slurry (US inj.); and (3) surface application of Acidified slurry (AS) and four increasing mineral N fertilizer application levels of 0, 40, 70, and 90 kg ha−1 (min-N). Surface-applied slurry was spread to the ley plots in bands at intervals of 0.25 m by a watering can, representing the band-spreading technique. In terms of injection technique, slurry was injected to a depth of 0.05–0.07 m by a plot-sized slurry spreader with 6 double disks, a capacity of 1 m3, and a working width of 1.5 m. The mineral fertilizers were surface-applied using a Tume RL 1500 spreader.
Due to technical challenges in 2017, slurry injection was carried out on July 3 and surface applications the day after, whereas all slurry applications took place on June 20 in 2018. In 2018, one US inj. plot received less slurry than the other three plots due to a blockage in one of the injection hoses, and this plot was therefore removed from the statistical analysis. Otherwise, the injected amounts were assumed to be equal, despite the fact that the possibility of fluctuating amounts of injected slurry could not be completely excluded among the three other US inj. plots in 2018. The mean content of total N (2.9–3.2 kg t−1), NH4+-N (1.6–2.1 kg t−1), total P (0.56–0.65 kg t−1), and total K (2.7–3.4 kg t−1) were quite similar between the different slurries, with the notable exception of S, which averaged 0.4–0.5 kg t−1 for the untreated slurries and 1.8–2.2 kg t−1 for the acidified slurries. For the second cut, no additional mineral fertilizers were applied to the slurry plots. Mineral P and K fertilizer applications were provided to the treatments with increasing min-N application levels, equal to the amounts in slurries. Application rates of S were not adjusted equally between treatments. Application rates of nutrients (N, P, K, and S) for the second cut in 2017–2018 are given in Table 1. For the first cut in 2017–2018, all experimental plots were fertilized equally using mineral fertilizers (100 kg N ha−1, 0 kg P ha−1, 28 kg K ha−1, and 16 kg S ha−1). Similarly, all plots received an equal mineral fertilization (50 kg N ha−1, 0 kg P ha−1, 52 kg K ha−1, and 7 kg S ha−1) for the third cut in 2018, while the third cut in 2017 was unfertilized.
Acidification procedure and slurry analysis
One week before slurry applications for the grass plots, cattle slurry was collected by a tractor-drawn tank from a covered storage pit (100 m3) serving as a pre-tank for a farm-scale biogas plant on a dairy farm of the Natural Resources Institute Finland in Maaninka, Kuopio. After thorough mixing in the tank, slurry was poured gradually into four pre-weighed open plastic containers (each 1 m3). In the containers, the slurry was weighed and then diluted by adding tap water with a 1:7.5–8.5 (v:w) ratio, targeting a Dry matter (DM) of about 7.7% to enable flow through the slurry spreader. Two containers were preserved untreated at the original pH, and the slurry in the other two containers was acidified, targeting a pH of 5.5 with commercially available concentrated H2SO4 (KemAcipro TECH™ 93%; density of 1.82 kg dm−3). Prior to treatments and in situ pH measurements (Hanna Instruments HI98191, with pH sensor HI72911B, Pietiko Oy, Finland), all slurries were stirred with a container mixer (HLS 0.75/90, Mamec Oy, Finland) at 90 rpm for about 3 min, with the exception of slower stirring to avoid foaming during the pouring of H2SO4. The containers were stored uncovered in an unheated storehouse until field applications. In situ pH measurement was conducted at the end of the short storage period. The acidification treatment represented short-term storage or spreading tank acidification techniques. The acidified slurry, in which the final pH was closer to the targeted value of 5.5, was selected for field applications. In the acidified slurries, the pH was 5.5 in 2017 and 5.6 in 2018, and the DM content was 7.5 and 8.1% respectively. For the untreated slurries, the pH averaged 7.3, with DM 7.4–8.1%. In total, 3.3 and 2.6 l H2SO4 were needed per tonne of slurry to achieve the target pH in 2017 and 2018 respectively.
Soil analysis
After the last harvest of the year, soil samples were taken from each plot with an auger at the depths of 0–0.02, 0.02–0.10, and 0.10–0.25 m on October 23, 2017 and September 25, 2018. Easily available total N and inorganic N (NH4+-N and NO3−-N) were extracted from fresh soil samples with 2 M KCl at a soil:solution ratio of 1:5 (w:v) for 2 h. The suspensions were filtered and frozen until analyzed for NH4+-N and NO3−-N with a Skalar San++ autoanalyzer. Soluble organic N (SON) was taken as the difference between soluble total N acquired after oxidative digestion and inorganic N. Soil S was analyzed from acid ammonium acetate (AAAc, 0.5 M CH3COONH4, 0.5 M CH3COOH, pH 4.65, at a soil:solution ratio of 1:10 (v/v) for 1 h) extracts obtained according to Vuorinen and Mäkitie (1955). Soil pH was measured in a soil–water suspension (1:2.5 v:v).
Yields and plant analyses
The grass plots were harvested by a Haldrup 1500 plot harvester from a 12 m2 area (1.5 m × 8 m) to a stubble height of 0.07 m three times per growing season. In 2017, the first cut was on June 28 and the second on August 11, and the third cut for the unfertilized grass ley was carried out on October 11. In 2018, the corresponding cuts were on June 15, July 27, and September 10.
The DM content was determined gravimetrically by drying fresh samples at 60 °C for 40–48 h, after which the DM yield was calculated. The content of crude protein and digestibility value (D value) of forage grass were analyzed from each cut using the Near-infrared spectroscopy (NIR) technique (Foss NIRSystems XDS analyzer, Valio Ltd). To calculate the total N content (g kg−1 DM) and N yield (kg ha−1), the content of crude protein was divided by a protein-to-N conversion factor of 6.25. The apparent recovery of total and available N was calculated using Eqs. 1 and 2 respectively, where N yieldmin-N 0 = N yield of min-N 0 treatment, where no N fertilizer was applied. Available N represents NH4+-N in terms of slurry, and NH4+-N and NO3−-N in terms of mineral fertilizers.
Mineral N equivalences for the slurry treatments were calculated from the yield response curve of the ley to increasing min-N additions. Determination of total S was carried out according to Miller (1998), using a microwave wet digestion method in a closed vessel. Dry plant samples were digested in concentrated HNO3-30% H2O2 mixtures in a microwave (CEM Mars 6). The concentration of S in extracts was analyzed by an iCAP 6500 DUO ICP emission spectrometer. The concentrations of P and K were determined using the X-ray fluorescence (XRF) method (Valio Ltd).
