Abstract
Production of sterile mono-sex fish is of great significance for sustainable aquaculture as well as germ cell transplantation. In this study, we aimed to produce mono-sex triploid yellow drum, including genotypic females (XXX female) and sex-reversed phenotypic males (XXX male). Firstly, the mono-female triploids were produced through cold-shock treatment on eggs fertilized with sperm from neo-males. Then, the mono-male triploids were produced by the sex reversal of mono-female triploids with oral administration of letrozole (LZ). We comparatively investigated the growth and gonadal development in the mono-sex triploids. The results showed that the triploids displayed similar growth performance to their diploids throughout their first year, but had impaired gonadosomatic index and gametogenesis. No mature gametes were produced in the triploids during their first spawning season. Meanwhile, we analyzed the process of gametogenesis in the both sex of triploids. Ultrastructure of gametogenesis showed that the germ cells arrested at abnormal metaphase 1 in females, while males had irregular meiotic divisions, variable-sized spermatid and degenerated cells. The expression levels of meiosis-related genes (i.e., sycp3 and rec8) confirmed the abnormal meiosis in the triploids. Furthermore, the gonadal development was also determined by the expression patterns of vasa, dmrt1 and cyp19a1a. Abnormal expression of vasa mRNA and protein were detected in triploids. High cyp19a1a expression levels suggested the sex steroid hormones production might be at least partially functional in triploid females. In addition, high dmrt1 expression levels confirmed the masculinization and testicular development of sex-reversed triploid males by LZ. Our findings provide an efficient protocol to produce sterile mono-sex triploid yellow drum and provide new insights into the mechanism of gonadal sterility of triploid fish.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Triploidy is a condition of having three sets of chromosomes (3n) in an organism or cell. In aquaculture, inducing triploidy is a commonly-used method to produce sterile fish with improved growth performance and reduced reproductive problems (Tiwary et al. 2004; Maxime 2010). In general, triploid fish are functionally sterile because they cannot produce normal gametes due to the irregular meiotic division of chromosomes. Therefore, triploid fish can avoid the energy consumption for sexual maturation and allocate more energy towards somatic growth. Moreover, sterile triploid fish can minimize the environmental impacts of escaped culture fish and avoid genetic interactions of the wild population.
In aquaculture, the triploidy has been achieved in many fish species, such as rainbow trout (Oncorhynchus mykiss) (Lincoln and Scott 1983), blue tilapia (Oreochromis aureus) (Mol et al. 1994), crucian carp (Carassius auratus) (Zhang et al. 2021) and sea bass (Dicentrarchus labrax) (Felip et al. 2001). Most of triploid fish have a growth advantage over diploid fish (Okomoda et al. 2020; Tiwary et al. 2004). However, the growth advantage of triploids is not always apparent (Aydın et al. 2022; do Nascimento et al. 2017; Mol et al. 1994), and some studies have found that triploid fish have greater mortality than diploid fish (Cotter et al. 2000; Koenig et al. 2011). Meanwhile, there are also numerous studies of gonadal development in triploid fish. For instance, impaired gonadal development and abnormal gametogenesis were found in the most of triploid fish (Comai 2005; Golpour et al. 2016; Takeuchi et al. 2018). However, some studies also have found the triploids could produce small amounts of mature gametes (do Nascimento et al. 2017; Hamasaki et al. 2013; Zhang et al. 2021). Therefore, the characteristic of growth and gonadal development of triploid fish is species-specific. On the other hand, many fish species have sexual dimorphic in growth, with female fish would grow faster (Wu and Gui 1999; Bye and Lincoln 1986; Chen et al. 2007) or slower (Beardmore et al. 2001; Farley et al. 2014) than males. Therefore, the production of mono-sex populations of triploid fish is of great significant for improving its yield and provide an effective mean for sustainable aquaculture. Meanwhile, mono-sex triploid fish also have the potential for scientific research, such as serving as surrogate recipients for germ cell transplantation (Okutsu et al. 2007; Yoshizaki et al. 2011).
Yellow drum (Nibea albiflora), which has an XY sex determining system, is one of the most commercially valuable Sciaenidae species distributed in East Asia (Sun et al. 2018). Especially in China, this species is economically important for fisher and aquaculture due to its high market demand and nutritional value (Xu et al. 2018; Chen et al. 2017). Yellow drum has a high tolerance to crowding and a wide range of physiological conditions, making it suitable for intensive aquaculture in sea cages along the coastal waters of China (Xu et al. 2018; Tian et al. 2020). Meanwhile, yellow drum also has a sexually dimorphic in growth, with females growing faster and larger than males at the same age (Qin et al. 2019). Previous studies have successfully established a system for producing all-female yellow drum by mating of normal females with phenotypic males (known as neo-males) (Chen et al. 2017; Xu et al. 2018; Qin et al. 2019). However, the studies about the production of mono-sex triploid yellow drum have not reported. Although the gonadal development of triploid Nibe croaker (Nibea mitsukurii) has reported in previous work (Takeuchi et al. 2018), the knowledge on the growth and detailed gonadal development of mono-sex triploid yellow drum also remains unclear.
In this study, we aimed to produce mono-sex triploid yellow drum, including genotypic female triploids (XXX female) and sex-reversed phenotypic male triploids (XXX male). Firstly, the mono-female triploids were produced through cold-shock treatment on eggs fertilized with sperm from neo-males. Then, the mono-male triploids were produced by the sex reversal of mono-female triploids with oral administration of letrozole (LZ), an aromatase inhibitor that can induce sex reversal in fish. We also investigated the growth performance, gonadal development, gametogenesis and gene expression of the triploids from 75 to 390 days post hatching (dph), a period that encompasses their first reproductive cycle. To our knowledge, this is the first study on the production and characterization of mono-sex triploid yellow drum.
Materials and methods
Broodstock management and gamete collection
At May 2020, yellow drum broodstock were obtained from the research station of Zhejiang Marine Fisheries Research Institute (Xishan Island, City of Zhoushan, China) and reared in 24 m2 indoor tank with flow seawater under controlled conditions (a photoperiod of 13 h light vs. 11 h dark and a water temperature of 18–22 ℃). All experiments were approved by the Institutional Animal Care and Use Ethics Committee of the Zhejiang Marine Fisheries Research Institute, China. To induce spawning, the females were intraperitoneally injected with luteinizing hormone releasing hormone (LHRH)-A2 (Ningbo Second Hormone Factory, Ningbo, China) at a dose of 1.5 ug/kg body weight. Thirty-two hours after the hormone injection, ovulated eggs were collected from each female by gently squeezing the abdomens by hands for use in artificial insemination. At the same time, milt was also collected from ripe males (without the injection of exogenous hormone) by pressing their abdomens and subsequently mixed. The eggs and milt were held separately in dry glass bowls and kept at 4 ℃ in the dark prior to use. One mL milt was mixed with approximately 16,000 eggs from each female, and sperm was subsequently activated by adding 100 mL 22 ℃ seawater. Five minutes (min) after fertilization, 500 mL seawater were added again.
