Abstract
Peat fires contribute to global warming and environmental destruction. Once ignited, the fires tend to spread deep, underground and are difficult to extinguish using solely water. Mixtures of soap-based firefighting agents and water are expected to improve suppression efficiency by enhancing the permeability of water, a cooling material. Nevertheless, peat fire suppression is rarely studied. We performed peat fire extinguishing experiments in Palangkaraya, Indonesia to evaluate the efficiency of an environmentally friendly soap-based agent; and we conducted field experiments on 1.5 m × 1.5 m as well as 7 m × 7 m of peatlands. We conducted firefighting activities by applying (1) groundwater as well as (2) a solution of groundwater and 1 vol% of a soap-based firefighting agent. Surface temperatures of peat fires were approximately 160°C and 66°C after initial firefighting activities using solely water and a 1 vol% soap-based solution, respectively. The quantity of water required to extinguish the fires was 7.2 L/m2 using solely water, and decreased to 3.6 L/m2 using the soap-based solution. The soap-based solution exhibited a higher heat removal effect on the peat soil surface and higher permeability into peat soil than solely water, and can therefore be used to quickly extinguish peat fires. 10 months after the experiment, experimental sites sprayed with the soap-based solution demonstrated recovery of vegetation to the same degree as the sites sprayed solely with water. Thus, the soap-based firefighting agent is environmentally friendly, has promising firefighting properties, and is a reasonable tool for mitigating peat fires.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The frequency of devastating wildfires is increasing around the world [1, 2]. The occurrence of wildfires is hypothesized to be related to climate change and deforestation affected by human activities [3]. The El Nino phenomenon also reduces precipitation and soil moisture content, which contributes to the widespread nature and severity of wildfires [4]. Lierop [5] reported that between 2003 and 2012, approximately 67 million hectares (Mha) of forest land burned annually, mostly in tropical South America and Africa. In Indonesia, 0.79 Mha burned on Kalimantan Island in 1997, releasing 0.19–0.23 Gt of carbon into the atmosphere via peat combustion [6]. Approximately 4.6 Mha were burned during the 2015 fire season and 0.89 Gt of carbon dioxide equivalents were released [7]. Forest fires thus pose substantial environmental problems, such as deforestation and greenhouse gas emissions. Indonesia has particularly vast peatland deposits, which serve as the largest storage of organic carbon in the country [8]. The burning of Indonesian peat produces large quantities of greenhouse gases [6]; and causes haze pollution, health risks, as well as economic damage to neighboring countries including Singapore and Malaysia [9]. The damage caused by Indonesian forest and peat fires in 2015 was estimated to exceed US $16 billion [10]. To address this problem, various measures have been applied for peat fires in Indonesia through technical cooperation between government agencies; including preventing peat fires, early fire detection, and post-fire extinguishing activities. Extensive efforts have been made to prevent peat fires by creating dams to maintain the level of the groundwater in the rainy season. Honma et al. [11] developed a peat fire detection system that uses satellite data to detect the early stages of peat fires and disseminates important information to relevant parties. Takahashi et al. [12] implemented a compact fire extinguishing system using tricycles to extinguish peat fires. Additionally, because it is also useful to dig ditches in the soil to create fire protection zones, corresponding research is also being conducted [13].
Once drained, peatland is flammable, and the organic soils (because of the high organic content) tend to burn for a long time once ignited (weeks to months) [14]. Smoldering peat fires can spread in horizontal and vertical directions. To some degree, peat fires tend to survive below ground level because of the better conditions in terms of preventing heat loss than smoldering on the surface. Smoldering is slow, low-temperature, flameless burning; and can reach deep into the soil where there are large cracks or natural piping systems [15]. Because the combustion tends to progress underground, it is difficult to directly extinguish by using solely water, which is the typical procedure in responding to surface flaming wildfires. In peat fires, most hot spots are located below ground level; water poorly penetrates through the peat soil and thus does not reach these hot spots because of the relatively high surface tension of the water. This leads to the large quantities of water (approximately 6 L/kg) required to extinguish peat fires [16].
