Abstract
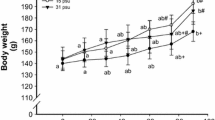
Golden pompano (Trachinotus ovatus) is a commercially important marine fish and is widely cultured in the coastal area of South China. Salinity is one of the most important environmental factors influencing the growth and survival of fish. The aims of this study are to investigate the growth, physiological, and molecular responses of juvenile golden pompano reared at different salinities. Juveniles reared at 15 and 25‰ salinity grew significantly faster than those reared at the other salinities. According to the final body weights, weight gain rate, and feed conversion ratio, the suitable culture salinity range was 15–25‰ salinity. The levels of branchial NKA activity showed a typical “U-shaped” pattern with the lowest level at 15‰ salinity, which suggested a lower energy expenditure on osmoregulation at this level of salinity. The results of this study showed that the alanine aminotransferase, aspartate aminotransferase, and cortisol of juveniles at 5‰ were higher than those of other salinity groups. Our results showed that glucose-6-phosphate dehydrogenase significantly increased at 5‰ and 35‰ salinity. Our study showed that osmolality had significant differences in each salinity group. GH, GHR1, and GHR2 had a wide range of tissue expression including the liver, intestine, kidneys, muscle, gills and brain. The expression levels of GH, GHR1 and GHR2 in the intestine, kidneys, and muscle at 15‰ salinity were significantly higher than those in other three salinity groups. Based on the growth parameters and physiological and molecular responses, the results of the present study indicated that the optimal salinity for rearing golden pompano was 21.36‰ salinity.



Similar content being viewed by others
References
Ababutain IM (2011) Antimicrobial activity of ethanolic extracts from some medicinal plant. Aust J Basic Appl Sci 5:678–683
Abou Anni IS, Bianchini A, Barcarolli IF, Varela Junior AS, Robaldo RB, Tesser MB, Sampaio LA (2016) Salinity influence on growth, osmoregulation and energy turnover in juvenile pompano Trachinotus marginatus Cuvier 1832. Aquaculture 455:63–72
Ágústsson T, Sundell K, Sakamoto T, Ando M, Björnsson BT (2003) Pituitary gene expression of somatolactin, prolactin, and growth hormone during Atlantic salmon parr-smolt transformation. Aquaculture 222:229–238
Ahmmed MK, Ahmmed F, Kabir KA, Faisal M, Ahmed SI, Ahsan MN (2017) Biochemical impacts of salinity on the catfish, Heteropneustes fossilis (Bloch, 1794), and possibility of their farming at low saline water. Aquac Res 48:4251–4261
Almeida DV, Martins CMG, Figueiredo MA, Lanes CFC, Bianchin A, Marins LF (2013) Growth hormone transgenesis affects osmoregulation and energy metabolism in zebrafish (Danio rerio). Transgenic Res 22:75–88
Amiri BM, Xu EG, Kupsco A, Giroux M, Hoseinzadeh M, Schlenk D (2018) The effect of chlorpyrifos on salinity acclimation of juvenile rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 195:97–102
Arnason T, Magnadottr B, Bjornsson B, Steinarsson A, Bjornsson BT (2013) Effects of salinity and temperature on growth, plasma ions, cortisol and immune parameters of juvenile Atlantic cod (Gadus morhua). Aquaculture 380-383:70–79
Bertucci JI, Tovar MO, Blanc AM, Gómez-Requeni P, Unniappan S, Canosa LF (2017) Influence of water salinity on genes implicated in somatic growth, lipid metabolism and food intake in Pejerrey (Odontesthes bonariensis). Comp Biochem Physiol B Biochem Mol Biol 210:29–38
Boeuf G, Payan P (2001) Review: how should salinity influence fish growth? Comp Biochem Physiol C Toxicol Pharmacol 130:411–423
