Abstract
The physiology of ectothermic animals, including fish, is strictly regulated by season-related external factors such as temperature or photoperiod. The immune response and the production of hormones, such as estrogens, are therefore also subject to seasonal changes. This study in common carp aimed to determine how the season affects the estrogen system and the immune response, including the antibacterial response during Aeromonas salmonicida infection. We compared the immune reaction in spring and autumn in the head kidney and liver and found that carp have higher levels of blood 17β-estradiol in autumn, while in the liver of these fish there is a higher constitutive expression of genes encoding vitellogenin, estrogen receptors and Cyp19 aromatase than in spring. Fish sampled in autumn also exhibited higher expression of immune-related genes in the liver. In contrast, in the head kidney from fish sampled in the autumn, the expression of genes encoding estrogen receptors and aromatase was lower than in spring, and a similar profile of expression was also measured in the head kidney for inos, arginases and il-10. In turn, during bacterial infection, we observed higher upregulation of the expression of inos, il-12p35, ifnγ-2, arginase 2 and il-10 in the liver of carp sampled in spring. In the liver of carp infected in spring a higher upregulation of the expression of the genes encoding CRPs was observed compared to fish infected during autumn. The opposite trend occurred in the head kidney, where the upregulation of the expression of the genes involved in the immune response was higher in fish infected in autumn than in those infected in spring. During the infection, also season-dependent changes occurred in the estrogen system. In conclusion, we demonstrated that season differentially affects the estrogenic and immune activity of the head kidney and liver. These results reinforce our previous findings that the endocrine and immune systems cooperate in maintaining homeostasis and fighting infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The immune response of fish is significantly influenced by various external factors, including temperature and light (Magnadottir 2010). These factors drastically differ between seasons and cause season-dependent changes in the immunological parameters such as hematocrit, the number of leukocytes and their activity (respiratory burst, phagocytosis and nitrite production (Saha et al. 2002; Buchtíková et al. 2011; Montero et al. 2022; Zheng et al. 2022). Moreover, the production of antibacterial proteins and IgM, as well as the activity of the complement system, is often reported to be season dependent (Saha et al. 2002; Buchtíková et al. 2011; Dolan et al. 2016; Kondera et al. 2019; Bhardwaj et al. 2022). Furthermore, exposure to many parasites and pathogens varies seasonally, following the parasite life cycle or because of intra-annual variation in the infectivity of pathogens (Banerjee and Bandyopadhyay 2010; Rohlenová et al. 2011; Szwejser et al. 2017b).
Interactions between the endocrine and immune systems enable the body to maintain homeostasis and to adapt to changing internal and external environmental factors. They are based on a common language of signaling molecules and receptors (Verburg-van Kemenade et al. 2017). However, these relationships are quite complex, and this complexity increases even more, due to the fact that the activity of the endocrine system, including the estrogen system, is also affected by external factors such as ambient temperature, light, water quality, oxygen level and salinity (Hjernquist et al. 2012; Szwejser et al. 2017b; Gotthard 2001). The seasonal nature of hormone concentration levels in fish is well-known. For example, in common carp higher concentrations of 17β-estradiol (E2) were usually observed in April and November, while their lowest concentrations occur in August (Saha et al. 2002; Taghizadeh et al. 2013). In turn, in rainbow trout, a higher level of E2 was reported in May and in September (Chen et al. 2021). In addition, high E2 concentrations affect the level of vitellogenin, as well as the expression of estrogen receptors and aromatase (Nelson et al. 2007; Nagler et al. 2012; Ye et al. 2022).
It must be underlined that in fish, like in other vertebrates, steroid hormones, including estrogens, are important regulators of the immune system (Segner et al. 2017; Verburg-van Kemenade et al. 2017). Fish leukocytes express nuclear (ERα, ERβ) and membrane (GPER1/GPR30) estrogen receptors (Iwanowicz et al. 2014; Kovats 2015; Szwejser et al. 2017a), and the most common cellular effect of estrogen-induced immunoregulation is the inhibition of NFκB activity and the regulation of MAPK kinase activity (Biswas et al. 2005). These pathways decrease the expression of proinflammatory mediators (Ghisletti et al. 2005; Burgos-Aceves et al. 2016), reduce the production of reactive oxygen species (ROS) and nitric oxide (NO) (Shelley et al. 2013; Iwanowicz et al. 2014), phagocytosis (Seemann et al. 2015) and lysozyme activity (Iwanowicz et al. 2014). Moreover, E2 affects the adaptive immune response, but in this case, results obtained in different fish species are conflicting (Suzuki et al. 1996; Cuesta et al. 2007; Thilagam et al. 2009). For example, E2 increased B-cell proliferation and antibody production in rainbow trout and crucian carp (Suzuki et al. 1996; Thilagam et al. 2009; Cook 1994), while it decreased B-cell activity in sea bream (Cuesta et al. 2007). Moreover, E2 exposure induced thymic involution and thymocyte differentiation (Seemann et al. 2015). E2 also decreased mitogen-stimulated proliferation of blood leukocytes of channel catfish and rainbow trout (Shelley et al. 2013; Iwanowicz et al. 2014).
Both in mammals and in fish, estrogen-induced immunomodulation is strictly dependent on the hormone concentration and the exposure time (Cabas et al. 2013). For example, Cabas and co-workers (Cabas et al. 2013) showed a decrease of phagocytosis in seabream head kidney cells treated in vitro with E2 for 1 h, while the opposite phenomenon was found in cells incubated with E2 for 16 and 48 h. Moreover, high concentrations of estradiol (10–1000 nM) reduced phagocytosis in the head kidney leukocytes of carp (Yamaguchi et al. 2001), while a lower concentration (5.5 nM) decreased the ROS production in the head kidney cells of seabream (Chaves-Pozo et al. 2003). In turn, in goldfish, high E2 dose (5 μM) suppressed the in vitro chemotaxis of cells from a kidney macrophage cell line (Wang and Belosevic 1995), while 1 μM of E2 downregulated both the expression of pro- and anti-inflammatory mediators in carp monocytes/macrophages (Maciuszek et al. 2020a). However, macrophages of seabream that were stimulated with Vibrio anguillarum genomic DNA (VaDNA) increased their expression of pro-inflammatory mediators upon E2 treatment (18.35 or 183.55 nM) (Liarte et al. 2011).
Furthermore, in vivo studies in fish demonstrated that estradiol influenced the outcome of disease. It significantly reduced the mortality of zebrafish infected with spring viraemia of carp virus (SVCV) (López-Muñoz et al. 2015), while it increased the mortality of goldfish infected with Trypanosoma danilewsky (Wang and Belosevic 1994). Also, in rainbow trout infected with Yersinia ruckeri, both long (at least 6 months) and short-term (14 days) exposure to E2 resulted in increased mortality (Wenger et al. 2011). Such E2-exposure also decreased the expression of complement components (c3-1, c3-3 and factor-H) in the liver of Yersinia ruckeri-infected fish. In turn, our studies found that in carp fed for 14 days with an E2-containnig diet, the expression of anti-inflammatory mediators during Aeromonas salmonicida infection was higher than in fish fed with control diet (Maciuszek et al. 2020a).