Production of genotypic female and sex-reversal phenotypic male triploid yellow drum
In order to produce genotypic triploid females (XXX female), the eggs were separately obtained from 5 genotypic diploid females of three years-old (XX, female), and fertilized with sperm obtained from 3 diploid neo-males of two years-old (XX, male). After artificial insemination, the fertilized eggs received a cold-shock treatment to suppress second polar body extrusion, according to the protocol optimized by Chen et al. (2017). Briefly, the eggs were maintained in seawater at 3 ℃ for 10 min at 2.5 min after fertilization, and then cultured in 1000-L seed production tank under 21–22 ℃.
At 25 dph, a total of 1200 fish of genotypic triploid female survived. In our previous study, the diploid yellow drum were 100% sex reversed into neo-males by orally administered of 10 mg/kg LZ for 60 days (from 25 to 85 dph) (Qin et al. 2019). Therefore, in this study, a half of genotypic triploid females (approximately 600) were selected and transferred to 3 fiber-reinforced plastic (FRP) tanks (200-L, n = 200 fish per tank), and were orally administered a dose of 10 mg/kg LZ from 30 to 90 dph to produce sex-reversed phenotypic triploid males (XXX, male). Briefly, the LZ (Macklin, Shanghai, China) was dissolved in 100% ethanol, and slowly poured onto the commercial feed pellets to make pellets containing dose of 10 mg/kg LZ. On the other hand, the eggs (from 3 genotypic diploid females of three years-old) fertilized using sperm obtained from 3 normal diploid males of two years-old (XY) and treated without cold-shock treatment were used as controls. The experimental design is illustrated in Fig. 1. Larvae and juvenile fish were reared as described in Xu et al. (2018). Briefly, the fish were fed twice-daily (larvae were fed with Brachionus rotundiformis and Artemia salina, and juveniles were fed with the commercial pellets). At 150 dph, fish of each group was individually marked by injection of different dye into the dorsal fin, then mixed and reared together in 36 m2 tank.
Experiment design for production of mono-sex triploid yellow drum. Genotypic triploid females (3n♀, XXX) were produced by cold-shock treatment (at 3 ℃ for 10 min at 2.5 min after fertilization) on eggs fertilized with sperm from neo-males, and phenotypic triploid males (3n♂, XXX) were produced from sex-reversal of genotypic triploid females by oral administration of letrozole (LZ) from 30 to 90 dph (A). The eggs (from 3 genotypic diploid females of three years-old) fertilized using sperm obtained from 3 normal diploid males of two years-old (XY) and treated without cold-shock treatment were used as controls (B). LZ, letrozole; dph, days post hatching
At 12 h post fertilization, 200 to 300 eggs at the blastula stage were collected and examined under a stereo microscope. The fertilization rates were calculated by dividing the number of developing embryos by the total number of eggs and expressed as a percentage. The hatching rate were evaluated approximately 24 h after hatching from 200 to 300 fertilized eggs, and determined as the number of morphologically normal larvae divided by the number of fertilized eggs, which was also expressed as a percentage.
Ploidy determination
In order to determine ploidy of triploids, the relative DNA content of hatched larvae at 1 dph and the number of chromosomes of 5-month-old fish were measured. Hatched larvae obtained from cold-shock treatment and control were cleaned with distilled water twice and placed in 1.5 mL microcentrifuge tubes (5 to 10 larvae per tube) with 400 uL Nuclei Extraction buffer (CyStain UV Precise P, Sysmex, Japan). After shredding treatment, 1.6 mL staining buffer with 4,6-diamidino-2-phenyl-indole (DAPI) was added to each of the microcentrifuge tubes. Then, the mixture was filtered by a 40 um filter and placed in a new test tube. Finally, fluorescence intensity of DAPI-stained cells was measured by a flow cytometer (CyFlow Ploidy Analyzer, Sysmex, Japan). The operation and measurements were repeated 5 times.
Five-month-old cold-shock treated and control fish were intraperitoneally injected with 5 mL/kg−1 (body weight) of 0.1% phytoagglutinin and 5 mL/kg−1 (body weight) of 0.5% colchicine. Three hours after injection, the kidneys were removed into 0.075 mol/L KCL solution, minced to dissociate the cells, and held for 30 min at room temperature. Then the supernatant liquid was replaced with freshly prepared Carnoy’s solution (methanol/glacial acetic acid = 3:1) for 90 min at 4 ℃, and dropped onto slides. The chromosomes were stained with 15% Giemsa solution and photographed under light microscope (model BX-51N-34FL, Olympus, Japan). More than 30 metaphases were examined (10 fish were examined for both cold-shock and control groups).
Sampling and growth performances analysis
In order to examine the growth performance and gonadal development, triploids (genotypic females and sex-reversal phenotypic males) and diploids were sampled at 75, 90, 120, 150, 180, 210, 270, 300, 360 and 390 dph. Thirty randomly selected fish were sampled for each group. At the same time, the body length (BL) and weight (BW) of each fish were recorded.
Gonadal histology, sex ratios and gonadosomatic index (GSI)
Randomly selected six fish of each group at each time for gonadal histology. After anesthetization, each fish was dissected, then the left gonad was fixed in Bouin’s solution for histological analysis and immunohistochemistry, and the right gonad was fixed in RNAwait (Biosharp, Guangzhou, China) and then stored at -80 ℃ for Quantitative real-time PCR (qPCR). For histological analysis, the fixed gonads were embedded in paraffin, cut into 5-um-thick sections, and stained with hematoxylin and eosin (HE). Ovaries were confirmed by the presence of an ovarian cavity in the transverse section of the gonads. Testes were identified by the presence of sperm duct and spermatocytes in the section of the gonads. Sections were examined and photographed using a light microscopy (model BX-51N-34FL; Olympus, Japan).
At 90 and 120 dph, a total of 120 fish including 40 genotypic triploid females, 40 sex-reversal phenotypic triploid males and 40 normal diploids were used in the sex ratios analysis. Besides, the GSI (gonad weight/body weight) were calculated at 270 and 300 dph.
Transmission electron microscopy (TEM) analysis
In order to further analyze the ultrastructure of germ cells, 12 individuals from both triploid females and males at 360 and 390 dph were selected for TEM assay. The dissected gonads were cut into fragments (< 1 mm) and immersed them by 2.5% glutaraldehyde in 1 × phosphate buffered saline (1 × PBS) (pH 7.4) for 24 h at 4 ℃. Then the gonads were washed with 1 × PBS, fixed with 2% osmium tetroxide (OsO4) for 4 h at 4 ℃ and dehydrated by using a graded series of ethanol (30, 50, 75, 95, 100 and 100%) for 10–20 min each. They were then embedded in Epon-Araldite for subsequent ultrathin sectioning. Ultrathin Sects. (75–80 nm) were cut using the Ultracut E ultramicrotome, stained with uranyless EM stain, counterstained them with 1% lead citrate, and examined under Zeiss Libra 120 transmission electron microscope (Zeiss, Oberkochen, Germany).