Borski RJ, Yoshikawa JSM, Madsen SS, Nishioka RS, Zabetian C, Bern HA, Grau EG (1994) Effects of environmental salinity on pituitary growth hormone content and cell activity in the euryhaline tilapia, Oreochromis mossambicus. Gen Comp Endocrinol 95:483–494
Breves JP, Fox BK, Pierce AL, Hirano T (2010a) Gene expression of growth hormone family and glucocorticoid receptors, osmosensors, and ion transporters in the gill during seawater acclimation of Mozambique tilapia, Oreochromis mossambicus. J Exp Zoology A 313,432–441
Breves JP, Hasegawa S, Yoshioka M, Fox BK, Davis LK, Lerner DT, Takei Y, Hirano T, Grau EG (2010b) Acute salinity challenges in Mozambique and Nile tilapia: differential responses of plasma prolactin, growth hormone and branchial expression of ion transporters. Gen Comp Endocrinol 167:135–142
Butler AA, Funk B, Breier BH, LeRoith D, Roberts CT Jr, Gluckman PD (1996) Growth hormone (GH) status regulates GH receptor and GH binding protein mRNA in a tissue- and transcript-specific manner but has no effect on insulin-like growth factor-I receptor mRNA in the rat. Mol Cell Endocrinol 116:181–189
Canli EG, Canli M (2015) Low water conductivity increases the effects of copper on the serum parameters in fish (Oreochromis niloticus). Environ Toxicol Pharmacol 39:606–613
Chang JCH, Wu SM, Tseng YC, Lee YC, Baba O, Hwang PP (2007) Regulation of glycogen metabolism in gills and liver of the euryhaline tilapia (Oreochromis mossambicus) during acclimation to seawater. J Exp Biol 210:3494–3504
Chang CH, Lo WY, Lee TH 2016. The antioxidant peroxiredoxin 6 (Prdx6) exhibits different profiles in the livers of seawater- and fresh water-acclimated milkfish, Chanos chanos, upon hypothermal challenge. Frontiers in Physiology, 29
Chourasia TK, D'Cotta H, Baroiller JF, Slosman T, Cnaani A (2018) Effects of the acclimation to high salinity on intestinal ion and peptide transporters in two tilapia species that differ in their salinity tolerance. Comp Biochem Physiol A Mol Integr Physiol 218:16–23
da Silva Aires M, Paganini CL, Bianchini A (2018) Biochemical and physiological effects of nickel in the euryhaline crab Neohelice granulata (Dana, 1851) acclimated to different salinities. Comparative Biochemistry and Physiology Part C, toxicology & Pharmacology 204:51–62
Deane EE, Woo NYS (2004) Differential gene expression associated with euryhalinity in sea bream (Sparus sarba). Am J Phys 287:1054–1063
Deane EE, Woo NYS (2005) Upregulation of the somatotropic axis is correlated with increased G6PDH expression in black sea bream adapted to isoosmotic salinity. Ann N Y Acad Sci 1040:293–296
Deane EE, Woo NYS (2009) Modulation of fish growth hormone levels by salinity, temperature, pollutants and aquaculture related stress: a review. Rev Fish Biol Fish 19:97–120
Divino JN, Monette MY, McCormick SD, Yancey PH, Flannery KG, Bell MA, Rollins JL, Hippel FA, Schultz ET (2016) Osmoregulatory physiology and rapid evolution of salinity tolerance in threespine stickleback recently introduced to fresh water. Evol Ecol Res 17:179–201
Downie AT, Kieffer JD (2016) The physiology of juvenile shortnose sturgeon (Acipenser brevirostrum) during an acute saltwater challenge. Can J Zool 94:677–683
Guo H, Ma Z, Jiang S, Zhang D, Zhang N, Li Y (2014) Length-weight relationship of oval pompano, Trachinotus ovatus (Linnaeus 1758) (Pisces; Carangidae) cultured in open sea floating sea cages in South China Sea. Indian Jouranl of Fisheries 61:93–95
Guo HZ, Gui JY, Li M, Liu HH, Zhang MZ, Meng FX, Shi G, Wang RX, He XY, Zhao YM (2018) Effect of feeding frequency on growth performance, antioxidant status, immune response and resistance to hypoxia stress challenge on juvenile dolly varden char Salvelinus malma. Aquaculture 486:197–201
Hajirezaee S, Mirvaghefi AR, Farahmand H, Agh N (2018) A metabolic approach to understanding adaptation to sea water by endangered Persian sturgeon, Acipenser persicus fingerlings. Aquac Res 49:341–351