Interestingly, immune cells not only respond to hormones but are also capable to produce hormones. For example, carp lymphoid organs (thymus, spleen, head kidney) and leukocytes (lymphocytes, monocytes/macrophages and neutrophilic granulocytes) express the cyp19a and cyp19b encoding aromatase cytochrome P450, involved in the conversion of C19 steroids to estrogens (Szwejser et al. 2017b).
Data about estrogen-induced gender-dependent differences in the immune response in vertebrates are very limited as most studies are performed with male individuals. In medaka infected with Edwardsiella tarda, high endogenous E2 levels caused higher mortality of female fish than male fish (Dong et al. 2017). Also in zebrafish, cyp19a1a was identified as a putative factor involved in this feature as during SVCV infection, cyp19a1a −/− zebrafish males have higher expression of ifnφ1 and lower expression of the viral svcv-n gene than cyp19a1a+/+ males and females. Moreover, it was demonstrated that MITA, a crucial mediator of virus-triggered type I IFN signaling, colocalizes with Cyp19a1a in the endoplasmic reticulum and that Cyp19a1a is a negative regulator of expression of type I IFNs (Lu et al. 2022).
It however remains poorly understood to what extent the estrogen system in fish is responsible for the seasonal changes of the immune response.
The trade-off theory presumes that during the reproductive period, with higher levels of E2, other processes and physiological functions are inhibited to save energy in favor of reproduction (Sueiro and Palacios 2016). However, it must be emphasized that this compromise is not a fixed value and can be optimized according to the actual needs of the organism. Also, costs related to the immune system include costs for recovery and tissue repair after infection or reproduction, e.g., increased protein synthesis (Vargas-Villavicencio et al. 2009).
In the present study, we aim to establish whether the season-related differences in the antimicrobial/inflammatory response of carp are related to the seasonal changes in the estrogen system.
Materials and methods
Animals
Young sexually immature individuals of common carp (Cyprinus carpio L; 9–12 months; line R3×R8; 60–90 g) were obtained from the Institute of Ichthyobiology and Aquaculture, Polish Academy of Science, Golysz, Poland. Studies were conducted in the autumn (November/December 2018) and in the spring (March/April 2019). Fish were exposed to the natural photoperiod occurring in Poland during mid-autumn (9 h of light and 15 h of dark) and mid-spring (12 h of light and 12 h of dark). The water temperature was constant at 20–21 °C. Prior to the experiments, fish were adapted to their new environment for 4 weeks at 21 °C in recirculating tap water at the Institute of Zoology and Biomedical Research in Krakow, Poland. Fish were kept in tanks (volume 375 l, flow rate 4 l/min, density 45 fish/tank and 60 g/l), and all unnecessary interferences were avoided. Fish were fed pelleted dry food (Aller Master, Aller Aqua, Poland) at a daily maintenance rate of 1% of their estimated body weight. To avoid additional stress and/or differences in handling, all samplings were performed by the same person and at the same time of day (at 9.00 am).
All animals were handled in strict accordance with good animal practice as defined by the relevant national and local animal welfare bodies, and procedures were approved by the local ethical committee (2nd Local Institutional Animal Care and Use Committee (IACUC) in Krakow, Poland, license number 291/2017).
Infection
In both seasons, fish were kept in 6 tanks (40 l, with water circulation and aeration). Experiments were performed 2 times independently in both seasons, with 3–4 fish per group/time point every time (CTR, 24 hpi, 96 hpi).
Aeromonas salmonicida subsp. salmonicida from Polish origin was obtained from the Department of Fish Diseases, National Veterinary Research Institute, Pulawy. Bacteria were grown in lysogeny broth (LB) medium for 18 h at 25 °C, centrifuged at 1600×g for 10 min and the bacterial pellet reconstituted in sterile PBS (280 mOsM) as described previously (Maciuszek et al. 2020a, b). Optical density was measured at 625 nm, and data were aligned with a previously derived McFarland scale to determine the bacterial concentration.
Fish were anesthetized (5 min) with tricaine methane sulphonate (TMS; Sigma-Aldrich, St. Louis, MO, USA; 0.2 g/l) buffered with NaHCO3 (POCH, Gliwice, Poland; 0.4 g/l) and intraperitoneally injected with a non-lethal dose of A. salmonicida (4 × 108 bacteria in 250 μl PBS per fish) as described previously (Falco et al. 2012). Fish were sacrificed at 24 and 96 h post-injection (hpi, n=7 per each group). Control animals were kept untreated. All fish were sacrificed by deep and prolonged anesthesia with TMS.
Serum hormone level
Fish were bled through puncture of the caudal vein using a needle attached to a 5 ml syringe. The samples were taken midline just posterior of the anal fin. Every time approximately 5 ml of blood was removed from the caudal vein into a syringe. Blood clots were removed by centrifuging at 800×g for 10 min, and serum was collected and stored at −20 °C for future use.
Hormone levels were determined using commercial kits: (i) for estradiol: DRG, Marburg, Germany (range 10.6–2000 pg/ml, sensitivity 10.60 pg/ml, (ii) for cortisol: Neogen Kit, Lexington, USA (range 0.04–10.0 ng/ml; sensitivity 0,04 ng/ml). Analyses were performed according to the manufacturer’s protocol. All standards and samples from every individual fish were analyzed in duplicate, in the same batch.
Gene expression
Head kidneys and livers of control and infected fish were carefully removed and immediately transferred to fix RNA (EURx, Gdansk, Poland) and kept at −20 °C for further analysis.
RNA was isolated from tissues with GeneMATRIX Universal RNA Purification Kit (EURx, Gdansk, Poland) according to the manufacturer’s protocol. Final elution was carried out in 30 μl of nuclease-free water, to maximize the concentration of RNA. Before proceeding with further analyses, RNA was quantified, and its integrity checked (Tecan Spark NanoQuant PlateTM). Samples were stored at −80 °C.
For each sample, a non-RT (non-reverse transcriptase) control was included. The cDNA synthesis was performed with High-Capacity cDNA Reverse Transcription Kits (Applied Biosystems, Waltham, Massachusetts, USA) according to the manufacturer’s protocol. Briefly, 1 μg of total RNA was added to 10 μl RT master mix containing 2 μl 10X RT Buffer, 0.8 μl 25XdNTP Mix (100 mM); 2 μl 10XRT Random Primers; 1 μl MultiSribe™Reverse Transcriptase and 4.2 μl of nuclease-free water. Samples were then placed into the thermal cycler (Ditabis AG, Pforzheim, Germany, 25 °C at 10 min; 37 °C at 120 min; 85 °C at 5 min followed by rapid cooling to 4 °C). Samples were set at 100 μl with nuclease-free water and stored at −20 °C until further use.