Quantitative real-time PCR (qPCR)
At 270, 300, 360 and 390 dph, the expression patterns for gonad-specific genes including vasa and cyp19a1a in ovary, and vasa and dmrt1 in testis were examined via qPCR. At the same time, the relative abundances of meiosis-related genes (rec8 and sycp3) in gonads of both sexes at 300 and 360 dph were also examined via qPCR. The specific primers of the genes were designed using Primer Premier 5.0 and listed in Table 1. β-actin and 18 s was chosen as the reference genes. Total RNA was extracted from ovaries and testes using the RNA extraction kit (Solarbio, China). The first-strand cDNA was synthesized using a Transcript First-strand cDNA Synthesis Kit (TranStart, China). The qPCR amplifications were performed using 2 × SYBY Premix Ex TapTM II (Takara, China) according to the manufacturer’s instructions. PCR amplification was performed using a reaction volume of 20 μL, with 6.8 μL ddH2O, 0.4 μL forward and reverse primers (10 μmol/L), 2 μL cDNA, 0.4 μL ROX Reference Dye I and 10 μL 2 × SYBY Premix Ex TapTM II. The PCR reaction was performed under the following conditions: 30 s at 95 ℃; 5 s at 95 ℃ and 30 s at 60 ℃ for 33 cycles; finally 15 s at 95 ℃, 30 s at 60 ℃, and 15 s at 95 ℃ was used for the dissociation stage. Each assay included a no-reverse transcriptase and a no-template control. The relative gene expression levels were analyzed using the 2−ΔΔCT method.
Immunohistochemistry
The localization of Vasa in gonads of both the triploids and diploids at 360 dph was detected using Vasa antibody (ab209710, Abcam, UK). Specifically, the gonadal samples fixed in Bouin’s solution were embedded in paraffin wax, and then sliced into 5–7 um serial sections. The tissue sections were rehydrated with PBS, digested with proteinase K (10 ug/mL) for 30 min, and then fixed in 4% PFA for 20 min, washed with PBST for 5 min four times. Subsequently the samples were incubated with Vasa antibody at a 1: 1000 dilution at 4 ℃ overnight, then washed in PBST and incubated with the secondary antibody at a 1:2000 dilution for 30 min at room temperature. Finally, the samples were stained with DAPI (E607303, Sangon, China) for 10 min, and washed in PBST. Fluorescence images were captured with a fluorescence microscope (model BX-51N-34FL, Olympus, Japan).
Statistical analysis
All data were presented as means ± standard error of the mean (SEM) using GraphPad Prism 9.0 software. Significant differences in BL and BW were determined using one-way ANOVA followed by the Kruskal-Waillis multiple comparisons test (P < 0.05). Significant difference in GSI, gene expression, fertilization and hatching rates were determined using the Student’s t-test (Mann–Whitney test) (P < 0.05).
Results
Fertilization, hatching and survival rates
The fertilization rates of the cold-shock treated and control embryos were 63.64 ± 6.56% and 84.25 ± 7.63%, respectively. The hatching rates of the cold-shock treated and control embryos were 22.32 ± 3.73% and 70.03 ± 3.03%, respectively. Obviously, the fertilization and hatching rates were significantly affected by the cold-shock treatment in the present study. From 30 dph, the survival rates of sex-reversed triploid males and genotypic triploid females were high (> 90%), and there was no significant difference (P > 0.05).
Ploidy determination in yellow drum
As shown in Fig. 2A, the DNA content of diploid and triploid yellow drum was 124 and 186, respectively. The DNA content of the triploid fish was 1.5 times than that of the diploid. Microscopic observation of metaphase plates showed that the chromosome number (3n = 72) of triploid fish was 1.5 times larger than that of diploid fish (2n = 48) (Fig. 2B). Triploids had a whole set of chromosomes more than diploid fish.
Ploidy determination in triploid yellow drum. The relative DNA content of fin cells from diploid and triploid yellow drum. The horizontal coordinate indicates the DNA content, and the vertical coordinate indicates the cell counts. The DNA contents of diploid and triploid yellow drum were 124 and 186, respectively (A). The chromosomes numbers of diploid (2n) and triploid (3n) yellow drum were 48 and 72, respectively (B). Scale bars = 10 μm
Sex ratios of triploid and diploid yellow drum
The sex ratios were confirmed by histological examinations and shown in Table 2. The sex ratios were 100% female in genotypic triploid females and 100% male in sex-reversal phenotypic triploid males. However, the sex ratios were close to 1:1 (55% and 60% male) in normal diploids.
Growth performances in triploid yellow drum
The BL and BW of the genotypic triploid females, sex-reversal phenotypic triploid males and normal diploids had been analyzed and shown in Fig. 3. A rapid growth on BL and BW of yellow drum was determined from 75 to 180 dph, and a slow growth was determined from 180 to 390 dph. The BL of triploid fish was significantly higher than that of diploid fish from 75 to 150 dph. However, no significant differences were observed from 150 to 390 dph (Fig. 3A). In terms of BW, significant differences between the triploid and diploid fish were observed from 75 to 300 dph (Fig. 3B). Similar values of BW were observed from 300 to 390 dph (Fig. 3B).
GSI and external morphology of triploid yellow drum gonads
The GSI and external morphology of diploid and triploid gonads were analyzed and summarized in Fig. 4. In females, the GSI of diploid females was increased and significantly higher than that of triploid females from 270 to 390 dph (Fig. 4A). However, the triploid females had low GSI and small ovaries during these periods including the first spawning season (from 360 to 390 dph) (Fig. 4A and Cab). This data suggested that oogenesis was impaired in triploid females. In males, the GSI of triploids was significantly lower than that of diploids at 270 and 300 dph (Fig. 4B). While no significant differences in GSI and external morphology of testes were observed between diploid and triploid males at 360 and 390 dph (Fig. 4A and Ccd). Meanwhile, no abnormalities in external morphology were observed in triploid testes.
Gonadosomatic index (GSI) and external morphology of diploid (2n) and triploid (3n) yellow drums. In females, the GSI of triploid females maintained small and significantly lower than that of triploid females (A). In males, the GSI of triploids was significantly lower than that of diploids at 270 and 300 dph, while no significant differences in GSI at 360 and 390 dph (B). External features of gonads in diploid and triploid yellow drum (C): female at 360 dph (a), female at 390 dph (b), male at 360 dph (c) and male at 390 dph (d). Double asterisks indicate significant differences between the two groups (P < 0.01). Scale bars = 1.5 cm (a, b), 0.9 cm (c, d). dph, days post hatching
Gonadal histology of triploid yellow drum
The ovaries of diploid females consisted of perinucleolus stage oocytes (PNOs; Fig. 5A-D) from 75 to 180 dph. Furthermore, previtellogenic oocytes (EVOs) were observed in the ovaries of diploid females at 270 dph (Fig. 5I). However, only oogonia (OG) and chromatin-nucleolus stage oocytes (CNOs) were observed in the ovaries of triploid females from 75 to 270 dph (Fig. 5E-H, M). At 360 dph, late vitellogenic oocytes (LVOs) began appeared in diploid ovaries (Fig. 5K). Meanwhile, during the first spawning season (390 dph), many post vitellogenic oocytes (PVOs) were observed in diploid ovaries (Fig. 5L). While only a few scattered PNOs and a large number of CNOs were observed in triploid ovaries (Fig. 5N-P) from 300 to 390 dph. These data suggested that oogenesis was impaired in triploid females.