Hiroi J, McCormick SD (2012) New insights into gill ionocyte and ion transporter function in euryhaline and diadromous fish. Respir Physiol Neurobiol 184:257–268
Hora MSC, Joyeux JC, Rodrigues RV, Sousa-Santos LP, Gomes LC, Tsuzuki MY (2016) Tolerance and growth of the longsnout seahorse Hippocampus reidi at different salinities. Aquaculture 463:1–6
Imanpoor MR, Najafi E, Kabir M (2012) Effects of different salinity and temperatures on the growth, survival, haematocrit and blood biochemistry of goldfish (Carassius auratus). Aquac Res 43:332–338
Imsland AK, Gustavsson A, Gunnarsson S, Foss A, Arnason J, Arnarson I, Jonsson AF, Smaradottir H, Thorarensen H (2008) Effects of reduced salinities on growth, feed conversion efficiency and blood physiology of juvenile Atlantic halibut (Hippoglossus hippoglossus L.). Aquaculture 274:254–259
Kiilerich P, Kristiansen K, Madsen SS, (2007) Hormone receptors in gills of smolting Atlantic salmon, Salmo salar: Expression of growth hormone, prolactin, mineralocorticoid and glucocorticoid receptors and 11β-hydroxysteroid dehydrogenase type 2. Gen Comp Endocr, 152: 295–303
Laiz-Carrión R, Fuentes J, Redruello B, Guzmán JM, Martín del Río MP, Power D, Mancera JM (2009) Expression of pituitary prolactin, growth hormone and somatolactin is modified in response to different stressors (salinity, crowding and food-deprivation) in gilthead sea bream Sparus auratus. Gen Comp Endocrinol 162:293–300
Lalitha S (2000) Primer Premier 5. Biotech Software & Internet Report 1:270–272
Laverty G, Skadhauge E (2012) Adaptation of teleosts to very high salinity. Comp Biochem Physiol A Mol Integr Physiol 163:1–6
Lee, SH., Lee, MC., Puthumana, J, Park, JC., Kang, S, Hwang, DS, Shin, KH, Park, HG, Souissi, S, Om, AS, Lee, JS, Han, J, (2017). Effects of salinity on growth, fatty acid synthesis, and expression of stress response genes in the cyclopoid copepod Paracyclopina nana. Aquaculture, 470: 182–189
Lin JD, Lin PY, Chen LM, Fang WH, Lin LP, Loh CH (2010) Serum glutamic- oxaloacetic transaminase (GOT) and glutamic-pyruvic transaminase (GPT) levels in children and adolescents with intellectual disabilities. Res Dev Disabil 31:172–177
Lisboa V, Barcarolli IF, Sampaio LA, Bianchini A (2015) Effect of salinity on survival, growth and biochemical parameters in juvenile Lebranch mullet Mugil liza (Perciformes: Mugilidae). Neotropical Ichthyology 13:447–452
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2 (−Delta Delta C(T)) method. Methods 25:402–408
Ma Z, Guo H, Zheng P, Wang L, Jiang S, Qin JG, Zhang D (2014) Ontogenetic development of digestive functionality in T. ovatus Trachinotus ovatus (Linnaeus 1758). Fish Physiol Biochem 40:1157–1167
Ma Z, Guo H, Zhang D, Hu C, Jiang S (2015) Food ingestion, consumption and selectivity of pompano, Trachinotus ovatus (Linnaeus 1758) under different rotifer densities. Aquac Res 46:2593–2603
Ma Z, Guo H, Zheng P, Wang L, Jiang S, Zhang D, Qin JG (2016a) Effect of salinity on the rearing performance of juvenile Trachinotus ovatus (Linnaeus 1758). Aquac Res 47:1761–1769
Ma Z, Zheng P, Guo H, Jiang S, Qin J, Zhang D, Liu X (2016b) Salinity regulates antioxidant enzyme and Na+-K+-ATPase activities of juvenile T. ovatus Trachinotus ovatus (Linnaeus 1758). Aquac Res 47:1481–1487
Mao JN, Cogburn LA, Burnside J (1997) Growth hormone down-regulates growth hormone receptor mRNA in chickens but developmental increases in growth hormone receptor mRNA occur independently of growth hormone action. Mol Cell Endocrinol 129:135–143
Mattioli CC, Takata R, Leme FOP, Costa DC, Filho RM, Silva WS, Luz RK, (2017) The effects of acute and chronic exposure to water salinity on juveniles of the carnivorous freshwater catfish Lophiosilurus alexandri. Aquaculture, 481: 255–266