Carp-specific primers (5′–3′) for immune-related (inos, il-1β, il-12p35 ifn-γ2, c3, crp1, crp2, arginase 1, arginase 2, il-10) and endocrine-related (erα, erβ, gper1, cyp19a, cyp19b, vtg) RNA detection were used. The 40S ribosomal protein s11 (40s11) gene served as an internal standard. Accession numbers, primer sequences and their concentrations are listed in Supplementary Table 1.
For RT-qPCR 4 μl cDNA and forward and reverse primers (2 μl each) were added to 7 μl SYBR®Select Master Mix (Applied Biosystems, Waltham, Massachusetts, USA). RT-qPCR (2 min at 50 °C, 2 min at 95 °C, 40 cycles of 15 s at 95 °C, 60 s at 60 °C) was carried out with a Rotor-Gene Q (Qiagen, Hilden, Germany). Following each run, melt curves were collected by detecting fluorescence from 60 to 90 °C at 1 °C intervals.
Constitutive expression was rendered as a ratio of target gene vs. reference gene (40S ribosomal protein s11 gene) and was calculated according to the following equation:
where E is the amplification efficiency and Ct is the number of PCR cycles needed for the signal to exceed a predetermined threshold value (Pfaffl 2001).
Changes in gene expression upon seasons, between autumn and spring, were rendered as a ratio of target gene vs. reference gene (40S ribosomal protein s11 gene) relative to expression in control samples according to the following equation:
Statistical analysis
Analysis mRNA levels were performed in Microsoft Excel, and statistical analysis was performed with GraphPad 8 Software (San Diego, CA, USA). Data were expressed as mean and standard error (SE). The assumptions of normality and equality of variance were found. Shapiro-Wilk and Gaussian distribution for normality test, and F test and Brown-Forsythe test for variances, were used. Differences in the serum level of E2 and constitutive gene expression between autumn and spring were compared using the Mann-Whitney U test. Significance of differences in in vivo study were compared by two-way analysis of variance (ANOVA), followed by post hoc Tukey’s test for multiple comparisons. Results of interactions in two way ANOVA analysis are included in Supplementary Table 2. The differences were considered statistically significant at p<0.05.
Results
Season-dependent differences in the estrogen system
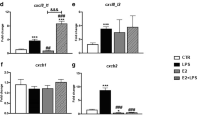
In autumn, the level of plasma 17β-estradiol and the gene expression of vitellogenin (vtg) in the liver were significantly higher than in the spring (Fig. 1a, b). The constitutive expression of genes encoding estrogen receptors (ERα, GPER1) and cyp19a in the head kidney was significantly lower in autumn compared to spring (Fig. 1c). In contrast, in the liver, constitutive expression of genes encoding estrogen receptors (ERβ and GPER1), as well as enzymes involved in the E2 conversion (CYP19a and b) was higher in the autumn than in the spring (Fig. 1d).
Season-dependent differences in the estrogen system. a—level of E2 in blood plasma, b—gene expression of vitellogenin in the liver, c, d—gene expression of estrogen receptors (erα, erβ and gper1) and enzymes involved in E2 conversion (cyp19a and b) in the head kidney (HK) and liver. Constitutive gene expression was determined by quantitative RT-qPCR and expressed relative to the expression of the 40S ribosomal protein s11 gene. Averages and SE (n=5–7). Stars indicates significant differences between autumn (orange bar) and spring (green bar) (* p ≤ 0.05, ** p ≤ 0.01)
Season-dependent differences in the constitutive gene expression of inflammatory markers
Lower constitutive expression of inos, arginase 1 and 2, il-10 was observed in the head kidney in the autumn (Fig. 2a, b). In the liver, only ifn-γ2 expression was lower during this season (Fig. 2c). In the liver constitutive expression of inos, il-1β, arginase 1, arginase 2 and il-10 was lower in the spring than in the autumn (Fig. 2c, d). Constitutive expression of genes encoding C3, CRP1 and CRP2 did not differ between both seasons (Fig. 2e).
Season-dependent differences in the constitutive expression of inflammatory mediators in the head kidney (a, b—HK) and liver (c—e). Constitutive gene expression of proinflammatory (inos, il-1β, il-12p35, ifn-γ2), anti-inflammatory (arginase 1, arginase 2, il-10) mediators and acute phase proteins (c3, crp1, crp2) was determined by quantitative RT-qPCR and expressed relative to the expression of the 40S ribosomal protein s11 gene. Averages and SE (n=5–7). Stars indicates significant differences between autumn (orange bar) and spring (green bar) (* p ≤ 0.05, ** p ≤ 0.01)
Season-dependent and infection-induced differences in gene expression of inflammatory markers during bacterial infection
In both seasons, at 24 hpi, the expression of genes encoding for IL-1β and IL-10 was upregulated in the head kidney (Fig. 3a, b, g). Upregulation of inos expression was significantly higher in autumn than in spring. Upregulation of the expression of il-12p35, ifn-γ2 and arginase 2 in the head kidney was only found in the autumn (Fig. 3c, d, f). In contrast, in the liver, the infection-induced upregulation of the expression of inflammatory markers was mainly observed in the spring. In spring, infection induced upregulation of inos at 24 hpi (Fig. 4a), while upregulated expression of il-12p35, ifn-γ2, arginase 2 and il-10 was observed only in the spring both at 24 and 96 hpi (Fig. 4c–g). Upregulation of il-1β expression was also found in the liver in the autumn at 24 hpi (Fig. 4b).
Season-dependent differences in the gene expression of inflammatory markers in the head kidney of Aeromonas salmonicida infected fish. Fish were i.p. injected with A. salmonicida (4 × 108 bacteria in 250 μl PBS per fish). At 24 and 96 h post-infection (hpi) the head kidneys were collected, and gene expression of proinflammatory (a—inos, b—il-1β, c—il-12p35, d—ifn-γ2) and anti-inflammatory (e—arginase 1, f—arginase 2, g—il-10) mediators was measured by quantitative RT-qPCR. Averages and SE (n=5–7). Changes in gene expression are shown as x-fold increase compared to the control group without infection (CTR) and standardized for the housekeeping gene 40S ribosomal protein s11. Mean values not sharing letters are statistically different between autumn (orange bar) and spring (green bar)
Season-dependent differences in the gene expression of inflammatory markers in the liver in Aeromonas salmonicida infected fish. Fish were i.p. injected with A. salmonicida (4 × 108 bacteria in 250 μl PBS per fish). At 24 and 96 h post-infection (hpi) the livers were collected, and gene expression of proinflammatory (a—inos, b—il-1β, c—il-12p35, d—ifn-γ2), anti-inflammatory (e—arginase 1, f—arginase 2, g—il-10) mediators was measured by quantitative RT-qPCR. Averages and SE (n=5-7). Changes in gene expression are shown as x-fold increase compared to the control group without infection (CTR) and standardized for the housekeeping gene 40S ribosomal protein s11. Mean values not sharing letters are statistically different between autumn (orange bar) and spring (green bar)
In the liver, infection downregulated the expression of crp1 (at 24 hpi in the autumn and spring and at 96 hpi only in the autumn) and crp2 (at 24 and 96 hpi in the autumn) (Fig. 5), while in the spring, at 96 hpi, expression of crp2 was upregulated (Fig. 5c).