Ammonia measurements
The NH3 volatilization rates from slurry-treated plots were measured using the equilibrium concentration technique (JTI method) as in Hagner et al. (2021). The method combines the stirred dynamic chamber method with a passive diffusion sampler technique and enables simultaneous field measurements in small plots (Svensson 1994; Mattila 2006). Briefly, in the L-type passive diffusion samplers, adsorption filters (Whatman® Grade 40), which were impregnated with 2% oxalic acid (C2H2O4 × 2 H2O)–methanol (CH3OH) solution and dried in a desiccator, were mounted at the top of the sampler and directly exposed to the ambient air. In turn, in the C-type samplers, the adsorption filters were placed at the bottom and membranes (Fluoropore® PTFE, hydrophobic) on top of the samplers. In each slurry plot, a steel frame was mounted in the ground, covering two slurry bands/slots and an area of 0.12 m2, and the chamber was set then on top of the frame. A white plastic container (length 0.40 m × width 0.32 m × height 0.20 m) served as the ventilated chamber, which was equipped with a battery-operated air-mixing fan. A metal plate was placed in front of the opening of the chamber to exclude the influence of external wind. Both types of passive diffusion samplers were mounted with two replicates on the vertically adjustable holders and placed between the bands/slots both inside the chamber and outside, at about 1.5 m from the chamber.
In 2017, the slurry injection treatment was carried out on July 3, during which three series of NH3 measurements (1.6–2.0 h) were implemented on these plots. Band-applied slurries were spread a day after the injection, and in these plots, three series of measurement periods of 1.5–2.0 h were conducted on the first day (July 4), and one longer measurement period (5.0 h) on both the second (July 5) and third (July 6) day. In 2018, three series of measurement periods of 1.9–2.9 h were carried out during the day of slurry applications on June 20, and two series of 3.0 and 4.7 h on the following day (June 21). Surface applications of slurries were performed within about 1 h at 10–11 a.m., and slurry injection within about 0.5 h at around noon but before 1 p.m. The NH3 measurements were started in each plot within 0.17 h after the slurry application. Furthermore, background concentrations of NH3 were measured from a similar untreated grass ley and soil type within 50 m of the experimental site with two to three replicates, and their time and duration coincided with the NH3 measurements in the experimental plots. After the measurements, the NH3 filters were extracted with 8 ml of H2O for 0.5 h, and the NH4+-N concentrations of the extracts were then analyzed with a Skalar San++ autoanalyzer. The ammonia volatilization rate was calculated using equations as fully described by Svensson (1994) and Mattila (2006). The data from the separate measurement series were extrapolated over the entire period until the end of the last measurement episode, covering an average period of 53 h in 2017 and 29 h in 2018. Exceptionally, the cumulative emissions from the plots that had received injected slurry in 2017 were estimated only over 9 h on the day of slurry application due to technical problems. For the approximate estimate of cumulative NH3 emission, the averages of two consecutive measurements were applied for the intervals between the measurement periods, and the emission during the night (from 9 p.m. to 8 a.m.) was assumed to be insignificant. This was supported by Häni et al. (2016), who reported negligible nighttime emissions of NH3 after application of livestock slurry to grassland.
Weather conditions during NH3 measurements
In the experimental area, precipitation, temperature, and wind speed were recorded by a weather station (a-Weather, a-Lab Oy, Finland) at a sampling rate of 0.25 h. The wind speed was measured by a cup anemometer at a height of 1.7 m. During the growing season, soil moisture (matric suction) was followed by tensiometers (Irrometer®) at depths of 0.20 and 0.40 m with two replicates. To determine the dry bulk density at depths of 0−0.05, 0.05−0.10, 0.10−0.15, and 0.15−0.20 m, soil profile samples were taken by a window-type auger (Ø 0.048 m) with eight replicates and dried at 105 °C for 48 h. During the NH3 measurements, soil samples were also taken by an auger (Ø 0.023 m) to determine the gravimetric soil water content. At depths of 0−0.05, 0.05−0.10, 0.10−0.15, and 0.15−0.20 m, the respective dry bulk density averaged 1.17, 1.27, 1.42, and 1.43 Mg m−3, and was used to convert gravimetric soil water content into volumetric water content.
On July 3–4 in 2017, the injected and band-spread slurries were applied, and the first three NH3 measurement series were conducted in calm and warm weather, with mean and maximum daily temperatures of 16.2–18.6 °C and 21.2–24.4 °C respectively (Supplementary Fig. 2). Thereafter, the weather cooled significantly, and during the last two measurement series, the mean and maximum daily temperatures had dropped to 8.4–11.3 °C and 9.6–13.1 °C respectively. Light rain, with total precipitations of 1.2 and 3.1 mm, fell between NH3 measurements on July 3 and 4, and 4 and 5 respectively. Compared to 2017, the slurry applications and the following three NH3 measurements series were conducted in cooler and windy weather conditions on June 20 in 2018. During the measurement periods on June 20–21, the air temperature varied from 7.3–9.9 °C in the night to 18.1–20.8 °C in the day, with a mean daily temperature of 14.0–15.3 °C. On the day of slurry applications in 2018, wind speed averaged 4.2 m s−1 during spreading. After the last measurement period, rainfall appeared with a total precipitation of 35 mm on June 22. During slurry applications, the plough layer tended to be drier in 2017 than in 2018, as monitored by tensiometers (Supplementary Fig. 3). At depths of 0–0.05, 0.05–0.10, 0.10–0.15, and 0.15–0.20 m, the respective volumetric water contents averaged 25, 27, 31, and 32%, being significantly drier only at a depth of 0.05–0.10 m in 2017 than 2018.
Statistical analyses
Statistical analyses were performed using ANOVA (the MIXED procedure of the SAS software 9.4.; SAS Institute Inc. Cary, NC, USA). In the case of plant and NH3 emission analyses, treatment was considered a fixed, and replicate a random, effect. Years and measurement periods were analyzed separately. Negative NH3 emissions were assumed to be zero. A few outliers were removed from the analysis when required (a blockage in one of the injection hoses in 2018, or unconvincing NH3 emission). Logarithmic or square root transformation was used if the assumption of the equality of variance was invalid. When transformation was used, the Standard error of the mean (SEM) is not presented. In the case of NH3 emission, a 95% confidence interval is presented instead of SEM.