Countermeasures for large-scale forest fires include fire spread prevention and fire extinguishing. In fire spread prevention, a fire retardant is sprayed in advance; whereas in fire extinguishing, water and a firefighting agent solution containing a surfactant are sprayed on a burning fire. The mixing of a surfactant with water lowers the surface tension of water and thus substantially enhances its permeability into the burning material [17]. This approach enhances the cooling effect on combustibles and enables water to be used more efficiently. Fire extinguishing is further enhanced by foaming; which improves adhesion and is effective for extinguishing combustion flames, both in terms of the required time and quantity of water [18]. However, a large quantity of synthetic firefighting agent tends to flow into the water system during firefighting and foam tends to persist for a long time, thus introducing relatively high environmental toxicity for aquatic organisms.
To address these issues and environmental concerns, Mizuki et al. [17] and Kawahara et al. [19] developed a soap-based firefighting agent for general building fires and forest fires using soap as a surfactant. Here, we report the effectiveness of the soap-based firefighting agent for fighting peat fires in a series of experiments conducted in Central Kalimantan Palangkaraya City (Indonesia) and analyzed the peat fire situation in detail. We also devised an effective firefighting method for peat fires. We conducted a JICA (Japan International Cooperation Agency) project field survey of peat fires and a demonstration experiment in Palangkaraya City. Rivai et al. [20] and Subekti et al. [21] have also reported on the effectiveness of extinguishing peat fires using soap-based firefighting agents derived from palm oil.
We conducted meso-scale and large-scale peat fire extinguishing experiments with the University of Palangkaraya to verify the effectiveness of the soap-based firefighting agent. In the meso-scale experiments, we investigated the spread of peat fires and quantity of water required to extinguish the fire. In the large-scale experiments, we carried out practical firefighting activities and verified the effectiveness of the soap-based firefighting agent compared with water treatment. We also confirmed the low eco-toxicity of the soap-based firefighting agent by observing the natural regeneration of the vegetation over a 10 months period.
2 Materials and Methods
2.1 Soap-Based Firefighting Agent Preparation
Lauric acid and oleic acid were purchased from Cognis Oleochemicals Co., Ltd., Malaysia, and Miyoshi Oil & Fat Co., Ltd., Japan, to prepare potassium laurate and potassium oleate. Methylglycine diacetic acid (MGDA, manufactured by BASF, Germany) was used as the chelating agent. Water, propylene glycol (PG), and hexylene glycol (HG) were used as diluents.
Purified water and 20.4 wt.% MGDA were combined, and stirred at 50°C for 1 h (Figure 1). Next, 12.9 wt.% PG, 7.0 wt.% HG, 4.6 wt.% potassium hydroxide (48%), 4.3 wt.% lauric acid, and 6.8 wt.% oleic acid were then added in sequence; stirred at 50°C for 1 h; and the pH was adjusted. The pH of the soap-based firefighting agent was 10.73, the specific gravity was 1.119, the viscosity was 21.6 cSt, and the surface tension (1 vol%) was 31.1 dyn/cm (which is half the value for water, 72 dyn/cm).