Season-dependent differences in the gene expression of acute phase proteins in the liver in Aeromonas salmonicida infected fish. Fish were i.p. injected with A. salmonicida (4 × 108 bacteria in 250 μl PBS per fish). At 24 and 96 h post-infection (hpi) the livers were collected, and gene expression of acute phase proteins (a—c3, b—crp1, c—crp2) was measured by quantitative RT-qPCR. Averages and SE (n=5–7). Changes in gene expression are shown as x-fold increase compared to the control group without infection (CTR) and standardized for the housekeeping gene 40S ribosomal protein s11. Mean values not sharing letters are statistically different between autumn (orange bar) and spring (green bar)
Season-dependent and infection-induced changes in the expression of receptors and enzymes from the estrogen system
In the spring, expression of erα, gper1 and cyp19b was upregulated in the head kidney at 96 hpi compared to the expression observed in control animals (Fig. 6a, c, e). A similar phenomenon was not observed in the autumn. In this season only infection-induced upregulation of cyp19a expression was found at 24 hpi (Fig. 6d).
Season-dependent and infection-induced changes in estrogen system in the head kidney. Fish were i.p. injected with A. salmonicida (4 × 108 bacteria in 250 μl PBS per fish). At 24 and 96 h post-infection (hpi) the head kidneys were collected, and gene expression of estrogen receptors (a—erα, b—erβ and c—gper1) and enzymes involved in E2 conversion (d—e cyp19a and b) was measured by quantitative RT-qPCR. Averages and SE (n=5–7). Changes in gene expression are shown as x-fold increase compared to the control group without infection (CTR) and standardized for the housekeeping gene 40S ribosomal protein s11. Mean values not sharing letters are statistically different between autumn (orange bar) and spring (green bar)
In the liver, during spring, A. salmonicida infection induced upregulation of gper1, cyp19a and cyp19b (at 24 hpi) (Fig. 7c–e) and erα (at 96 hpi) (Fig. 7a).
Season-dependent and infection-induced changes in the expression of estrogen system genes in the liver. Fish were i.p. injected with A. salmonicida (4 × 108 bacteria in 250 μl PBS per fish). At 24 and 96 h post-infection (hpi) the livers were collected, and gene expression of estrogen receptors (a—erα, b—erβ and c—gper1) and enzymes involved in E2 conversion (d—e, cyp19a and b) was measured by quantitative RT-qPCR. Averages and SE (n=5–7). Changes in gene expression are shown as x-fold increase compared to the control group without infection (CTR) and standardized for the housekeeping gene 40S ribosomal protein s11. Mean values not sharing letters are statistically different between autumn (orange bar) and spring (green bar)
The level of 17β-estradiol in the blood plasma did not differ between control and infected animals in the spring, while in the autumn it was lower in infected fish (at 24 hpi) than in control uninfected animals and in fish at 96 hpi (Fig. S1). The levels of cortisol in the blood plasma did not differ in all examined samples (Fig. S2).
Discussion
We identified seasonal differences in the antimicrobial/inflammatory response of common carp and observed distinctive differences between the estrogen system in the head kidney and liver.
When comparing the activity of the estrogen system of carp throughout spring and autumn we found that fish sampled in the autumn exhibited higher levels of plasma E2. Previously such differences, both in the level of blood and gonadal E2, were found in several fish species (Saha et al. 2002; Taghizadeh et al. 2013; Soranganba 2022).
In the present studies, in the liver of fish sampled during springtime, we also observed higher constitutive expression of genes encoding vitellogenin, a precursor of egg yolk, estrogen receptors (ERα, GPER1) and estrogen synthase (CYP19), responsible for the biosynthesis of estrogens. Like in mammals, fish liver plays a crucial role in digestion, in energy metabolism, xenobiotic detoxification, biosynthesis of serum proteins and also in endocrine and immune responses (Taylor et al. 2022). The liver also participates in steroidogenesis (Rajakumar and Senthilkumaran 2020). In oviparous animals, such as teleost fish, the female liver is the main organ for the synthesis of oocyte constituents, such as vitellogenins and the zona pellucida proteins (choriogenins and other minor vitamin-binding proteins). Their synthesis is under direct control of estrogens (Qiao et al. 2016). In many fish species, the liver undergoes seasonal variations in size and in the content of fat and glycogen, and these changes depend on the fish gender and estrogen concentrations, as they can be abolished either by ovariectomy in females or by the administration of estrogen to mature males (Ishii and Yamamoto 1970). Recently, Chen and coworkers (Chen et al. 2021) described season-related differences in the gene expression of estrogen receptors and vitellogenin in the liver of rainbow trout. Similar to our observation for carp, the constitutive expression of these genes in rainbow trout was higher in the course of autumn (October–November) than in the spring. Also, compared to the breeding females (sampled in April), non-breeding females of the orange-spotted grouper (sampled in February) had lower expression of vtg, erα, erβ1 and erβ2 in the liver (Ye et al. 2022).
Interestingly, in the liver of carp we now also observed season-related differences in the expression of the genes involved in the immune response. During autumn, the constitutive expression of genes encoding iNOS, IL-1β, arginase 1 and 2 as well as IL-10 was higher than in the spring. We did not observe such differences in the constitutive expression of genes encoding for acute phase proteins. In contrast, a recent study of Zheng and coworkers (Zheng et al. 2022) in females of Chinese sturgeons showed lower levels of plasma CRP in autumn and winter than in spring and summer, whereas in males lower CRP values were registered throughout the first three seasons and they increased in winter. Previously, seasonal differences in the expression of genes encoding immune mediators were also observed in the liver of Nile tilapia, for which higher expression of these genes was measured in summer and late summer. However, for this experiment, the fish were sampled in their natural living environment, and therefore the authors concluded that these differences correlated with variations during different seasons in the water temperature, oxygen level, concentrations of ammonia and nitrite, as well as with water contamination (Haredi et al. 2020).