The model for soil analyses was constructed using treatment, soil profile, and treatment × soil profile interaction as fixed effects, while replicate and replicate × soil profile interaction were used as random effects. Years were analyzed separately. The soil profile was considered a repeated effect using a Compound Symmetry covariance structure. A few outliers were removed from the analysis when required. Logarithmic transformation was used if the assumption of the equality of variance was invalid. The analysis of pH was calculated by transforming pH to H3O because of the logarithmic scale of the pH. When transformations were used, SEM is not presented. In the case of volumetric water content, year was considered a fixed, and replicate a random effect.
The Residual maximum likelihood (REML) estimation method and the Kenward-Roger approximation for degrees of freedom were used in all models. Pairwise comparisons of means were determined using Tukey–Kramer’s test with a significance level of 0.05.
Results
Ammonia emissions
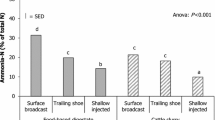
In both study years, slurry injection and acidification treatments reduced NH3 emissions in comparison to band-spread US (Table 2, Fig. 1). However, the reduction in NH3-N volatilization rates was higher in AS compared to US inj., which did not differ significantly from US directly after the slurry application (the first measurement period). In contrast to US, higher NH3 emissions were recorded from the injection treatment in 2018 than in 2017. The acidification treatment consistently produced the lowest NH3 emissions but was not discerned from the US inj. in 2017. Variability between replicate measurements was partly large, generating the substantially wide confidence intervals.
Approximate cumulative average ammonia emission (kg NH3-N ha−1) during a an average period of 53 h after slurry application in 2017, and b 29 h in 2018, except for 9 h for the slurry injection treatment in 2017. Bars represent the lower and upper bounds of 95% confidence intervals. Means (n = 4) for estimated cumulative NH3-N emissions marked with a different letter differ significantly at p ≤ 0.05 (Tukey’s test) within treatments after 1, 2, or 3 days. Note the difference in the scale of the Y-axis. US = untreated cattle slurry applied by band spreading, US inj. = untreated cattle slurry applied by injection, AS = cattle slurry acidified with sulfuric acid, applied by band spreading
The approximate cumulative NH3 losses from the band-spread US plots over the whole 28–54-h periods amounted to about 22 kg NH3-N ha−1 in 2017 (54 h) and 10 kg NH3-N ha−1 in 2018 (28 h) (Fig. 1), composing 31 and 12% of the \({\text {NH}}_{4}^{+}\)-N initially applied in the slurry respectively. For the US inj. (9 h) and AS (53 h), the estimated total NH3-N losses averaged about 0.7 kg ha−1 during the entire monitoring periods in 2017, reducing the cumulative NH3 emissions by 97% in relation to the band-spread US. In 2018, the corresponding reductions were 43% for US inj. and 95% for AS, the estimated total emissions reaching about 5.7 kg NH3-N ha−1 (30 h) and 0.5 kg NH3-N ha−1 (29 h) respectively. Acidification led to a significant decrease in the estimated total NH3-N emissions in both study years. During the day of slurry applications in 2017–2018, the average NH3 volatilization rate from the band-spread US plots was about 3 and 18 times higher than during the measurement series on the second and third day respectively. Consequently, the majority of NH3 losses occurred during the day of slurry application, accounting for an average of 66% of the estimated total losses in the band-spread US plots. Overall, acidification reduced the total NH3-N losses to a very low level, and on the day of AS spreading, the NH3-N losses amounted to 31–83% of the estimated cumulative losses.
Dry matter yield
The first forage cut in 2017, which preceded the slurry applications and was grown with equal mineral fertilization, showed a consistent yield response of an average of 5130 kg DM ha−1 (SEM 168, p = 0.26) and N yield of 93 kg ha−1 (SEM 3.8, p = 0.21) across the field. During the first harvest, the grass canopy was significantly lodged. Differences in the losses of NH3 between the slurry treatments applied for the second cut in 2017 were not reflected in the DM yields (Fig. 2, Supplementary Table 2). The third cut grown without additional fertilizer application remained low, on average 500 kg DM ha−1, and evidenced no residual N fertilizer effect for the slurry treatments in comparison to the min-N 70 treatment (Supplementary Table 2).
The yield response curves (DM yield, kg ha−1) of increasing mineral nitrogen (N) additions (kg ha−1) and the mineral-N equivalences of the slurry treatments in the second cut in a 2017 and b 2018. US = untreated cattle slurry applied by band spreading, US inj. = untreated cattle slurry applied by injection, AS = cattle slurry acidified with sulfuric acid, applied by band spreading
In 2018, sufficient mineral fertilization was again applied for the first cut, but somewhat higher DM yields were harvested from the US (5970 kg ha−1) and US inj. (5950 kg ha−1) than from the min-N 70 (5660 kg ha−1) and AS (5550 kg ha−1) treatments (SEM 129, p < 0.001). Average N yield was 108 kg ha−1 (SEM 4.6, p = 0.21). In the second cut, having received the slurry applications, the min-N 70 treatment was discerned with the highest yield level when compared to the slurry treatments (Fig. 2, Supplementary Table 2). Among the slurry treatments, AS was more productive than the untreated slurries, whether surface spread or injected. In the third cut with a uniform mineral N fertilizer application, a small positive residual effect was attained for US inj. (2150 kg DM ha−1) in comparison to the min-N 70 treatment (1740 kg DM ha−1), whereas US and AS did not differ significantly from the min-N 70 (Supplementary Table 2).
Plant samples
Plant sample analyses are reported only for the second cut, for which the slurry treatments were applied. In 2017, no differences between treatments were recorded in plant N and S concentrations, or in total uptakes of these elements (Table 3). Apparent N recovery was clearly higher from mineral fertilizer than from slurries. When confined to the NH4+-N content of the slurries, higher N recoveries of 23–30% were achieved. According to the yield response curves (Fig. 2), ca. 18% of the total N in US and 18–30% in US inj. and AS were equivalent to min-N. The average D value, K concentration, and P concentration over all treatments were 682 g kg−1 DM, 30 g kg−1 DM, and 3.5 g kg−1 DM respectively. The P concentration of AS (3.7 g kg−1 DM) was significantly higher than the P concentration of US (3.4 g kg−1 DM), but no other differences between treatments were observed.