McCormick SD, Regish M, Christensen K (2009) Distinct freshwater and seawater isoforms of Na+/K+-ATPase in gill chloride cells of Atlantic salmon. J Exp Biol 212:3994–4001
Meier KM, Figueiredo MA, Kamimura MT, Laurino J, Maggion R, Pinto LS, Dellagostin OA, Tesser MB, Sampaio LA, Marins LF, (2009) Increased growth hormone (GH), growth hormone receptor (GHR), and insulin-like growth factor I (IGF-I) gene transcription after hyperosmotic stress in the Brazilian flounder Paralichthys orbignyanus. Fish Physiol. Biochem. 35: 501–509
Montory JA, Cumillaf JP, Cubillos VM, Paschke K, Urbina MA, Gebauer P (2018) Early development of the ectoparasite Caligus rogercresseyi under combined salinity and temperature gradients. Aquaculture 486:68–74
Nakano K, Tagawa M, Takemura A, Hirano T (1997) Effects of ambient salinities on carbohydrate metabolism in two species of tilapia, Oreochromis mossambicus and O. niloticus. Fish Sci 63:338–343
Nakano K, Tagawa M, Takemura A, Hirano T (1998) Temporal changes in liver carbohydrate metabolism associated with seawater transfer in Oreochromis mossambicus. Comparative Biochemistry and Physiology. Part B, biochemistry & molecular biology. 119:721–728
Nakao N, Higashimoto Y, Ohkubo T, Yoshizato H, Nakai N, Nakashima K, Tanaka M (2004) Characterization of structure and expression of the growth hormone receptor gene of the Japanese flounder (Paralichtys olivaceus). J Endocrinol 182:157–164
Nguyen PTH, Do HTT, Mather PB, Hurwood DA (2014) Experimental assessment of the effects of sublethal salinities on growth performance and stress in cultured tra catfish (Pangasianodon hypophthalmus). Fish Physiol Biochem 40:1839–1848
Oliveira-Ribeiro CA, Pelletier E, Pfeiffer WC, Rouleau C (2000) Comparative up-take, bioaccumulation, and gill damages of inorganic mercury in tropical and nordic freshwater fish. Environ Res 83:286–292
Olson KR (2002) Cell signaling and ion transport across the fish gill epithelium. J Exp Zool 293:336–347
Ozaki Y, Fukada H, Tanaka H, Kagawa H, Ohta H, Adachi S, Hara A, Yamauchi K (2006) Expression of growth hormone family and growth hormone receptor during early development in the Japanese eel (Anguilla japonica). Comparative Biochemistry and Physiology Part B, biochemistry & molecular biology 145:27–34
Partridge GJ, Jenkins GI (2002) The effect of salinity on growth and survival of juvenile black bream (Acanthopagrus butcheri). Aquaculture 210:219–230
Ran Z, Chen H, Ran Y, Yu S, Li S, Xu J, Liao K, Yu X, Zhong Y, Ye M, Yan X (2017) Fatty acid and sterol changes in razor clam Sinonovacula constricta (Lamarck 1818) reared at different salinities. Aquaculture 473:493–500
Ray AJ, Lotz JM (2017) Comparing salinities of 10, 20, and 30‰ in intensive, commercial-scale biofloc shrimp (Litopenaeus vannamei) production systems. Aquaculture 476:29–36
Reindl KM, Sheridan MA (2012) Peripheral regulation of the growth hormone-insulin-like growth factor system in fish and other vertebrates. Comparative Biochemistry and Physiology. Part A, molecular & integrative physiology. 163:231–245
Reinecke M (2010) Influences of the environment on the endocrine and paracrine fish growth hormone-insulin-like growth factor-I system. J Fish Biol 76:1233–1254
Rhee JS, Kim BM, Seo JS, Kim IC, Lee YM, Lee JS, (2012) Cloning of growth hormone, somatolactin, and their receptor mRNAs, their expression in organs, during development, and on salinity stress in the hermaphroditic fish, Kryptolebias marmoratus. Comp. Biochem. Physiol. A 161: 436–442
Riley LG, Richman NH III, Hirano T, Grau EG (2002) Activation of the growth hormone/insulin-like growth factor axis by treatment with 17a-methyltestosterone and seawater rearing in the tilapia, Oreochromis mossambicus. Gen Comp Endocrinol 127:285–292