We also verified if the antibacterial immune response in carp differs between autumn and spring. Fish were infected with Aeromonas salmonicida which causes in carp erythrodermatitis manifested by hemorrhagic changes and inflammation (Falco et al. 2012; Pionnier et al. 2013; Maciuszek et al. 2020a, b). In common carp, A. salmonicida do not cause sepsis, although due to the influence of secondary infections and their consequences, it can cause fish death (mortality less than 20%) (Molnár et al. 2019).
In the liver of A. salmonicida-infected fish we found upregulation of the gene expression of several pro- and anti-inflammatory mediators, and surprisingly these increases were more pronounced in fish sampled in the spring compared to those sampled in the autumn. Previously, A. salmonicida-induced changes in the expression of some immune-related genes were observed by Pionnier and colleagues (Pionnier et al. 2013); however, the authors did not specify the season in which these experiments were performed. Similarly, Dietrich and co-workers (Dietrich et al. 2022) found that at 48 h of A. salmonicida infection, the expression of tnfα, il-6a, but not ifng-2a, is upregulated in the liver of carp. In turn, in turbot, an infection with A. salmonicida induced upregulation of immune related genes such as MHCI and II, IgGFcR1, C1qB and nitric oxide synthase trafficker, and downregulation of genes associated with the acute phase response and metabolic processes (Millán et al. 2011).
Moreover, in spring, bacterial infection also caused an upregulation of the expression of genes encoding for membrane estrogen receptor GPER1 and CYP19 (24 hpi) in the liver.
Throughout these seasons, we also measured the activity of the estrogen and immune system in the head kidney, which in teleost fish comprises a lymphoid organ, a major hematopoietic organ and an endocrine gland producing cortisol, catecholamines and thyroid hormones (Geven and Klaren 2017; Verburg-van Kemenade et al. 2017). In contrast to the results obtained for the liver, in the head kidney of fish that were sampled in the autumn, we found lower constitutive expression of the genes encoding estrogen receptors (ERα and GPER1) and CYP19a than in spring. Also, the constitutive expression of immune-related genes in the head kidney, such as inos, arginase 1 and 2 and il-10, was lower in autumn than in the spring.
In turn, the results in three-spined sticklebacks showed that during spring there is an increased expression of genes involved in the adaptive immune response (cd8a, foxp3b, orai1, il-1r-like, tbk1), while late winter was characterized by signatures of innate immunity (including IL-1 signaling and non-classical complement activity) and adjusted toll-like receptor signaling (Brown et al. 2016). Moreover, in goldfish, differences in the expression of genes related to the innate immune response were observed both between the breeding (November) and non-breeding season (March), but also between males and females (Zhong et al. 2014). During the breeding season they found an increased expression of tnfα1 and 2, ifn-γ, ccl-1 and cxcl8 in the head kidney of females, compared to females in non-breeding season. Moreover, in the head kidney of males they did not observe such changes. Interestingly, in the other organs (spleen, gill and liver) higher expression of these genes was noticed in females sampled during the breeding season, compared to non-breeding females and males.
Like in the liver, also in the head kidney A. salmonicida infection up-regulated expression of genes involved in the inflammatory reaction; however, in this case strong upregulations were observed in the head kidney of fish sampled in the autumn. Previous studies of carp found that after intraperitoneal injection with A. salmonicida, at 6 hpi there was an increase in the expression of genes encoding pro-inflammatory mediators (tnfα1 and 2) and anti-inflammatory il-10. Interestingly, no changes were observed at the later time points studied (12 h – 5 days) (Falco et al. 2012). Moreover, for A. salmonicida-infected crucian carp, Ling and coworkers (Ling et al. 2019) observed elevated expression of il-1β, tnfα, il-11 and c-lysozyme. Moreover, elevated expression of pro-inflammatory genes was also demonstrated in the head kidney of infected carp by the Pionnier and colleagues (Pionnier et al. 2013). They found increased expression of crp1, bf/c2, c3 and masp2. In, turn, in turbot, after infection with A. salmonicida an upregulation of genes related to the immune/defense response was observed (Millán et al. 2011). Unfortunately, these publications did not study the impact of season. In turn, Buchtíková et al. (2011) have shown in carp blood plasma higher activity of the alternative complement pathway in November and April than in other months, what may suggest its important protective role before wintertime and spawning period.
In wintertime, Montero and coworkers (Montero et al. 2022) found a decreased number of lymphoid cells in the head kidney, spleen and thymus of rainbow trout that were kept under constant photoperiod and water temperature throughout the year. In carp, changes in the number of T- and B-cells and their activity were observed in spring (May), summer (August) and autumn (October). An increase in the number of T-cells and a decrease in number of B-cells were demonstrated in summer and autumn (Rudenko et al. 2019).
Moreover, upon A. salmonicida infection of rainbow trout, seasonal differences in the immune response, with decreased B- and T-cell responses in the winter, were described. Also, a strong increase in the level of natural, but not specific, antibody levels only appeared in fish (at 12 hpi) during the wintertime. Similar results indicating a decrease of leukocyte numbers and immunosuppression in response to the antigen during winter were published for other fish species including carp (Wishkovsky and Avtalion 1987; Saha et al. 2002; Rijkers and Frederix-Wolters 1980). For example, in rainbow trout it was found that phagocytosis, complement lytic activity, respiratory burst activity and opsonization capacity of plasma were higher at 20 °C than at 5–10 °C (Nikoskelainen 2004).
In overwintering channel catfish that were challenged with Aeromonas hydrophila, Yang and coworkers (Yang et al. 2015) observed higher expression of il-1β and il-8 in the head kidney between December and April.
We observed a convincing seasonal correlation between the constitutive expression of genes that encode for the estrogen system and the inflammatory mediators both in the liver (higher expression of both groups of genes in the autumn than in the spring) and in the head kidney (higher expression of both groups of genes in the spring than in the autumn). We however do not have direct proof that E2, via estrogen receptors, differentially regulates the immune response in both organs in spring- and autumn-time. Nevertheless, our previous studies confirmed that, during spring time, E2-supplementation changes the expression of immune-related genes in the head kidney and liver of A. salmonicida-infected carp (Maciuszek et al. 2020a). Results describing immunomodulatory effects of E2 were also published for rainbow trout during Yersenia ruckeri infection (Wenger et al. 2011), for goldfish during parasite infection (Wang and Belosevic 1994), for Atlantic salmon during lice infection (Krasnov et al. 2015) and for zebrafish during viral infection (López-Muñoz et al. 2015) or Microcystis aeruginosa infection (Liu et al. 2018). E2 probably modulates the immune response by affecting the activity of leukocytes involved in the innate response. For example, rainbow trout infected with Yersenia ruckeri after feeding for two weeks (short exposure) or for 5 months (long exposure) with E2 displayed downregulation of the expression of several immune-related genes in the liver such as c3-1, c3-3 and factor-H (Wenger et al. 2011). Similar studies found that in Edwardsiella ictururi infected fish, E2 downregulated expression of the hepcidin 1 gene (Robertson et al. 2009).