Ovarian histology of diploid (2n) and triploid (3n) yellow drum sampled at 75 (A, E), 90 (B, F), 120 (C, G), 180 (D, H), 270 (I, M), 300 (J, N), 360 (K, O) and 390 (L, P) dph. The insets indicate the magnification of main germ cell types. The insets of E–H and M-P show the CNO with chromatin adjacent to the nuclear edge and OG. OG, oogonia; PNO, perinucleolar oocyte; CNO, chromatin-nucleus oocyte; EVO, previtellogenic oocyte; PVO, postvitellogenic oocyte; LVO, late vitellogenic oocyte; dph, days post hatching. Scale bars = 80 μm (A-J, M-P), 100 μm (K), 250 μm (L), 20 μm (insert in A-D), 8 μm (insert in E–H, M-P)
The testes of both diploid and triploid males at 75 dph were mainly composed of spermatogonia (SG; Fig. 6A and E). Furthermore, many cysts containing spermatocytes (SCs) were observed in both diploid and triploid testes at 90 and 120 dph (Fig. 6B-C and F-G). From 180 to 390 dph, the testes of diploid fish were composed of lobules filled with spermatozoon (SZ) (Fig. 6D and I-L); however, triploid testes consisted of cysts filled with either SC and spermatids (ST) (Fig. 6N-P), although a few SZ-like cells (indicated by the asterisk in Fig. 6O-P) were observed at 360 and 390 dph. Meanwhile, degenerated spermatocytes (DS) varied in size were observed in the triploid testes (Fig. 6P). DS showed highly condensed nuclei and were presumed to be undergoing apoptosis.
Testicular histology of diploid (2n) and triploid (3n) yellow drum sampled at 75 (A, E), 90 (B, F), 120 (C, G), 180 (D, H), 270 (I, M), 300 (J, N), 360 (K, O) and 390 (L, P) dph. Asterisk indicates SZ-like cells. DS showed highly condensed nuclei and were presumed to be undergoing apoptosis. DS, degenerated spermatocytes; SC, spermatocyte; SG, spermatogonia; ST, spermatid; SZ, spermatozoa; dph, days post hatching. Scale bars = 25 μm (A-H, K-L, O-P), 40 μm (I-J), 30 μm (M–N)
Ultrastructure of germ cells in triploid yellow drum
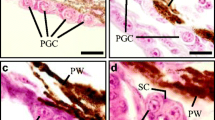
In triploid ovaries, three types of female germ cells including OG, CNO, and PNO were observed. The OG as a single isolated germ cell (approximately 10 μm in diameter) had a prominent nucleus (Fig. 7Aa). Meanwhile, some mitochondria in clusters were observed in the cytoplasm of OG. With duplication, the OG developed into CNO, which was often grouped in cysts (Fig. 7Ab-d). The CNO had a clear nucleus with a prominent nucleolus (NU) adjacent to the nuclear edge (Fig. 7Ab-e). It is worth mentioning that a number of high density and coarse chromatin material with synaptonemal complexes were observed in the NU of CNO (Fig. 7Ae). PNO contained numerous ovoid mitochondria in the cytoplasm and was surrounded by two layers of somatic cells, including the inner granulosa cell (GC) and outer thecal cell (TC) (Fig. 7Af-g). A great number of lysosomes were observed in the cytoplasm of TC (Fig. 7Ah).
Ultrastructure of gonads in triploid female (A) and male (B) yellow drums at 360 and 390 dph. OG, oogonia; CNO, chromatin-nucleus oocyte; PNO, perinucleolus oocyte; NU, nucleous; GC, granulosa cell; TC, thecal cell; L, lysosome; M, mitochondria; SG, spermatogonia; PS, pachytene spermatocyte; DIS, diplotene spermatocytes; MI; first meiotic division; A1, anaphase/telophase 1; A2, anaphase/telophase 2; DS, degenerated spermatocyte; ST, spermatid; dph, days post hatching. Scale bars = 2 μm (a, h), 5 μm (b-d, g), 2.5 μm (e), 4 μm (f) in A; 3 μm (a), 2.5 μm (b), 4 μm (c-e, g), 10 μm (f), 5 μm (h); 900 nm (i) in B
In triploid testes, seven types of male germ cells including SG, five types of SC and spermatid ST were observed (Fig. 7B). The isolated SG was the largest germ cell of spermatogenic lineage (10 μm in diameter). The SG was clearly characterized by its elliptical shape, voluminous nucleus and distinctive nucleolus (Fig. 7Ba). Spermatocytes included five different stages of spermatocytes: pachytene spermatocytes (PS), diplotene spermatocytes (DIS), spermatocytes undergoing first meiotic division (MI), anaphase/telophase 1 (A1), and anaphase/telophase 2 (A2) (Fig. 7Bb-g). A number of abnormal A2 and S varied in size were observed in triploid testes (Fig. 7Bf-g). Meanwhile, the sizes of NU of abnormal S were also variable (Fig. 7Bh). These results suggested the process of sperm maturation was impaired in triploid males.
Locations of Vasa in gonads of triploid yellow drum
The locations of Vasa in gonads of triploid and diploid fish at 360 dph were detected (Fig. 8). In triploid and diploid ovaries, Vasa protein was cytoplasmic and mainly expressed in PNOs and PGOs (Fig. 8E-H). Meanwhile, weaker signals of Vasa protein were also detected in CNOs in triploid ovaries (Fig. 8E and H). In diploid males, Vasa protein expressed in SG and SCs in the whole testes (Fig. 8M-P). However, in triploid testes, Vasa protein was mainly expressed in SG located at the edge of gonads (Fig. 8I-L).