Riley LG, Hirano T, Grau EG (2003) Effects of transfer from seawater to fresh water on the growth hormone/insulin-like growth factor-I axis and prolactin in the Tilapia, Oreochromis mossambicus. Comparative Biochemistry and Physiology Part B, biochemistry & Molecular Biology 136:647–655
Sakamoto T, McCormick SD (2006) Prolactin and growth hormone in fish osmoregulation. Gen Comp Endocrinol 147:24–30
Sakamoto T, Shepherd BS, Madsen SS, Nishioka RS, Siharath K, Richman NH, Bern HA, Grau EG (1997) Osmoregulatory actions of growth hormone and prolactin in an advanced teleost. Gen Comp Endocrinol 106:95–101
Sampaio LA, Bianchini A (2002) Salinity effects on osmoregulation and growth of the euryhaline flounder Paralichtys orbignyanus. J Exp Mar Biol Ecol 269:187–196
Schmitz M, Ziv T, Admon A, Baekelandt S, Mandiki SNM, L'Hoir M, Kestemon P (2017) Salinity stress, enhancing basal and induced immune responses in striped catfish Pangasianodon hypophthalmus (Sauvage). J Proteome 167:12–24
Scott GR, Schultel PM, Wood CM (2006) Plasticity of osmoregulatory function in the killifish intestine: drinking rates, salt and water transport, and gene expression after freshwater transfer. J Exp Biol 209:4040–4050
Seale AP, Riley LG, Leedom TA, Kajimura S, Dores RM, Hirano T, Grau G (2002) Effects of environmental osmolality on release of prolactin, growth hormone and ACTH from the tilapia pituitary. Gen Comp Endocrinol 128:91–101
Shui C, Shi Y, Hua X, Zhang Z, Zhang H, Lu G, Xie Y (2018) Serum osmolality and ions, and gill Na+/K+-ATPase of spottedtail goby Synechogobius ommaturus (R.) in response to acute salinity changes. Aquaculture and Fisheries 3:79–83
Silva Aires, M da, Paganini, CL, Bianchini, A, (2018). Biochemical and physiological effects of nickel in the euryhaline crab Neohelice granulata (Dana, 1851) acclimated to different salinities. Comp Biochem P hysiol C T oxicol P harmacol, 204: 51–62
Stewart HA, Noakes DLG, Cogliati KM, Peterson JT, Iversen MH, Schreck CB (2016) Salinity effects on plasma ion levels, cortisol, and osmolality in Chinook salmon following lethal sampling. Comp Biochem Physiol A Mol Integr Physiol 192:38–43
Sun L, Zhang D, Jiang S, Guo H, Zhu C (2013) Isolation and characterization of 21 polymorphic microstatellites in golden pompano Trachinotus ovatus. Conserv Genet Resour 5:1107–1109
Tan X, Lin H, Huang Z, Zhou C, Wang A, Qi C, Zhao S (2016) Effects of dietary leucine on growth performance, feed utilization, non-specific immune responses and gut morphology of juvenile Trachinotus ovatus. Aquaculture 465:100–107
Taylor JF, Migaud H, Porter MJ, Bromage NR (2005) Photoperiod influences growth rate and plasma insulin-like growth factor-I levels in juvenile rainbow trout, Oncorhynchus mykiss. Gen Comp Endocrinol 142:169–185
Tomy S, Chang YM, Chen YH, Cao JC, Wang TP, Chang CF (2009) Salinity effects on the expression of osmoregulatory genes in the euryhaline black porgy Acanthopagrus schlegeli. Gen Comp Endocrinol 161:123–132
Tran-Ngoc KT, Schrama JW, Le MTT, Nguyen TH, Roem AJ, Verreth JAJ (2016) Salinity and diet composition affect digestibility and intestinal morphology in Nile tilapia (Oreochromis niloticus). Aquaculture 469:36–43
Tsui, WC, Chen, JC, Cheng, SY, (2012). The effects of a sudden salinity change on cortisol, glucose, lactate, and osmolality levels in grouper Epinephelus malabaricus. Fish P hysiology and B iochemistry, 38: 1323–1329
Tsuzuki MY, Sugai JK, Maciel JC, Francisco CJ, Cerqueira VR (2007) Survival, growth and digestive enzyme activity of juveniles of the fat Snook (Centropomus parallelus) reared at different salinities. Aquaculture 271:319–325