Schematic for of making a soap-based firefighting agent. First, we added MGDA to purified water and stirred at 50°C for 1 h. After confirming that MGDA was completely dissolved, we proceeded to the next step. We added PG, HG, potassium hydroxide, lauric acid, and oleic acid in that order; and stirred the mixture at 50°C for 1 h. After confirming that the fatty acids completely dissolved, we adjusted the pH to complete the soap-based firefighting agent
2.2 Study Area
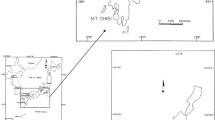
The selection and preparation of the experimental site were conducted in collaboration with the University of Palangkaraya. Fire extinguishing experiments were conducted in Palangkaraya City, Central Kalimantan Province, Kalimantan Island, Republic of Indonesia (2°17′23.1′′S, 114°01′57.3′′E), as shown in Figure 2. In the meso-scale experiments, peat soil blocks were collected and dried, and 1.5 m × 1.5 m experimental plots were created as shown in Figure 3. In the large-scale experiments, two sites of 7 m × 7 m of peat soil in the natural state was separated with galvanized iron plates to prevent the fire spreading outside the site. The experimental sites were conditioned as shown in Figure 4. The average local temperature at the time of experiments was 33.8°C and the average humidity was 77.8%. In addition, the average annual rainfall volume of Palangkaraya was 2,884 mm; whereas the average monthly rainfall in June and September were 200 and 100 mm, respectively [22, 23].
2.3 Moisture Content
The peat sample in meso and large-scale experiments were collected, and the moisture content was analyzed in June 2015 as well as September 2015, respectively. All samples were taken from the experiment sites. The moisture content of peat soil was calculated by the following procedure.
-
(1)
Measured the mass of wet peat sample
-
(2)
Dried at 105°C for 24 h
-
(3)
Measured the mass of the dried sample
-
(4)
The moisture content was the ratio between the mass difference before and after drying to the mass of the dried sample.
2.4 Water Analysis
The pH and hardness of the water used in the fire extinguishing experiments were measured with a pH meter (MM-60, DKK-TOA Corporation, Japan) and a portable total hardness meter (HI 96735, HANNA, Japan), respectively. The water used in meso and large-scale experiments were sampled and analyzed in June 2015 and September 2015, respectively.
2.5 Peat Soil Permeability Test
Permeability was evaluated by the water drop penetration time (WDPT) test by the following procedure [24].
-
(1)
Approximately 60 g of peat soil was collected in a glass petri dish (diameter approximately 10 cm)
-
(2)
Using a micropipette, 200 µL of water or 1 vol% soap-based firefighting agent solution was applied to the surface of peat soil
-
(3)
The penetration time of the water or the 1 vol% soap-based firefighting agent solution to the peat soil was visually measured
2.6 Experimental Protocol
2.6.1 Meso-scale Experiments
Meso-scale fire extinguishing experiments were conducted from June 9 to 11, 2015. The vegetation on the surface of the peat soil was first removed and the peat soil was then collected with an open-top metal box (0.3 m × 0.3 m × 0.3 m). One hundred peat soil blocks were collected and air-dried for 2 months or longer to ensure that the moisture content on the peat soil surface was approximately 54.6%. As shown in Fig. 3, two wooden compartments were prepared; each wooden compartment was divided into four plots of 1.5 m × 1.5 m, and four experimental plots were used for fire extinguishing experiments (two plots for fire extinguishing with water and the other two plots for fire extinguishing experiments with the soap-based firefighting agent solution). Twenty-eight igniters made of soft fiberboard were placed on the center of each experimental site. The first experiment was at the left bottom (water), the second experiment was at the left top (1 vol% soap-based firefighting agent solution), the third experiment was at the right bottom (water), and the fourth experiment was at the right top (1 vol% soap-based firefighting agent solution). Using soft fiber boards enables the highly reproducible combustion of peat soil because the board material is homogeneous and the calorific value is expected to remain constant during combustion. The ignition protocol was as follows. A soft fiber board was heated with a gas burner for 1 min and burned. After 40 min, the ash from the combustion of the fiberboard was removed; and then the measurement of peat soil temperatures at depths of 2, 4, 6, 8 and 10 cm was started (GL240, GRAPHTEC Corporation, Japan). A thermal imaging camera (E4, FLIR Systems Inc., America) was also used to measure the fire temperatures on the peat surface.