Location of Vasa protein in the gonads of diploid (2n) and triploid (3n) yellow drum by fluorescence immunohistochemistry. D, H, P and L are histological images stained by HE. The insets indicate the magnification of CNO in E and H. The red circles indicate the CNO. The inset shows the magnification of SG in I. PGO, primary growth oocyte; PNO, perinucleolus oocyte; EVO, previtellogenic oocyte; VO, vitellogenic oocyte; EVO, previtellogenic oocyte; CNO, chromatin-nucleus oocyte; SC, spermatocyte; SZ, spermatozoa; SG, spermatogonia; dph, days post hatching. Scale bars = 150 μm (A-D), 80 μm (E–G, I-L), 100 μm (M-P)
Expression patterns of gonad-specific genes in triploid yellow drum
At 270, 300, 360 and 390 dph, the expression patterns for gonad-specific genes including vasa and cyp19a1a in ovaries, and vasa and dmrt1 in testes were examined. At the same time, the relative abundances of meiosis-related genes (rec8 and sycp3) in gonads of both sexes at 300 and 360 dph were also examined. As shown in Fig. 9, the expression levels of germ cell marker gene, vasa, were similar in diploid and triploid ovaries. The expression of the female-specific gene, cyp19a1a, were significantly higher in diploid ovaries than that in triploid ovaries at 270 and 300 dph. On the contrary, at 360 and 390 dph, the lower expression levels of cyp19a1a were detected in diploid ovaries compared with sterile triploids. The meiosis-related genes, sycp3 and rec8 had higher expression levels in triploid ovaries than that in diploids.
In testes, vasa mRNA levels were significant lower in the triploid fish compared with diploids. The expression levels of the male-specific gene, dmrt1, were significantly higher in diploid fish than that in triploid fish at 270 and 300 dph. However, at 360 and 390 dph, no significant differences of dmrt1 expression levels were detected between diploid and triploid testes. Meanwhile, there also were no significant differences of sycp3 and rec8 expression levels between diploid and triploid males.
Discussion
In this study, we successfully produced mono-sex triploid yellow drum, including genotypic female triploids (XXX female) and sex-reversed phenotypic male triploids (XXX male). Firstly, the mono-female triploids were produced through cold-shock treatment on eggs fertilized with sperm from neo-males. Then, the mono-male triploids were produced by 100% sex reversal of mono-female triploids with oral administration of LZ. The growth performance and gonadal development in both the genotypic triploid females and sex-reversed phenotypic males during their first reproductive cycle were characterized. The results showed that triploids had similar growth performance to diploids during the first year. However, the gonadal development in both triploid females and sex-reversed males were impaired, with abnormal meiosis result in functional sterility. This study demonstrates an efficient protocol for producing sterile mono-sex triploid yellow drum.
In this study, we produced genotypic triploid females of yellow drum by cold-shock treatment of eggs fertilized with milt from neo-males. We confirmed that genotypic triploid females were 100% female by histological examinations. Further, we produced sex-reversed phenotypic triploid males by treating genotypic triploid females with oral administration of LZ for 60 days (from 30 to 90 dph) in this study. LZ is a triazole-containing aromatase inhibitor and has been used to inducing masculinization in fish, for example zebrafish (Danio rerio) (Uchida et al. 2004), rainbow trout (Xu et al. 2021), blue drum (Nibea mitsukurii) (Qin et al. 2019), diploid yellow drum (Qin et al. 2019) and yellow catfish (Pelteobagrus fulvidraco) (Shen et al. 2015; Yang et al. 2018). The sex-reversal phenotypic triploid males were also confirmed as 100% male by histological examinations. To the best of our knowledge, this is the first study to develop an effective method to produce genotypic triploid females and sex-reversal phenotypic triploid males in Teleostei.
Furthermore, we systematically investigated the growth performance and gonadal development of genotypic triploid females and sex-reversal males during their first year (from 75 to 390 dph). In teleosts, numerous studies have evaluated and compared the growth competences of triploids and diploids. A common thought has been acceptable that triploid fish should grow faster than their diploid counterpart, due to the reallocation of energy costs from gonadal to somatic growth in sterile triploids. However, to date, the literature on the growth rates of triploid fish is inconclusive, as triploid fish would grow faster (Qin et al. 1998; Okomoda et al. 2020) or slower (Felip et al. 2001; Ottera et al. 2016) than diploid fish. In this study, we found that the BL and BW of the triploid fish was significantly higher than that of diploid fish during the early growth period (from 75 to 180 dph). However, during the later growth period, there was no significant differences of BL and BW between triploid and diploid fish. The not-remarkable growth performance is consistent with the findings in sea bass (Felip et al. 2001) and Nile tilapia (Oreochromis niloticus L.) (Jamjun and Amararatne 2004). It seems that the advantage of growth of triploid fish is species-specific. However, it is worth mentioning that contradictory results of growth rates of triploid fishes between individuals of the same species have also been reported in Atlantic salmon Salmo salar (Maxime 2010). It is suggested that the growth differences between diploid and triploid fish are highly variable and closely dependent on the experimental conditions. Therefore, further studies of growth performance in triploid yellow drums are required.
In the present study, we observed impaired gonadal development in both the genotypic triploid females and sex-reversal males. In females, obvious smaller ovaries were observed in triploids compared to that of diploids, and no seasonal changes in the ovarian GSI of triploids were observed during the first spawning season. Histologically, although several scattered PNOs were observed, the ovaries of triploids were composed of a large number of CNOs. In teleosts, ultrastructural features of normal oogenesis are similar, the germ cells can be developed from oogonia into mature eggs in Bluefin Tuna (Thunnus thynnus) (Abascal and Medina 2005) and silver pomfret (Pampus argenteus) (Yang et al. 2021a, b). In this study, ultrastructure of germ cells showed only three types of germ cells including the OG, CNOs, and PNOs existed in triploid ovaries. Meanwhile, a number of high density and coarse chromatin material with synaptonemal complexes were observed in the NU adjacent to the nuclear edge of the CNOs. Furthermore, meiosis-related genes (rec8 and sycp3) expression levels in triploid females were significantly higher than those of diploid females. These results above suggested that the germ cells in the genotypic triploid females were abnormal and retained at metaphase 1. In males, the GSI of triploids was significantly lower than that of diploids during the early development period, while no significant differences in GSI and external morphology of testes were observed between diploid and triploid males during the first spawning season. Histologically, meiosis and a delay in germ cell development were observed in sex-reversed phenotypic triploid males. However, only small amounts of SZ-like cells were produced in the lobules in triploid testes during the first spawning season. Compared with ultrastructural features of normal spermatogenesis in fish species (Yang et al. 2021a, b; Liu et al. 2021; Papah et al. 2013), triploid male yellow drum also had seven types of male germ cells including SPG, five types of SCs and SZ. However, a large number abnormal anaphase/telophase II SCs and ST varied in size and DSs were also observed in triploid testes. Furthermore, the expression levels of sycp3 and rec8 were similar in diploid and triploid males, implying that partial spermatogenic cells of triploid male can enter meiosis but few ST could be produced. These sterile and abnormal gonadal development are consistent with previous reports on triploid males, such as crucian carp (Zhang et al. 2021) and rainbow trout (Han et al. 2010), Nibe croaker (Takeuchi et al. 2018), grass puffer (Takifugu niphobles) (Hamasaki et al. 2013), and tilapia (Okomoda et al. 2020). The mechanism underlying this phenomenon is not clear, but the findings in this study strongly indicate that abnormal meiosis may result in sterile triploid females and males.