Weng CF, Lee TH, Hwang PP (1997) Immune localization of prolactin receptor in the mitochondria-rich cells of the euryhaline teleost (Oreochromis mossambicus) gill. FEBS Lett 405:91–94
Whitehead A, Roach JL, Zhang SY, Galvez F (2012) Salinity- and population-dependent genome regulatory response during osmotic acclimation in the killifish (Fundulus heteroclitus) gill. J Exp Biol 215:1293–1305
William KFT 2014. The role of osmotic stress transcription factor 1 in fishes. Front Zool, 11
Wu H, Liu J, Lu Z, Xu L, Ji C, Wang Q, Zhao J (2017) Metabolite and gene expression responses in juvenile flounder Paralichthys olivaceus exposed to reduced salinities. Fish & Shellfish Immunology 63:417–423
Yada T, McCormick SD, Hyodo S (2012) Effects of environmental salinity, biopsy, and GH and IGF-I administration on the expression of immune and osmoregulatory genes in the gills of Atlantic salmon (Salmo salar). Aquaculture 362-363:177–183
Yamaguchi Y, Breves JP, Haws MC, Lerner DT, Grau EG, Seal AP (2018) Acute salinity tolerance and the control of two prolactins and their receptors in the Nile tilapia (Oreochromis niloticus) and Mozambique tilapia (O. mossambicus): a comparative study. Gen Comp Endocrinol 257:168–176
Yin SJ, Zhang L, Zhang L, Wan J, Song W, Jiang X, Park YD, Si YX (2018) Metabolic responses and arginine kinase expression of juvenile cuttlefish (Sepia pharaonis) under salinity stress. Int J Biol Macromol 113:881–888
Yuan M, Jia Q, Wang T, Lu Q, Tang L, Wang Y, Lu W (2017) Dynamic responses of prolactin, growth hormone and their receptors to hyposmotic acclimation in the olive flounder Paralichthys olivaceus. Gen Comp Endocrinol 254:8–13
Zacharia S, Kakati VS (2004) Optimal salinity and temperature for early developmental stages of Penaeus merguiensis De man. Aquaculture 232:373–382
Zhang YT, Huang S, Qiu HT, Li Z, Mao Y, Hong WS, Chen SX (2017) Optimal salinity for rearing Chinese black sleeper (Bostrychus sinensis) fry. Aquaculture 476:37–43
Zhang L, Feng Q, Sun L, Ding K, Huo D, Fang Y, Zhang T, Yan H (2018) Differential gene expression in the intestine of sea cucumber (Apostichopus japonicus) under low and high salinity conditions. Comparative Biochemistry and Physiology Part D, Genomics & Proteomics 25:34–41
Acknowledgements
This work was supported by the Guangdong Provincial Science and Technology Project (2019B030316030), China Agriculture Research System (CARS-47), China-ASEAN Maritime Cooperation Fund, National Infrastructure of Fishery Germplasm Resources Project (2019DKA30407) and Guangdong Provincial Special Fund For Modern Agriculture Industry Technology Innovation Teams.
Funding
This study was supported by the China Agriculture Research System (CARS-47-G07), China-ASEAN maritime cooperation fund, Natural Science Foundation of Guangdong Province (2015A030313818), Sanya city scientific and technology cooperation of academic and regional (2015YD06), National Science & Technology Infrastructure platform (2018DKA30470), and Guangdong Provincial Special Fund For Modern Agriculture Industry Technology Innovation Teams.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experiments in this study were approved by the Animal Care and Use Committee of South China Sea fisheries Research Institute, Chinese Academy of fishery Sciences (no. SCSFRI96-253) and performed according to the regulations and guidelines established by this committee.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, B., Guo, HY., Zhu, KC. et al. Growth, physiological, and molecular responses of golden pompano Trachinotus ovatus (Linnaeus, 1758) reared at different salinities. Fish Physiol Biochem 45, 1879–1893 (2019). https://doi.org/10.1007/s10695-019-00684-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-019-00684-9