Interestingly, we also found that in both seasons, infection differentially affected the level of E2. As mentioned before, we noted a high concentration of this hormone in the control fish during autumn (November/December), while it was low in the spring season (March/April). However, at 24 hpi, the level of E2 decreased in the autumn and now showed similarly low levels in both seasons. In the autumn, at 96 hpi the level of E2 increased to the level observed in control fish from this season, while it did not change in infected fish during springtime. Also, in other fish species, infection-induced changes in E2 levels were observed. For example, in Atlantic salmon, a decrease in E2 after infection with the ectoparasite salmon louse was shown at 3- and 16-days post-infection (Krasnov et al. 2015). Most probably, the changes in E2 level may have an immunomodulatory character.
In conclusion, we observed that the antibacterial/immune response of carp differed between seasons. We found season-dependent changes in the estrogen system, indicating an immunomodulatory role for this hormone. Moreover, we demonstrated that season differentially affects the estrogenic and immune activity of the head kidney and liver. These results reinforce our previous findings that the endocrine and immune systems cooperate in maintaining homeostasis and fighting infection.
Data availability
The data will be made available by the authors upon request. The datasets generated during and/or analyzed during the current study will be available in the https://uj.rodbuk.pl/.
References
Banerjee S, Bandyopadhyay PK (2010) Observation on prevalence of ectoparasites in carp fingerlings in two districts of West Bengal. J Parasit Dis 34:44–47. https://doi.org/10.1007/s12639-010-0003-6
Bhardwaj AK, Chandra RK, Pati AK, Tripathi MK (2022) Seasonal immune rhythm of leukocytes in the freshwater snakehead fish, Channa punctatus. J Comp Physiol B 192:727–736. https://doi.org/10.1007/s00360-022-01460-7
Biswas DK, Singh S, Shi Q et al (2005) Crossroads of estrogen receptor and NF-κB signaling. Sci STKE 2005. https://doi.org/10.1126/stke.2882005pe27
Brown M, Hablützel P, Friberg IM et al (2016) Seasonal immunoregulation in a naturally-occurring vertebrate. BMC Genom 17:369. https://doi.org/10.1186/s12864-016-2701-7
Buchtíková S, Šimková A, Rohlenová K et al (2011) The seasonal changes in innate immunity of the common carp (Cyprinus carpio). Aquaculture 318:169–175. https://doi.org/10.1016/j.aquaculture.2011.05.013
Burgos-Aceves MA, Cohen A, Smith Y, Faggio C (2016) Estrogen regulation of gene expression in the teleost fish immune system. Fish Shellfish Immunol 58:42–49. https://doi.org/10.1016/j.fsi.2016.09.006
Cabas I, Rodenas MC, Abellán E et al (2013) Estrogen signaling through the G-protein–coupled estrogen receptor regulates granulocyte activation in fish. J Immunol 191:4628–4639. https://doi.org/10.4049/jimmunol.1301613
Chaves-Pozo E, Pelegrin P, Mulero V et al (2003) A role for acidophilic granulocytes in the testis of the gilthead seabream (Sparus aurata L., Teleostei). J Endocrinol 179:165–174. https://doi.org/10.1677/joe.0.1790165
Chen H, Bi B, Kong L et al (2021) Seasonal changes in plasma hormones, sex-related genes transcription in brain, liver and ovary during gonadal development in female rainbow trout (Oncorhynchus mykiss). Fishes 6:62. https://doi.org/10.3390/fishes6040062
Cook J (1994) The effects of stress, background colour and steroid hormones on the lymphocytes of rainbow trout (Oncorhynchus Mykiss)'. PhD thesis. University of Luton. http://hdl.handle.net/10547/621946
Cuesta A, Vargas-Chacoff L, García-López A et al (2007) Effect of sex-steroid hormones, testosterone and estradiol, on humoral immune parameters of gilthead seabream. Fish Shellfish Immunol 23:693–700. https://doi.org/10.1016/j.fsi.2007.01.015
Dietrich MA, Adamek M, Teitge F et al (2022) Proteomic analysis of carp seminal plasma provides insights into the immune response to bacterial infection of the male reproductive system. Fish Shellfish Immunol 127:822–835. https://doi.org/10.1016/j.fsi.2022.07.019
Dolan BP, Fisher KM, Colvin ME et al (2016) Innate and adaptive immune responses in migrating spring-run adult chinook salmon, Oncorhynchus tshawytscha. Fish Shellfish Immunol 48:136–144. https://doi.org/10.1016/j.fsi.2015.11.015
Dong M, Seemann F, Humble JL et al (2017) Modification of the plasma complement protein profile by exogenous estrogens is indicative of a compromised immune competence in marine medaka (Oryzias melastigma). Fish Shellfish Immunol 70:260–269. https://doi.org/10.1016/j.fsi.2017.09.020
Falco A, Frost P, Miest J et al (2012) Reduced inflammatory response to Aeromonas salmonicida infection in common carp (Cyprinus carpio L.) fed with β-glucan supplements. Fish Shellfish Immunol 32:1051–1057. https://doi.org/10.1016/j.fsi.2012.02.028
Geven EJW, Klaren PHM (2017) The teleost head kidney: integrating thyroid and immune signalling. Dev Comp Immunol 66:73–83. https://doi.org/10.1016/j.dci.2016.06.025
Ghisletti S, Meda C, Maggi A, Vegeto E (2005) 17β-Estradiol inhibits inflammatory gene expression by controlling NF-κB intracellular localization. Mol Cell Biol 25(8):2957–2968. https://doi.org/10.1128/MCB.25.8.2957-2968.2005
Gotthard K (2001) Growth strategies of ectothermic animals in temperate environments. In: Atkinson D, Thorndyke M (eds) Environment and animal development: genes, life histories, and plasticity. BIOS Scientific Publishers, Oxford, pp 287–303
Haredi AMM, Mourad M, Tanekhy M et al (2020) Lake Edku pollutants induced biochemical and histopathological alterations in muscle tissues of Nile Tilapia (Oreochromis niloticus). Toxicol Environ Health Sci 12:247–255. https://doi.org/10.1007/s13530-020-00042-w
Hjernquist MB, Söderman F, Jönsson KI et al (2012) Seasonality determines patterns of growth and age structure over a geographic gradient in an ectothermic vertebrate. Oecologia 170:641–649. https://doi.org/10.1007/s00442-012-2338-4
Ishii K, Yamamoto K (1970) Sexual differences of the liver cells in the goldfish, Carassius auratus, L. Bull. Fac. Fish. Hokkaido Univ 21:161–168