Further, we investigated the expression of gonad-specific genes in sterile triploid females and males in this study. vasa has been demonstrated a germ cell marker and displayed differential expression during oogenesis and spermatogenesis in teleost (Chen et al. 2005; Yoshizaki et al. 2000). Generally, Vasa (RNA and protein) is most abundant with even distribution in the cytoplasm of the spermatogonia, primary spermatocytes and oocytes at early stages in fish (Chen et al. 2005; Yoshizaki et al. 2000). In the present study, the Vasa protein abundantly expressed in PNOs and PGOs of diploid ovaries, however in sterile triploid ovaries, signals of Vasa protein were detected in a few PNOs and PGOs, and a large number of CNOs. This result exhibited abnormal gonadal development in triploid females. On the other hand, in diploid and triploid ovaries of yellow drum, the vasa mRNA expression levels were similar, which was in accordance with the high vasa expression in triploid hybrids (red crucian carp X common carp) (Yu et al. 2015). In testes, Vasa protein expressed in SG and SCs in the whole testes of diploid fish. However, in triploid testes, Vasa protein was mainly expressed in SG located at the edge of gonads. Further, the vasa mRNA expression levels were lower in triploids than in fertile diploid fish during both the non-breeding season and breeding season. Previous study has also shown that vasa expression is significantly reduced in triploid males (Yu et al. 2015). Thus, our results indicates that triploid sterility might be related to vasa mRNA and protein expression levels.
Sex steroid hormones produced by gonadal somatic cells play essential roles in germ cell development. It is well known that cyp19a1a, a female-specific gene, regulates estrogen and aromatase production during oogenesis (Xu et al. 2018). In this study, cyp19a1a was highly expressed in triploid ovaries during the first spawning season, although low expression levels of cyp19a1a were detected in sterile triploid ovaries during early developmental period (at 270 and 300 dph). In general, triploid females are known to exhibit low levels of sex steroids. For example, the low plasma levels of E2 were detected in triploid brook trout (Salvelinus fontinalis) (Schafhauser-Smith and Benfey 2001) and catfish (Heteropneustes fossilis) (Tiwary et al. 2001). However, in triploid grass puffer (Hamasaki et al. 2013) and olive flounder (Paralichthys olivaceus) (Wu et al. 2023) females, the plasma levels of E2 were similar to those of diploids. It is worth pointing out that the gonadal E2 levels in triploid females of olive flounder were lower than those of the diploids (Wu et al. 2023). It seems that the levels of E2 in triploid females are species-specific. In fish, oogenesis especially vitellogenesis occurs in synchrony with the increase of plasm E2 level. However, in this study, the impaired oogenesis indicated there may be a low plasm level of E2. Therefore, we speculated that the plasma E2 production could be influenced by other factor except the cyp19a1a in triploid yellow drum. The plasma E2 and the genes of steroidogenesis will be studied in triploids in future. In a sense, the high cyp19a1a expression levels indicated the triploid ovarian follicles possess the potency to produce sufficient sex steroids for oogenesis. Another sex-related gene, dmrt1, is known to be a key determinant of testicular development in vertebrates (Adolfi et al. 2015; Marchand et al. 2000; Webster et al. 2017). In this study, although the levels of dmrt1 expression were lower in triploid males during early testicular development (270 and 300 dph), the dmrt1 expression levels were similar to those of diploids during the first spawning season (360 and 390 dph). The high dmrt1 expression levels further confirmed the masculinization and testicular development of genotypic female triploids (XXX, female) by LZ at the molecular level. In a word, those results demonstrated that the triploid yellow drum had partially functional ovarian and testicular development. This is an important characteristic as surrogate recipients used for germ cell transplantation. In this study, the aim of inducing genotypic triploid females and sex-reversal phenotypic triploid males is finally to conduct germ cell transplantation. The induction of sterile mono-sex triploids is the first step. The second step is the donor fish’s germ cells transplantation into those triploids to ultimately produce donor fish’s gametes on a large scale.
In conclusion, we successfully produced genotypic female and sex-reversed phenotypic male triploid yellow drum through the cold-shock treatment and oral administration of LZ. The gonadal development in both the triploid females and sex-reversed males were impaired, without mature eggs or sperm production during their first spawning season. Meanwhile, abnormal meiosis might lead to sterility in triploid yellow drum. Besides, the sex steroid hormones production might be at least partially functional in triploid yellow drum. These results showed that genotypic triploid females and sex-reversed phenotypic triploid males of yellow drum produced in this study have the potential for sustainable aquaculture and were suitable for utilization as surrogate recipients in germ cell transplantation in Sciaeniadae.
Data availability
No data was used for the research described in the article.
References
Abascal FJ, Medina A (2005) Ultrastructure of oogenesis in the bluefin tuna, Thunnus thynnus. J Morphol 264:149–160. https://doi.org/10.1002/jmor.10325
Adolfi MC, Carreira AC, Jesus LW, Bogerd J, Funes RM, Schartl M, Sogayar MC, Borella MI (2015) Molecular cloning and expression analysis of dmrt1 and sox9 during gonad development and male reproductive cycle in the lambari fish, Astyanax altiparanae. Reprod Biol Endocrinol 13:2. https://doi.org/10.1186/1477-7827-13-2
Aydın I, Terzi Y, Polat H, Küçük E, Oztürk RÇ (2022) Growth, gonadal development, and fish fillet quality in diploid and triploid turbot, Scophthalmus maximus, originated from the Black Sea. Aquaculture 560:738558. https://doi.org/10.1016/j.aquaculture.2022.738558
Beardmore JA, Mair GC, Lewis RI (2001) Monosex male production in finfish as exemplified by tilapia: applications, problems, and prospects. Aquaculture 197:283–301. https://doi.org/10.1016/B978-0-444-50913-0.50015-1