In 2018, the N concentration of the grass fertilized with AS was higher than that derived from the US treatment (Table 3). The N yields followed DM yields. In apparent N recovery, AS was discerned as superior among the slurry treatments. The apparent N recoveries were higher overall in 2018 than in 2017. Of the NH4+-N content, 44% was recovered from AS, and nearly 30% from the other slurry treatments. The substantial S addition within AS was reflected as a slightly higher S concentration in the grass in 2018 (Table 3). The D value of min-N 70 was significantly lower than the D value of slurry treatments (638 g kg−1 DM vs. 666 g kg−1 DM). The average grass K concentration and P concentration over all treatments were 26 g kg−1 DM and 2.8 g kg−1 DM respectively. The K concentration of US (24 g kg−1 DM) was significantly lower than the K concentration of min-N 70 and AS (27 g kg−1 DM).
Soil
Soil analyses revealed that the non-acidified slurries (US and US inj.) tended to increase the soil pH in the uppermost 0.02-m layer in comparison to AS and min-N treatments (Table 4). A similar trend also occurred at a depth of 0.02–0.10 m, though the differences did not prove significant. High S-containing AS enriched S, especially in the deeper soil layers (0.02–0.10 and 0.10–0.25 m).
In soil easily available (KCl-extractable) N concentrations, no differences between treatments were observed at the end of the 2017 growing season (Table 5). In 2018, the concentration of NO3−-N was slightly higher in US than in the min-N 70 treatment in the 0–0.02 m surface layer and when averaged over all soil depths. In the top 0–0.02 m, the NO3−-N concentration in US was also identified from that in AS. The slurry treatments tended to exhibit somewhat higher SON and easily available total N concentrations in the 0.02–0.10 m layer than those found in the min-N 70 treatment, but the difference proved significant only to US. In practice, the differences in soil easily available N among treatments were marginal. Nearly 90% of the acquired N occurred in organic form at all the studied depths in both years.
Discussion
Acidification was more dependable than injection in reducing ammonia emissions
Acidification proved efficient in minimizing NH3 emissions to insignificant levels relative to band-spread US, because the AS treatment resulted in 90–99% reductions of NH3 emission rates during three measurements periods on the day of slurry applications in the 2017 and 2018 study years. This finding agrees with the study by Hagner et al. (2021) conducted in the same experimental field area in 2019 with cattle slurry acidified by H2SO4 or pyrolysis liquid before band spreading to grass ley. During the consecutive years of 2017–2019 in these two studies, the average cumulative NH3-N losses were estimated to represent roughly only 2% of the NH4+-N (58–87 kg ha−1) applied in slurry within about two days following band spreading of acidified slurries. Indeed, these studies confirmed that a decrease of cattle slurry pH below 6 along with acidification may also enable remarkable reductions in NH3 emissions associated with band-spread slurry in grassland farming in boreal conditions, and offer an alternative low-emission application technique for injection as proposed by Fangueiro et al. (2017, 2018). Although acidification treatment eradicated the liming effect of manure, it was not found to cause soil acidification beyond the level in soil that had received min-N.
Of the slurry application techniques, injection of slurry into the soil is generally regarded as a highly efficient mitigation practice to minimize NH3 losses after field application (Misselbrook et al. 2002; Mattila and Joki-Tokola 2003; Webb et al. 2010; Goedhart and Huijsmans 2017). A re-analysis of 199 Dutch emission experiments conducted on grasslands confirmed a clear ascending trend of total NH3 emissions among manure application techniques as follows: shallow injection < narrow band < surface broadcast; although the results from individual experiments overlapped within these techniques (Goedhart and Huijsmans 2017). In the previous Finnish experiments on grass leys by Mattila and Joki-Tokola (2003), NH3 volatilization was almost completely prevented with a shallow injection at an application rate of 33–62 t cattle slurry ha−1 on mineral and peat soils. In the present study, slurry injection tended to strongly reduce NH3 emission rates on the day of slurry applications in 2017 relative to US treatment, and the achieved reductions (83–96%) in the mean emission rates corresponded to those obtained from AS treatment. However, in 2018, the average emission rates from US inj. treatment were 3.4–8.5 times higher than in 2017, and the respective reductions relative to US remained substantially lower (35–64%). Hansen et al. (2003) demonstrated that the NH3 reduction potential of slurry injection techniques increased linearly when the volume of the slots increased, depending on the injection depth. The lower abatement efficiency achieved using shallow injection in the present study was therefore accounted for by an injection depth that was too shallow in relation to the amount of the applied slurry. This was the probable cause of the slurry surplus in the open slots and exposed slurry at the soil surface to the atmosphere.
As observed in the present study, especially in band-spread US, high emission rates are reported to appear during the first few hours after slurry applications, and consequently, a large proportion of the total NH3 losses typically occur within 24 h (Sommer et al. 1991; Misselbrook et al. 2002; Pfluke et al. 2011; Häni et al. 2016). This general trend in the NH3 emission pattern highlights the importance of abatement efforts to reduce NH3 emissions immediately after slurry applications. The estimated total NH3 losses of applied NH4+-N following band-spread US were in the same range, with an average 16–31% of ammoniacal N reported by Mattila and Joki-Tokola (2003) and Pfluke et al. (2011) for untreated cattle slurry applied in bands to grass ley at rates of 25–62 t ha−1. In addition to application technique and slurry pH, the NH3 emission potential from field-applied slurry is known to be related to e.g. air temperature, air humidity, wind speed, and the content of slurry DM and total ammoniacal N (e.g. Sommer et al. 1991; Sommer and Hutchings 2001; Häni et al. 2016). Compared with the NH3 emission rates from band-spread US in 2018, the higher emissions recorded in 2017 in the present study and in 2019 in the study by Hagner et al. (2021) were probably attributed to the higher air temperature prevailing on the slurry spreading day. Relative dry topsoil conditions were assumed to allow infiltration of slurry into the soil, whereas organic matter-related soil water repellency may have a restrictive effect on water infiltration in grasslands (Rasa et al. 2007). Moreover, surface crusting of field-applied slurry along with drying have been associated with a decrease in NH3 volatilization, with drying affected by the temperature, wind speed, and thickness of the slurry in bands (Sommer et al. 1991; Pfluke et al. 2011). On the slurry spreading day in 2018, the higher wind speed may have furthered the higher emission potential, but also crust formation. According to visual observations, a gust of wind had an impact on the slurry bands, which were distributed to a wider area, resulting in thinner slurry layers. In consequence, the lower emission rates may be partly explained by hastened crust formation. Recently, Pedersen et al. (2021) recommended the measurement of the exposed surface area, i.e. the contact area between the applied manure on the soil surface and the atmosphere, which may help explain differences in abatement efficiency achieved in the prevailing circumstances.