Iwanowicz LR, Stafford JL, Patiño R et al (2014) Channel catfish (Ictalurus punctatus) leukocytes express estrogen receptor isoforms ERα and ERβ2 and are functionally modulated by estrogens. Fish Shellfish Immunol 40:109–119. https://doi.org/10.1016/j.fsi.2014.06.021
Kondera E, Ługowska K, Witeska M (2019) Annual changes in hematological parameters of common carp juveniles under laboratory conditions. Ann Wars Univ Life Sci - SGGW - Anim Sci 58:143–151. https://doi.org/10.22630/AAS.2019.58.2.15
Kovats S (2015) Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol 294:63–69. https://doi.org/10.1016/j.cellimm.2015.01.018
Krasnov A, Wesmajervi Breiland MS, Hatlen B et al (2015) Sexual maturation and administration of 17β-estradiol and testosterone induce complex gene expression changes in skin and increase resistance of Atlantic salmon to ectoparasite salmon louse. Gen Comp Endocrinol 212:34–43. https://doi.org/10.1016/j.ygcen.2015.01.002
Liarte S, Chaves-Pozo E, Abellán E et al (2011) 17β-Estradiol regulates gilthead seabream professional phagocyte responses through macrophage activation. Dev Comp Immunol 35:19–27. https://doi.org/10.1016/j.dci.2010.07.007
Ling X, Dong W, Zhang Y et al (2019) Comparative transcriptomics and histopathological analysis of crucian carp infection by atypical Aeromonas salmonicida. Fish Shellfish Immunol 94:294–307. https://doi.org/10.1016/j.fsi.2019.09.006
Liu G, Ke M, Fan X et al (2018) Reproductive and endocrine-disrupting toxicity of Microcystis aeruginosa in female zebrafish. Chemosphere 192:289–296. https://doi.org/10.1016/j.chemosphere.2017.10.167
López-Muñoz A, Liarte S, Gómez-González NE et al (2015) Estrogen receptor 2b deficiency impairs the antiviral response of zebrafish. Dev Comp Immunol 53:55–62. https://doi.org/10.1016/j.dci.2015.06.008
Lu L-F, Jiang J-Y, Du W-X et al (2022) Fish female-biased gene cyp19a1a leads to female antiviral response attenuation between sexes by autophagic degradation of MITA. PLOS Pathog 18:e1010626. https://doi.org/10.1371/journal.ppat.1010626
Maciuszek M, Pijanowski L, Pekala-Safinska A et al (2020a) 17β-Estradiol affects the innate immune response in common carp. Fish Physiol Biochem 46:1775–1794. https://doi.org/10.1007/s10695-020-00827-3
Maciuszek M, Pijanowski L, Pekala-Safinska A et al (2020b) 17α-ethinylestradiol and 4-tert-octylphenol concurrently disrupt the immune response of common carp. Fish Shellfish Immunol 107:238–250. https://doi.org/10.1016/j.fsi.2020.10.005
Magnadottir B (2010) Immunological control of fish diseases. Mar Biotechnol 12:361–379. https://doi.org/10.1007/s10126-010-9279-x
Millán A, Gómez-Tato A, Pardo BG et al (2011) Gene expression profiles of the spleen, liver, and head kidney in Turbot (Scophthalmus maximus) Along the infection process with Aeromonas salmonicida using an immune-enriched oligo-microarray. Mar Biotechnol 13:1099–1114. https://doi.org/10.1007/s10126-011-9374-7
Molnár K, Székely C, Láng M (2019) Field guide to the control of warmwater fish diseases in Central and Eastern Europe, the Caucasus and Central Asia. In: FAO Fisheries and Aquaculture Circular No.1182. FAO, Ankara
Montero R, Chan JTH, Müller C et al (2022) Variations in rainbow trout immune responses against A. salmonicida: evidence of an internal seasonal clock in Oncorhynchus mykiss. Biology 11:174. https://doi.org/10.3390/biology11020174
Nagler JJ, Cavileer TD, Verducci JS et al (2012) Estrogen receptor mRNA expression patterns in the liver and ovary of female rainbow trout over a complete reproductive cycle. Gen Comp Endocrinol 178:556–561. https://doi.org/10.1016/j.ygcen.2012.06.010
Nelson ER, Wiehler WB, Cole WC, Habibi HR (2007) Homologous regulation of estrogen receptor subtypes in goldfish (Carassius auratus). Mol Reprod Dev 74:1105–1112. https://doi.org/10.1002/mrd.20634
Nikoskelainen S (2004) Effect of environmental temperature on rainbow trout (Oncorhynchus mykiss) innate immunity. Dev Comp Immunol 28:581–592. https://doi.org/10.1016/j.dci.2003.10.003
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45–e445. https://doi.org/10.1093/nar/29.9.e45
Pionnier N, Falco A, Miest J et al (2013) Dietary β-glucan stimulate complement and C-reactive protein acute phase responses in common carp (Cyprinus carpio) during an Aeromonas salmonicida infection. Fish Shellfish Immunol 34:819–831. https://doi.org/10.1016/j.fsi.2012.12.017
Qiao Q, Le Manach S, Sotton B et al (2016) Deep sexual dimorphism in adult medaka fish liver highlighted by multi-omic approach. Sci Rep 6:32459. https://doi.org/10.1038/srep32459
Rajakumar A, Senthilkumaran B (2020) Steroidogenesis and its regulation in teleost-a review. Fish Physiol Biochem 46:803–818. https://doi.org/10.1007/s10695-019-00752-0
Rijkers GT, Frederix-Wolters EMH (1980) The immune system of cyprinid fish. Kinetics and temperature dependence of antibody-producing cells in carp (Cyprinus carpio). Immunology 41(1):91–97
Robertson LS, Iwanowicz LR, Marranca JM (2009) Identification of centrarchid hepcidins and evidence that 17β-estradiol disrupts constitutive expression of hepcidin-1 and inducible expression of hepcidin-2 in largemouth bass (Micropterus salmoides). Fish Shellfish Immunol 26:898–907. https://doi.org/10.1016/j.fsi.2009.03.023
Rohlenová K, Morand S, Hyršl P et al (2011) Are fish immune systems really affected by parasites? An immunoecological study of common carp (Cyprinus carpio). Parasit Vectors 4:120. https://doi.org/10.1186/1756-3305-4-120
Rudenko OP, Paranyak RP, Kovalchuk NA et al (2019) Influence of seasonal factors on carp fish immune reactivity. Ukr J Ecol 9:168–173. https://doi.org/10.15421/2019_75
Saha NR, Usami T, Suzuki Y (2002) Seasonal changes in the immune activities of common carp (Cyprinus carpio). Fish Physiol Biochem 26:379–387. https://doi.org/10.1023/B:FISH.0000009275.25834.67
Seemann F, Knigge T, Olivier S, Monsinjon T (2015) Exogenous 17 β -oestradiol (E2) modifies thymus growth and regionalization in European sea bass Dicentrarchus labrax: e2 affects thymus development in Dicentrarchus labrax. J Fish Biol 86:1186–1198. https://doi.org/10.1111/jfb.12626