Bye VJ, Lincoln RF (1986) Commercial methods for the control of sexual maturation in rainbow trout (Salmo gairdneri R.). Aquaculture 57:299–309. https://doi.org/10.1016/0044-8486(86)90208-5
Chen SL, Li J, Deng SP, Tian YS, Wang QY, Zhuang ZM, Sha ZX, Xu JY (2007) Isolation of Female-Specific AFLP Markers and Molecular Identification of Genetic Sex in Half-Smooth Tongue Sole (Cynoglossus semilaevis). Mar Biotechnol 9:273–280. https://doi.org/10.1007/s10126-006-6081-x
Chen R, Lou B, Xu D, Zhan W, Takeuchi Y, Yang F, Liu F (2017) Induction of meiotic gynogenesis in Yellow drum (Nibea albiflora, Sciaenidae) using heterologous sperm and evidence for female homogametic sex determination. Aquaculture 479:667–674. https://doi.org/10.1016/j.aquaculture.2017.07.009
Chen YG, Ye D, Song P, Lv DY, Gui JF (2005) Expression and distributing of vasa gene during gametogenesis of goldfish (Carassius auratus). Zool Res 26: 179–83. https://hdl.handle.net/1807/9351
Comai L (2005) The advantages and disadvantages of being polyploid. Nat Rev Genet 6:836–846. https://doi.org/10.1038/nrg1711
Cotter D, O’Donovan V, O’Maoileidigh N, Rogan G, Roche N, Wilkins NP (2000) An evaluation of the use of triploid Atlantic salmon (Salmo salar L.) in minimising the impact of escaped farmed salmon on wild populations. Aquaculture 186:61–75. https://doi.org/10.1016/S0044-8486(99)00367-1
do Nascimento NF, Pereira-Santos M, Piva LH, Manzini B, Fujimoto T, Senhorini JA, Yasui GS, Nakaghi LSO (2017) Growth, fatty acid composition, and reproductive parameters of diploid and triploid yellowtail tetra Astyanax altiparanae. Aquaculture 471:163–171. https://doi.org/10.1016/j.aquaculture.2017.01.007
Farley JH, Eveson JP, Davis TLO, Andamari R, Proctor CH, Nugraha B, Davies CR (2014) Demographic Structure, Sex Ratio and Growth Rates of Southern Bluefin Tuna (Thunnus maccoyii) on the Spawning Ground. PLoS ONE 9:e96392. https://doi.org/10.1371/journal.pone.0096392
Felip A, Piferrer F, Zanuy S, Carrillo M (2001) Comparative growth performance of diploid and triploid European sea bass over the first four spawning seasons. J Fish Biol 58:76–88. https://doi.org/10.1006/jfbi.2000.1427
Golpour A, Siddique MAM, Siqueira-Silva DH, Pšenička M (2016) Induced sterility in fish and its potential and challenges for aquaculture and germ cell transplantation technology: a review. Biologia 71:853–864. https://doi.org/10.1515/biolog-2016-0118
Hamasaki M, Takeuchi Y, Miyaki K, Yoshizaki G (2013) Gonadal Development and Fertility of Triploid Grass Puffer Takifugu niphobles Induced by Cold Shock Treatment. Mar Biotechnol 15:133–144. https://doi.org/10.1007/s10126-012-9470-3
Han Y, Liu M, Zhang L, Li H (2010) Research on reproduction and development of triploid male Oncorhynchus mykiss (in Chinese). J Northeast Agric Univ 41(7):94–99. https://doi.org/10.19720/j.cnki.issn.1005-9369.2010.07.019
Jamjun P, Amararatne Y (2004) A comparative study of growth and feed utilization efficiency of sex-reversed diploid and triploid Nile tilapia, Oreochromis niloticus L. Aquac Res 36:45–51. https://doi.org/10.1111/j.1365-2109.2004.01182.x
Koenig MK, Koenig JR, Meyer KA, Schill DJ (2011) Performance of Diploid and Triploid Rainbow Trout Stocked in Idaho Alpine Lakes. North Am J Fish Manag 31:124–133. https://doi.org/10.1080/02755947.2011.561163
Lincoln RF, Scott AP (1983) Production of all-female triploid rainbow trout. Aquaculture 30:375–380. https://doi.org/10.1016/0044-8486(83)90179-5
Liu Y, Liu Q, Xu S, Wang Y, Feng C, Zhao C, Song Z, Li J (2021) A deep insight of spermatogenesis and hormone levels of aqua-cultured turbot (Scophthalmus maximus). Front Mar Sci 7:592880. https://doi.org/10.3389/fmars.2020.592880
Marchand O, Govoroun M, Cotta HD, McMeel O, Lareyre JJ, Bernot A, Laudet V, Guiguen Y (2000) DMRT1 expression during gonadal differentiation and spermatogenesis in the rainbow trout, Oncorhynchus mykiss. Biochim Et Biophys Acta (BBA) 1493:180–187. https://doi.org/10.1016/S0167-4781(00)00186-X
Maxime V (2010) The physiology of triploid fish: current knowledge and comparisons with diploid fish. Fish Fish 9:67–78. https://doi.org/10.1111/j.1467-2979.2007.00269.x
Mol K, Byamungu N, Cuisset B, Yaron Z, Ofir M, Mélard C, Castelli M, Kühn ER (1994) Hormonal profile of growing male and female diploids and triploids of the blue tilapia, Oreochromis aureus, reared in intensive culture. Fish Physiol Biochem 13:209–218. https://doi.org/10.1007/BF00004359
Okomoda VT, Pradeep JP, Oladimeji AS, Abol-Munafi AB, Alabi KI, Ikhwanuddin M, Martins C, Umaru JA, Hassan A (2020) Effect of electric induced triploidization on sex ratio, growth and gonad histology of red hybrid tilapia. Aquaculture 520:734991. https://doi.org/10.1016/j.aquaculture.2020.734991
Okutsu T, Shikina S, Kanno M, Takeuchi Y, Yoshizaki G (2007) Production of trout offspring from triploid salmon parents. Science 317:1517. https://doi.org/10.1126/science.1145626
Ottera H, Thorsen A, Karlsen Ø, Fjelldal PG, Morton HC, Taranger GL (2016) Performance of triploid Atlantic cod (Gadus morhua L.) in commercial aquaculture. Aquaculture 464:699–709. https://doi.org/10.1016/j.aquaculture.2016.08.018
Papah MB, Kisia SM, Ojoo RO, Makanya AN, Wood CM, Kavembe GD, Maina JN, Johannsson OE, Bergman HL, Laurent P, Chevalier C, Bianchini A, Bianchini LF, Onyango DW (2013) Morphological evaluation of spermatogenesis in Lake Magadi tilapia (Alcolapia grahami): A fish living on the edge. Tissue Cell 45:371–382. https://doi.org/10.1016/j.tice.2013.06.004
Qin JG, Fast AW, Ako H (1998) Growout performance of diploid and triploid Chinese catfish Clarias fuscus. Aquaculture 166:247–258. https://doi.org/10.1016/S0044-8486(98)00287-7
Qin Z, Yang F, Tian L, Chen R, Xu D, Takeuchi Y (2019) Induction of sex reversal in blue drum (Nibea mitsukurii) and gynogenetic yellow drum (Nibea albiflora) by oral administration of letrozole. Aquac Res 00:1–8. https://doi.org/10.1111/are.14434
Schafhauser-Smith D, Benfey TJ (2001) The reproductive physiology of three age classes of adult female diploid and triploid brook trout (Salvelinus fontinalis). Fish Physiol Biochem 25:319–333. https://doi.org/10.1023/A:1023285008072