Only acidification generated a positive yield response
The manure N conserved by reduced NH3 emissions is prone to subsequent losses by runoff, leaching, or denitrification (N2O and/or N2 emissions) unless utilized by the crop. In addition to avoiding transfer of environmental impacts from one form to another, agronomic benefits are desired to compensate the farmer for the costs of applying a reduced emission technique (Webb et al. 2010). Previous studies have reported yield increases of up to 25–35% due to N-conserving actions (Pain et al. 1994; Maguire et al. 2011). In the current study, the yield-increasing effect of AS in comparison to US in 2018 fell in this range (29%), and was also reflected in increased grass N concentration and N yield. However, smaller or insignificant effects of the reduced emission techniques on crop yields or N uptake, as evidenced in 2017 in the current study, are also commonly reported (e.g. Smith et al. 2000; Rodhe and Etana 2005; Sadeghpour et al. 2015). Webb et al. (2010) considered the inconsistent responses and lack of significance more likely to indicate the experiments’ limited sensitivity than the lack of a true effect. The additional N uptake enabled by the conserved N may remain small in comparison to variation in manure composition, uniformity of spreading, inherent soil fertility and weather conditions. In the current study, relatively high variation between replicates in the second cut in 2017 (see SEM values in Supplementary Table 2) may have hidden possible treatment effects. Part of the variation probably resulted from unequal stubble height due to the lodged canopy in the preceding first cut, but the observed variability remained largely unexplained. In 2018, the D value of the grass was clearly lower in comparison to 2017, indicating the higher development stage of the grass canopy, which explains the lower average N and S concentrations of the second cut in 2018. Grass K and P concentrations were sufficiently high to assume that K and P fertilization had been sufficient.
Although an approved and established technique for reducing NH3 emissions, injection showed no positive yield effect in comparison to band spreading in either of the study years. Previous studies have shown that slurry injection may decrease crop yield due to mechanical damage caused to roots and the canopy (Rodhe et al. 2006; Maguire et al. 2011). Furthermore, negative effects related to the technique may arise from anaerobic conditions created around the injection band, which may lead to an increase in nitrous oxide (N2O) emissions (Duncan et al. 2017). However, Webb et al. (2010) stated that concern about N2O emissions should not discourage the adoption of NH3 emission-reducing techniques. An advantage of the injection technique is that manure nutrients are introduced close to the root zone, where they may be more easily available to the crop than from surface spreading (Rasmussen 2002). In this study, the difference between US inj. and AS in 2018 could have been smaller without uncertainty concerning the amount of injected slurry.
In the current study, apparent recoveries of manure total N remained generally low (11–15% in 2017 and 17–28% in 2018). Hagner et al. (2021) reported roughly similar values in a field experiment conducted in the same area in 2019. In their study, the apparent total N recovery was 10% from band-spread untreated cattle slurry and 18% from similarly applied acidified slurry. Min-N equivalents were likewise consistent between these studies. According to Powell et al. (2010), manure/fertilizer N use efficiencies (proportions of manure/fertilizer N recovered in the crop) range between 16 and 77%, with a high dependence on climate, crop species, and management factors. In this study, the apparent total N recovery was higher on average in 2018 than in 2017, which is mainly explained by the lower N yield of min-N 0 plots in 2018.
Due to their slowly mineralizable organic N component, manures are known to supply N over several years after application. This feature is emphasized in cattle/dairy manure in comparison to poultry or swine manures (Eghball et al. 2002; Gutser et al. 2005). In the current study, fall-centered growth of the third crop due to delayed first harvests and N applications for all harvests in 2018 prevented a thorough observation of the residual N effect. However, the first and third DM yields in 2018 indicated that US and US inj. had supplied additional N for the grass. Furthermore, at the end of the experiment in 2018, soil easily available N concentrations tended to be somewhat higher in the 0.02–0.10 m soil layer in the soils that had received slurry as the N source than in min-N fertilized soil, though only the difference between min-N and US proved significant and corresponded to merely a few kg N per ha. Schröder et al. (2007) found the first-year residual effect for cattle manures ranged between 1 and 10% of the initial total N input. In the second and third residual years, the corresponding ranges were 2–5% and 1–4% respectively. Comparable residual availabilities have been reported by e.g. Cusick et al. (2006).
Overall, the most interesting observation regarding the soil KCl-extractable N concentrations was the dominance of organic N compounds in the available N pool among all treatments. On a hectare basis, only roughly 1–3 kg N was recovered in inorganic forms (NH4+-N and NO3−-N), while the amount of SON corresponded to ca. 30–40 kg. This finding may reflect the high N demand of continuously growing perennial grass. When N immobilization by plants and microorganisms limits the supply of NH4+ to nitrifiers, the rates of nitrification remain low, whereas at an abundant N availability, lesser competition for NH4+ accelerates the conversion of NH4+ to NO3− (Schimel and Bennett 2004).
Acidification-induced extra sulfur drained in the soil profile but had minor effects on crop uptake
In this experiment, S application was unequal between treatments, but AS received markedly higher S application for the second cut (91 kg ha−1 in 2017 and 78 kg ha−1 in 2018) than the other treatments (1–21 kg ha−1). However, the effect of the high S application on the plant S uptake was only minor. An excess of S in the diet of dairy cattle can be detrimental because of decreased absorption of selenium (Se) and copper (Cu) (National Research Council 2001). If the diet consists only of grass forage, the safe S concentration of forage is below 4.0 g kg−1 DM (National Research Council 2001). In this study, the S concentration of forage was always below 2.0 g kg−1 DM, and the high S application in AS did not result in harmfully high S concentrations in the forage.