Segner H, Verburg-van Kemenade BML, Chadzinska M (2017) The immunomodulatory role of the hypothalamus-pituitary-gonad axis: proximate mechanism for reproduction-immune trade offs? Dev Comp Immunol 66:43–60. https://doi.org/10.1016/j.dci.2016.07.004
Shelley LK, Osachoff HL, van Aggelen GC et al (2013) Alteration of immune function endpoints and differential expression of estrogen receptor isoforms in leukocytes from 17β-estradiol exposed rainbow trout (Oncorhynchus mykiss). Gen Comp Endocrinol 180:24–32. https://doi.org/10.1016/j.ygcen.2012.09.014
Soranganba N (2022) Seasonal changes in the estradiol level in different age groups of Amur common carp in Terai region of Uttarakhand. Pharma Innovation J 11(10S):82–85. https://www.thepharmajournal.com/archives/2022/vol11issue10S/PartB/S-11-9-242-563.pdf
Sueiro MC, Palacios MG (2016) Immunological and health-state parameters in the Patagonian rockfish Sebastes oculatus. Their relation to chemical stressors and seasonal changes. Fish Shellfish Immunol 48:71–78. https://doi.org/10.1016/j.fsi.2015.11.021
Suzuki Y, Orito M, Iigo M et al (1996) Seasonal changes in blood IgM levels in goldfish, with special reference to water temperature and gonadal maturation. Fish Sci 62:754–759. https://doi.org/10.2331/fishsci.62.754
Szwejser E, Maciuszek M, Casanova-Nakayama A et al (2017a) A role for multiple estrogen receptors in immune regulation of common carp. Dev Comp Immunol 66:61–72. https://doi.org/10.1016/j.dci.2016.04.003
Szwejser E, Verburg-van Kemenade BML, Maciuszek M, Chadzinska M (2017b) Estrogen-dependent seasonal adaptations in the immune response of fish. Horm Behav 88:15–24. https://doi.org/10.1016/j.yhbeh.2016.10.007
Taghizadeh V, Imanpoor MR, Mehdinejad N (2013) Study the seasonal steroid hormones of common carp in Caspian Sea, Iran. SpringerPlus 2:193. https://doi.org/10.1186/2193-1801-2-193
Taylor RS, Ruiz Daniels R, Dobie R et al (2022) Single cell transcriptomics of Atlantic salmon (Salmo salar L.) liver reveals cellular heterogeneity and immunological responses to challenge by Aeromonas salmonicida. Front Immunol 13:984799. https://doi.org/10.3389/fimmu.2022.984799
Thilagam H, Gopalakrishnan S, Bo J, Wang K-J (2009) Effect of 17β-estradiol on the immunocompetence of japanese sea bass (Lateolabrax japonicus). Environ Toxicol Chem 28:1722. https://doi.org/10.1897/08-642.1
Vargas-Villavicencio JA, De León-Nava MA, Morales-Montor J (2009) Immunoendocrine mechanisms associated with resistance or susceptibility to parasitic diseases during pregnancy. Neuroimmunomodulation 16:114–121. https://doi.org/10.1159/000180266
Verburg-van Kemenade BML, Cohen N, Chadzinska M (2017) Neuroendocrine-immune interaction: evolutionarily conserved mechanisms that maintain allostasis in an ever-changing environment. Dev Comp Immunol 66:2–23. https://doi.org/10.1016/j.dci.2016.05.015
Wang R, Belosevic M (1994) Estradiol increases susceptibility of goldfish to Trypanosoma danilewskyi. Dev Comp Immunol 18:377–387. https://doi.org/10.1016/0145-305X(94)90003-5
Wang R, Belosevic M (1995) The in vitro effects of estradiol and cortisol on the function of a long-term goldfish macrophage cell line. Dev Comp Immunol 19:327–336. https://doi.org/10.1016/0145-305X(95)00018-O
Wenger M, Sattler U, Goldschmidt-Clermont E, Segner H (2011) 17Beta-estradiol affects the response of complement components and survival of rainbow trout (Oncorhynchus mykiss) challenged by bacterial infection. Fish Shellfish Immunol 31:90–97. https://doi.org/10.1016/j.fsi.2011.04.007
Wishkovsky A, Avtalion RR (1987) Induction of immunosuppression to bacterial antigens in carp, Cyprinus carpio. J Fish Biol 31:101–106. https://doi.org/10.1111/j.1095-8649.1987.tb05300.x
Yamaguchi T, Watanuki H, Sakai M (2001) Effects of estradiol, progesterone and testosterone on the function of carp, Cyprinus carpio, phagocytes in vitro. Comp Biochem Physiol Part C Toxicol Pharmacol 129:49–55. https://doi.org/10.1016/S1532-0456(01)00176-4
Yang B, Wang C, Hu H et al (2015) Repeated handling compromises the immune suppression and improves the disease resistance in overwintering channel catfish (Ictalurus punctatus). Fish Shellfish Immunol 47:418–428. https://doi.org/10.1016/j.fsi.2015.09.010
Ye Z, Zhao T, Wei Q et al (2022) Distinct roles of estrogen receptors in the regulation of vitellogenin expression in orange-spotted grouper (Epinephelus coioides). Int J Mol Sci 23:8632. https://doi.org/10.3390/ijms23158632
Zheng Y, Zhang Y, Xie Z et al (2022) Seasonal changes of growth, immune parameters and liver function in wild Chinese Sturgeons under indoor conditions: implication for artificial rearing. Front Physiol 13:894729. https://doi.org/10.3389/fphys.2022.894729
Zhong H, Zhou Y, Yu F et al (2014) Seasonal changes and human chorionic gonadotrophin (hCG) effects on innate immune genes expression in goldfish (Carassius auratus). Fish Shellfish Immunol 38:303–310. https://doi.org/10.1016/j.fsi.2014.03.033
Acknowledgements
Authors want to thank Dr A. Safinska-Pekala for providing of A. salmonicida.
Funding
This work was supported by the Polish National Science Center (grant no. 2015/19/B/NZ6/00895).
Author information
Authors and Affiliations
Contributions
MM: co-designed and performed the experiments, collected, analyzed and discussed data and wrote the first version of the manuscript. LP: performed some experiments. LVK: discussed the data and edited the manuscript. MC: co-designed experiments, supervised the study and co-wrote the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Ethical approval
The animal study was reviewed and approved by the 2nd Local Institutional Animal Care and Use Committee (IACUC) in Krakow, Poland, license number 291/2017.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 78.0 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maciuszek, M., Pijanowski, L., Kemenade, L.Vv. et al. Season affects the estrogen system and the immune response of common carp. Fish Physiol Biochem 50, 797–812 (2024). https://doi.org/10.1007/s10695-023-01286-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-023-01286-2