Shen ZG, Fan QX, Yang W, Zhang YL, Wang HP (2015) Effects of 17α-Methyltestosterone and Aromatase Inhibitor Letrozole on Sex Reversal, Gonadal Structure, and Growth in Yellow Catfish Pelteobagrus fulvidraco. Biol Bull 228:108–115. https://doi.org/10.1086/BBLv228n2p108
Sun S, Li W, Xiao S, Lin A, Han Z, Cai M, Wang Z (2018) Genetic sex identification and the potential sex determination system in the yellow drum (Nibea albiflora). Aquaculture 492:253–258. https://doi.org/10.1007/s10126-011-9366-7
Takeuchi Y, Yatabe T, Yoshikawa H, Ino Y, Kabeya N, Yazawa R, Yoshizaki G (2018) Production of functionally sterile triploid Nibe croaker Nibea mitsukurii induced by cold-shock treatment with special emphasis on triploid aptitude as surrogate broodstock. Aquaculture 494:45–56. https://doi.org/10.1016/j.aquaculture.2016.05.030
Tian L, Tan P, Yang L, Zhu W, Xu D (2020) Effects of salinity on the growth, plasma ion concentrations, osmoregulation, non-specific immunity, and intestinal microbiota of the yellow drum (Nibea albiflora). Aquaculture 528:735470. https://doi.org/10.1016/j.aquaculture.2020.735470
Tiwary BK, Kirubagaran R, Ray AK (2001) Plasma levels of gonadotropin-II and gonadal sex steroids in triploid catfish, Heteropneustes fossilis (Bloch). Fish Physiol Biochem 24:9–14. https://doi.org/10.1023/A:1011159615057
Tiwary BK, Kirubagaran R, Ray AK (2004) The biology of triploid fish. Rev Fish Biol Ray, Fish 14:391–402. https://doi.org/10.1007/s11160-004-8361-8
Uchida D, Yamashita M, Kitano T, Iguchi T (2004) An aromatase inhibitor or high water temperature induce oocyte apoptosis and depletion of P450 aromatase activity in the gonads of genetic female zebrafish during sex-reversal. Comp Biochem Physiol a: Mol Integr Physiol 137:11–20. https://doi.org/10.1016/S1095-6433(03)00178-8
Webster KA, Schach U, Ordaz A, Steinfeld JS, Draper BW, Siegfried KR (2017) Dmrt1 is necessary for male sexual development in zebrafish. Dev Biol 422:33–46. https://doi.org/10.1016/j.ydbio.2016.12.008
Wu CJ, Gui JF (1999) Fish Genetics and Breeding Engineering (in Chinese). Shanghai Scientific and Technical Publishers, Shanghai
Wu Q, Song Z, Wang L, Wu Z, Zou C, Shu C, Liang S, Wang W, Sun Y, Yue X, Peng Q, You F (2023) Comparative study on the gonadal development in the diploid and artificially induced triploid olive flounder Paralichthys olivaceus. Aquaculture 565:739106. https://doi.org/10.1016/j.aquaculture.2022.739106
Xu D, Yang F, Chen R, Lou B, Zhan W, Hayashida T, Takeuchi Y (2018) Production of neo-males from gynogenetic yellow drum through 17α-methyltestosterone immersion and subsequent application for the establishment of all-female populations. Aquaculture 489:154–161. https://doi.org/10.1016/j.aquaculture.2018.02.015
Xu G, Huang T, Gu W, Liu E, Wang B (2021) Effects of letrozole and 17α-methyltestosterone on gonadal development in all-female triploid rainbow trout (Oncorhynchus mykiss). Aquac Res 00:01–10. https://doi.org/10.1111/are.15095
Yang T, Yang X, Cheng D, Guo W, Liu H, Gui J, Mei J (2018) Production of XX male yellow catfish by sex-reversal technology (in Chinese). Acta Hydrobiologia Sinica 42:871–878. https://doi.org/10.7541/2018.107
Yang Y, Ning C, Li Y, Wang Y, Hu J, Liu Y, Zhang M, Sun Y, Gu W, Zhang Y, Sun J, Xu S (2021a) Dynamic changes in mitochondrial DNA, morphology, and fission during oogenesis of a seasonal-breeding teleost. Pampus Argenteus Tissue Cell 72:101558. https://doi.org/10.1016/j.tice.2021.101558
Yang Y, Li Y, Wang Y, Hu J, Zhang M, Sun Y, Gu W, Zhang Y, Sun J, Jacques KJ, Xu S (2021) The ultrastructure of spermatogenic cells and morphological evaluation of testicular development in the silver pomfret (Pampus argenteus). Anat Histol Embryol 50:1034–1042. https://doi.org/10.1111/ahe.12747
Yoshizaki G, Sakatani S, Tominaga H, Takeuchi T (2000) Cloning and characterization of a vasa-like gene in rainbow trout and its expression in the germ cell lineage. Mol Reprod Dev 55:364–371. https://doi.org/10.1002/(SICI)1098-2795(200004)55:4%3c364::AID-MRD2%3e3.0.CO;2-8
Yoshizaki G, Fujinuma K, Iwasaki Y, Okutsu T, Shikina S, Yazawa R, Takeuchi Y (2011) Spermatogonial transplantation in fish: a novel method for the preservation of genetic resources. Comp Biochem Physiol, Part D 6:55–61. https://doi.org/10.1016/j.cbd.2010.05.003
Yu F, Zhong H, Liu G, Liu S, Zhang Z, Zhou Y, Tao M, Liu Y (2015) Characterization of vasa in the gonads of different ploidy fish. Gene 574:337–344. https://doi.org/10.1016/j.gene.2015.08.016
Zhang C, Li Q, Zhu L, He W, Yang C, Zhang H, Sun Y, Zhou L, Sun Y, Zhu SJ (2021) Abnormal meiosis in fertile and sterile triploid cyprinid fish. Sci China Life Sci 64:1917–1928. https://doi.org/10.1007/s11427-020-1900-7
Acknowledgements
We would like to thank Editage (http://www.editage.cn) for English language editing.
Funding
This work was supported by the Natural Science Foundation of Zhejiang Province for Distinguished Young Scientists (No. LR21C190001), the National Natural Science Foundation of China (No.31972785, 32202925), the Natural Science Foundation of Zhejiang Province (No. LQ23C19001), the Non-profit Technology Application Research Project of Zhejiang Province (No. LGN22C190018).
Author information
Authors and Affiliations
Contributions
Yang Yang: Data analysis, Writing-Review and Editing; Lei Lu: Methodology, Experiment manipulation, Data analysis; Ruiyi Chen: Methodology, Preliminary experiment; Liechao Yu: Methodology, Data analysis; Weihua Hu: Conceptualization, Preliminary experiment; Dongdong Xu: Experiment Design, Revision and Editing, Supervision, Funding acquisition.
Corresponding author
Ethics declarations
Ethical approval
This study was conducted strictly under the Marine Fisheries Research Institute of Zhejiang Province, China. All the animal experiments were approved by the Institutional Animal Care and Use Ethics Committee of the Marine Fisheries Research Institute of Zhejiang Province, China.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, Y., Lu, L., Chen, R. et al. Production of sterile mono-sex triploid yellow drum (Nibea albiflora): genotypic females and sex-reversed phenotypic males with emphasis on utilization as surrogate broodstock. Fish Physiol Biochem 49, 1277–1294 (2023). https://doi.org/10.1007/s10695-023-01256-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-023-01256-8