In the current study, the status of soil S was satisfactory, and it was assumed that S fertilizer application would not affect the DM yield as observed in the study of Hahtonen and Saarela (1995), and in a one-year study in the same experimental field area by Hyrkäs and Virkajärvi (2014). When comparing the N and S concentrations of this experiment with the critical S equations of Mathot et al. (2009), two observations from the AS treatment placed in the area were “probably sufficient,” and the rest of the data points in the area were “certainly sufficient.” In this study, the S yields of the second cut were 3.9–6.6 kg ha−1, resulting in positive S balances. However, the balances of S in Finnish crop production have generally proved close to zero or negative, which has raised concern about the sufficiency of S (Yli-Halla et al. 2011; Keskinen et al. 2016). The plant availability of total S contained in cattle slurry is less than 40% (Eghball et al. 2002), while S in H2SO4 appears completely as plant available inorganic sulfate (SO42−). In S deficiency conditions, slurry acidification may therefore be beneficial due to S fertilization.
Sulfate is known to be highly mobile in soil. In this study, the excess S had already drained more deeply into the soil profile during the fall soil sampling. Different S-containing additions or amendments may accelerate the leakage of SO42− from fields to waterbodies, contributing to aquatic biogeochemistry. As a side effect of gypsum amendments applied on clayey fields for reducing P losses to coastal water, Ekholm et al. (2011) measured an average of 1.6 to 3.2 times higher mean SO42− concentrations in runoff after amendments in a small catchment, in relation to the mean SO42− concentration of 19 mg l−1 in the reference period. In a leaching test with repacked topsoil columns for ryegrass and cereal crops, Loide et al. (2020) also found an increase in leaching losses of SO42−, with concomitant Ca and K mobilization, from soil columns treated with H2SO4-acidified slurries. Large-scale use of gypsum is not recommended in Finnish catchments with SO42−-poor lakes due to sulfate-induced eutrophication, in which hydrogen sulfide, as the end product of SO42− reduction, reacts with iron oxides to form iron sulfide, accelerating the release of benthic P (e.g. Ekholm et al. 2011). According to rough estimates, approximately 18 times more S was applied in gypsum at a rate of 4.1 t ha−1 in the study by Ekholm et al. (2011) than in the present study. However, to minimize the leaching losses of SO42−, Loide et al. (2020) recommended that the timing of acidified slurry applications coincide with crops’ high nutrient demand.
Conclusions
The two-year field experiment on grass ley evidenced significantly lower NH3 emission rates from acidified slurry and injected slurry than in the prevailing practice of band spreading untreated slurry. Both injection and acidification can therefore be considered credible techniques for reducing NH3 emissions following field application of slurry in boreal conditions, though acidification appeared more reliable. Injection seemed somewhat susceptible to technical difficulties, leaving slurry exposed on the soil surface and potentially destructive to perennial crops. More detailed studies on recommended slot volumes in grass production that consider both the damaging and covering aspects are required. The effects of both acidification and injection on the availability of other nutrients, especially phosphorus, also deserve more attention in future studies. Regardless of the emission-reducing technique used, observable yield benefits resulting from the conserved N are uncertain and depend on the growing conditions.
Data availability
The data are available from the authors on reasonable request.
References
Bouwman AF, Beusen AHW, Billen G (2009) Human alteration of the global nitrogen and phosphorus soil balances for the period 1970–2050. Global Biogeochem Cycles. https://doi.org/10.1029/2009GB003576
Bristow AW, Whitehead DC, Cockburn JE (1992) Nitrogenous constituents in the urine of cattle, sheep and goats. J Sci Food Agric 59:387–394. https://doi.org/10.1002/jsfa.2740590316
Cusick PR, Kelling KA, Powell JM, Muñoz GR (2006) Estimates of residual dairy manure nitrogen availability using various techniques. J Environ Qual 35:2170–2177. https://doi.org/10.2134/jeq2005.0287
Duncan EW, Dell CJ, Kleinman PJA, Beegle DB (2017) Nitrous oxide and ammonia emissions from injected and broadcast-applied dairy slurry. J Environ Qual 46:36–44. https://doi.org/10.2134/jeq2016.05.0171
Eghball B, Wienhold BJ, Gilley JE, Eigenberg RA (2002) Mineralization of manure nutrients. J Soil Water Conserv 57:470–473. https://digitalcommons.unl.edu/biosysengfacpub/139
Ekholm P, Jaakkola E, Kiirikki M, Lahti K, Lehtoranta J, Mäkelä V, Näykki T, Pietola L, Tattari S, Valkama P, Vesikko L, Väisänen S (2011) The effect of gypsum on phosphorus losses at the catchment scale. The Finnish Environment 33/2011. Finnish Environment Institute, Helsinki, 47 p. http://hdl.handle.net/10138/37061
Fangueiro D, Hjorth M, Gioelli F (2015) Acidification of animal slurry – a review. J Environ Manage 149:46–56. https://doi.org/10.1016/j.jenvman.2014.10.001
Fangueiro D, Pereira JLS, Macedo S, Trindade H, Vasconcelos E, Coutinho J (2017) Surface application of acidified cattle slurry compared to slurry injection: Impact on NH3, N2O, CO2 and CH4 emissions and crop uptake. Geoderma 306:160–166. https://doi.org/10.1016/j.geoderma.2017.07.023
Fangueiro D, Pereira JLS, Fraga I, Surgy S, Vasconcelos E, Coutinho J (2018) Band application of acidified slurry as an alternative to slurry injection in a Mediterranean double cropping system: Agronomic effect and gaseous emissions. Agric Ecosyst Environ 267:87–99. https://doi.org/10.1016/j.agee.2018.08.011
Finnish Meteorological Institute (2019) The Finnish Meteorological Institute's open data. https://en.ilmatieteenlaitos.fi/open-data. Creative Commons Attribution 4.0 International Public License. Accessed 2 January 2019.
Goedhart PW, Huijsmans JFM (2017) Accounting for uncertainties in ammonia emission from manure applied to grassland. Soil Use Manag 33:595–602. https://doi.org/10.1111/sum.12381
Gutser R, Ebertseder Th, Weber A, Schraml M, Schmidhalter U (2005) Short-term and residual availability of nitrogen after long-term application of organic fertilizers on arable land. J Plant Nutr Soil Sci 168:439–446. https://doi.org/10.1002/jpln.200520510
Hagner M, Räty M, Nikama J, Rasa K, Peltonen S, Vepsäläinen J, Keskinen R (2021) Slow pyrolysis liquid in reducing NH3 emissions from cattle slurry - Impacts on plant growth and soil organisms. Sci Total Environ 784:147139. https://doi.org/10.1016/j.scitotenv.2021.147139
Hahtonen M, Saarela I (1995) The effects of sulphur application on yield, sulphur content and N/S-ratio of grasses for silage at six sites in Finland. Acta Agric Scand Sect B Soil Plant Sci 45:104–111. https://doi.org/10.1080/09064719509413091
Häni C, Sintermann J, Kupper T, Jocher M, Neftel A (2016) Ammonia emission after slurry application to grassland in Switzerland. Atmospheric Environ 125:92–99. https://doi.org/10.1016/j.atmosenv.2015.10.069
Hansen MN, Sommer SG, Madsen NP (2003) Reduction of ammonia emission by shallow injection: Injection efficiency and additional energy demand. J Environ Qual 32:1099–1104. https://doi.org/10.2134/jeq2003.1099
Hartung J, Phillips VR (1994) Control of gaseous emissions from livestock buildings and manure stores. J Agric Eng Res 57:173–189. https://doi.org/10.1006/jaer.1994.1017
Hyrkäs M, Virkajärvi P (2014) Säilörehunurmen rikkilannoitus 2013. In: Kanninen J (ed) Lannoitus- ja kasvinsuojelukokeiden tuloksia 2013. Maa- ja elintarviketalouden tutkimuskeskus (MTT), Yara Suomi Oy, pp 35–36 (in Finnish).
Kai P, Pedersen P, Jensen JE, Hansen MN, Sommer SG (2008) A whole-farm assessment of the efficacy of slurry acidification in reducing ammonia emissions. Eur J Agron 28:148–154. https://doi.org/10.1016/j.eja.2007.06.004
Keskinen R, Ketoja E, Heikkinen J, Salo T, Uusitalo R, Nuutinen V (2016) 35-year trends of acidity and soluble nutrients in cultivated soils of Finland. Geoderma Reg 7:376–387. https://doi.org/10.1016/j.geodrs.2016.11.005
Kirchmann H, Witter E (1992) Composition of fresh, aerobic and anaerobic farm animal dungs. Bioresour Technol 40:137–142. https://doi.org/10.1016/0960-8524(92)90199-8
Leip A, Britz W, Weiss F, de Vries W (2011) Farm, land, and soil nitrogen budgets for agriculture in Europe calculated with CAPRI. Environ Pollut 159:3243–3253. https://doi.org/10.1016/j.envpol.2011.01.040
Liu Q, Wang J, Bai Z, Ma L, Oenema O (2017) Global animal production and nitrogen and phosphorus flows. Soil Res 55:451. https://doi.org/10.1071/SR17031
Loide V, Saue T, Võsa T, Tamm K (2020) The effect of acidified slurry on crop uptake and leaching of nutrients from a loamy topsoil. Acta Agric Scand B Soil Plant Sci 70:31–38. https://doi.org/10.1080/09064710.2019.1665705
Maguire RO, Kleinman PJA, Dell CJ, Beegle DB, Brandt RC, McGrath JM, Ketterings QM (2011) Manure application technology in reduced tillage and forage systems: a review. J Environ Qual 40:292–301. https://doi.org/10.2134/jeq2009.0228
Martinelle K, Häggström L (1997) On the dissociation constant of ammonium: effects of using an incorrect pK a in calculations of the ammonia concentration in animal cell cultures. Biotechnol Tech 11:549–551. https://doi.org/10.1023/A:1018482520832
Mathot M, Thélier-Huché L, Lambert R (2009) Sulphur and nitrogen content as sulphur deficiency indicator for grasses. Eur J Agron 30:172–176. https://doi.org/10.1016/j.eja.2008.09.004
Mattila PK, Joki-Tokola E (2003) Effect of treatment and application technique of cattle slurry on its utilization by ley: I. Slurry properties and ammonia volatilization. Nutr Cycl Agroecosystems 65:221–230. https://doi.org/10.1023/A:1022619304798
Mattila P (2006) Ammonia emissions from pig and cattle slurry in the field and utilization of slurry nitrogen in crop production. Dissertation, University of Helsinki.
McCrackin ML, Gustafsson BG, Hong B, Howarth RW, Humborg C, Savchuk OP, Svanbäck A, Swaney DP (2018) Opportunities to reduce nutrient inputs to the Baltic Sea by improving manure use efficiency in agriculture. Reg Environ Change 18:1843–1854. https://doi.org/10.1007/s10113-018-1308-8
Michalovicz L, Dick WA, Tormena CA, Müller MML, Cervi EC (2021) Temporal trends of sulfur levels in soils of northwest Ohio (USA) between 2002 and 2014. Land Degrad Dev 32:573–582. https://doi.org/10.1002/ldr.3745
Miller RO (1998) Microwave digestion of plant tissue in a closed vessel (chapter 8). In: Kalra YP (ed) Handbook of reference methods for plant analysis, CRC Press, Taylor & Francis Group. Florida, Boca Raton, pp 69–73
Misselbrook TH, Smith KA, Johnson RA, Pain BF (2002) SE - Structures and environment: Slurry application techniques to reduce ammonia emissions: Results of some UK field-scale experiments. Biosyst Eng 81:313–321. https://doi.org/10.1006/bioe.2001.0017
National Research Council (2001) Nutrient requirements of dairy cattle, seventh, revised. National Academy Press, Washington, DC, p 381
Ndegwa PM, Hristov AN, Arogo J, Sheffield RE (2008) A review of ammonia emission mitigation techniques for concentrated animal feeding operations. Biosyst Eng 100:453–469. https://doi.org/10.1016/j.biosystemseng.2008.05.010
Oenema O, Oudendag D, Velthof GL (2007) Nutrient losses from manure management in the European Union. Livest Sci 112:261–272. https://doi.org/10.1016/j.livsci.2007.09.007
Pain BF, Misselbrook TH, Rees YJ (1994) Effects of nitrification inhibitor and acid addition to cattle slurry on nitrogen losses and herbage yields. Grass Forage Sci 49:209–215. https://doi.org/10.1111/j.1365-2494.1994.tb01994.x
Pedersen J, Andersson K, Feilberg A, Delin S, Hafner S, Nyord T (2021) Effect of exposed surface area on ammonia emissions from untreated, separated, and digested cattle manure. Biosyst Eng 202:66–78. https://doi.org/10.1016/j.biosystemseng.2020.12.005
Pfluke PD, Jokela WE, Bosworth SC (2011) Ammonia volatilization from surface-banded and broadcast application of liquid dairy manure on grass forage. J Environ Qual 40:374–382. https://doi.org/10.2134/jeq2010.0102
Pirinen P, Simola H, Aalto J, Kaukoranta J-P, Karlsson P, Ruuhela R (2012) Climatological statistics of Finland 1981–2010. Reports 2012:1. Finnish Meteorological Institute, Helsinki, Finland, 83 p.
Powell JM, Gourley CJP, Rotz CA, Weaver DM (2010) Nitrogen use efficiency: A potential performance indicator and policy tool for dairy farms. Environ Sci Policy 13:217–228. https://doi.org/10.1016/j.envsci.2010.03.007
Rasa K, Horn R, Räty M, Yli-Halla M, Pietola L (2007) Water repellency of clay, sand and organic soils in Finland. Agric Food Sci 16:267–277. https://doi.org/10.2137/145960607783328218
Rasmussen K (2002) Influence of liquid manure application method on weed control in spring cereals. Weed Res 42:287–298. https://doi.org/10.1046/j.1365-3180.2002.00286.x
Rodhe L, Etana A (2005) Performance of slurry injectors compared with band spreading on three Swedish soils with ley. Biosyst Eng 92:107–118. https://doi.org/10.1016/j.biosystemseng.2005.05.017
Rodhe L, Pell M, Yamulk S (2006) Nitrous oxide, methane and ammonia emissions following slurry spreading on grassland. Soil Use Manag 22:229–237. https://doi.org/10.1111/j.1475-2743.2006.00043.x
Rotz CA (2004) Management to reduce nitrogen losses in animal production. J Anim Sci 82:E119–E137. https://doi.org/10.2527/2004.8213_supplE119x
Sadeghpour A, Hashemi M, Weis SA, Spargo JT, Mehrvarz S, Herbert SJ (2015) Assessing tillage systems for reducing ammonia volatilization from spring-applied slurry manure. Commun Soil Sci Plant Anal 46:724–735. https://doi.org/10.1080/00103624.2015.1005223
Schimel JP, Bennett J (2004) Nitrogen mineralization: challenges of a changing paradigm. Ecology 85:591–602. https://doi.org/10.1890/03-8002
Schröder JJ, Uenk D, Hilhorst GJ (2007) Long-term nitrogen fertilizer replacement value of cattle manures applied to cut grassland. Plant Soil 299:83–99. https://doi.org/10.1007/s11104-007-9365-7
Sims JT, Bergström L, Bowman BT, Oenema O (2005) Nutrient management for intensive animal agriculture: policies and practices for sustainability. Soil Use Manag 21:141–151. https://doi.org/10.1111/j.1475-2743.2005.tb00118.x
Smith KA, Jackson DR, Misselbrook TH, Pain BF, Johnson RA (2000) PA - Precision agriculture: Reduction of ammonia emission by slurry application techniques. J Agric Eng Res 77:277–287. https://doi.org/10.1006/jaer.2000.0604
Sommer SG, Hutchings NJ (2001) Ammonia emission from field applied manure and its reduction – invited paper. Eur J Agron 15:1–15. https://doi.org/10.1016/S1161-0301(01)00112-5
Sommer SG, Olesen JE, Christensen BT (1991) Effects of temperature, wind speed and air humidity on ammonia volatilization from surface applied cattle slurry. J Agric Sci 117:91–100. https://doi.org/10.1017/S0021859600079016
Svensson L (1994) A new dynamic chamber technique for measuring ammonia emissions from land-spread manure and fertilizers. Acta Agric Scand B Soil Plant Sci 44:35–46. https://doi.org/10.1080/09064719409411255
Van Kessel JS, Reeves JBIII, Meisinger JJ (2000) Nitrogen and carbon mineralization of potential manure components. J Environ Qual 29:1669–1677. https://doi.org/10.2134/jeq2000.00472425002900050039x
Vuorinen J, Mäkitie O (1955) The method of soil testing in use in Finland. Agrogeol Publ 63:1–44
Webb J, Menzi H, Pain BF, Misselbrook TH, Dämmgen U, Hendriks H, Döhler H (2005) Managing ammonia emissions from livestock production in Europe. Environ Pollut 135:399–406. https://doi.org/10.1016/j.envpol.2004.11.013
Webb J, Pain B, Bittman S, Morgan J (2010) The impacts of manure application methods on emissions of ammonia, nitrous oxide and on crop response - A review. Agric Ecosyst Environ 137:39–46
Webb J, Sørensen P, Velthof G, Amon B, Pinto M, Rodhe L, Salomon E, Hutchings N, Burczyk P, Reid J (2013) Chapter seven An assessment of the variation of manure nitrogen efficiency throughout Europe and an appraisal of means to increase manure-N efficiency. In: Sparks DL (ed) Adv Agron 119:371–442
Yli-Halla M, Pietola L, Kauppila R (2011) Retrospect of sulphur fertilization in Finland. In: Hera C, Dumitru M, Schnug E, Dorneau A, Haneklaus S, Popescu A, Anton I, Panoiu I (eds) Proceedings of the 15th World Fertilizer Congress of the International Scientific Centre for Fertilizers (CIEC), 29 August – 2 September 2010, Bucharest, Romania, pp 133–138.
Acknowledgements
The authors thank the research technicians Jenni Laakso, Johanna Kanninen, and Arto Pehkonen at Natural Resources Institute Finland in Maaninka, Kuopio for the establishment and management of the field experiment and help in NH3 measurements, and Jussi Sepponen for his help in preliminary statistical analysis. The personnel at Luke’s Jokioinen laboratory and commercial laboratories are thanked for their chemical analysis.
Funding
Open access funding provided by Natural Resources Institute Finland (LUKE). This study was part of the “LantaLogistiikka” project (No. 10347) funded by the European Agricultural Fund for Rural Development through the Centre for Economic Development, Transport and the Environment, North Savo, Finland.
Author information
Authors and Affiliations
Contributions
Conceptualization: MR, SL. Methodology: MR, TS. Formal analysis: MT. Investigation: MR. Writing–original draft preparation: RK, MT, MR. Writing–review and editing: All authors. Funding aquisition: MR, SL.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Consent to participate
All authors consented to participating in the study and all persons entitled to authorship have been so named.
Consent for publication
The submission of this work for publication has been approved by all authors and their institution.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Keskinen, R., Termonen, M., Salo, T. et al. Slurry acidification outperformed injection as an ammonia emission-reducing technique in boreal grass cultivation. Nutr Cycl Agroecosyst 122, 139–156 (2022). https://doi.org/10.1007/s10705-021-10190-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10705-021-10190-1