The initial firefighting activity was carried out 240 min after the start of combustion. The ignition reaches the end of the plot approximately 480 min after ignition based on the distance from the soft fiberboard to the edge of the experimental plot, and the average burning rate of the peat fire [22]. Because it is not possible to reproduce peat fire in its natural state when the combustion reaches the edge of the plot, the burn time was set at 240 min, enabling a margin of error. Groundwater from the experimental site was used to extinguish the fire. The water had a pH of 5.2 and hardness of 0 ppm. Soap loses its function depending on the pH and hardness. Under acidic conditions soap turns into fatty acids, and under high hardness conditions soap becomes water-insoluble and metallic in nature. To investigate whether it is possible to use soap, the pH and hardness of the water were investigated. The results of the water analysis indicate that there were no problems with using soap-based firefighting agents. Water or 1 vol% soap-based firefighting agent solutions were used for firefighting activity. A backpack-type water tank (Jet Shooter EV, Ashimori Industry Co., Ltd., Japan) was used to extinguish the fire until the peat surface temperature decreased to < 100°C. Additional firefighting activity was then carried out for hot spots where the peat surface temperature exceeded 100°C. This procedure was carried out until the peat surface temperature decreased to < 50°C.
The meso-scale fire extinguishing experiments were conducted to determine the following three experimental conditions for the large-scale fire extinguishing experiments: how to burn peat soil, initial water-spray density, and the standard ground surface temperature to assess the fire extinguishing approach.
2.6.2 Large-Scale Experiments
Large-scale fire extinguishing experiments were conducted from September 14 to 16, 2015. As shown in Fig. 4, peat lands with vegetation remaining on the peat surface were divided into 7 m × 7 m plots with galvanized iron plates to make two experimental sites, and 416 soft fiberboards were placed on the center of each site. The moisture content on the peat soil surface was 57.9%. The combustion method was as follows. The soft fiberboards were heated with a gas burner for 10 min and burned. After 40 min from the start of ignition, the ash was removed. A thermal imaging camera was used to measure the fire temperatures on the peat surface. Image analysis was performed with FLIR Thermal Studio, which is software from FLIR.
The initial firefighting activity was carried out 240 min after the start of combustion using groundwater from the experimental site. The water had a pH of 5.6 and a hardness of 0 ppm. The firefighting activity was undertaken in two stages. The initial firefighting activity was carried out at a spray density of 3.0 L/m2 with a portable pump (Fyr Pak, Hale Products Inc., US), Quadra Fog Nozzle (NH-40QF, YONE CORPORATION, Japan), line proportioner (FP40.360, YONE CORPORATION), and water tank; as shown in Fig. 5. The flow rate of the pump was 3.8 L/min. Additional firefighting activity was carried out with a backpack-type water tank against the hot spots where the peat surface temperature exceeded 50°C after the initial firefighting activity.
Fire extinguishing method in the large-scale fire extinguishing experiments. We transported water in the water tank with a pump, and sprayed water or the 1 vol% soap-based firefighting agent solution from the nozzle. We mixed the soap-based firefighting agent with a 1 vol% solution with a line proportioner
The water-spray density was 3.0 L/m2 in the initial firefighting activity and firefighting against hot spots continued until the peat surface temperature decreased to < 50°C.
2.7 Vegetation Recovery
The sites of the large-scale experiments were photographed 10 months after combustion and vegetation recovery was compared between the soap-based firefighting agent solution as well as the water-sprayed sites.
3 Results and Discussion
3.1 Meso-scale Experiments
The peat surface temperature of the burning peat soil reached > 280°C, as shown in Fig. 6, 40 min after the start of combustion. The underground temperature of the burning peat soil is shown in Fig. 7. The underground temperature of the burning peat soil reached approximately 300°C. After 80 min from the start of the temperature measurement, the temperature reached 300°C at a depth of 2 cm; after 180 min, the temperature reached 300°C at a depth of 4 cm.
Burning peat surface temperature in the meso-scale fire extinguishing experiments. We removed the ash from the combustion of the soft fiberboard and measured the peat surface temperature with a thermal imaging camera. The peat surface temperature exceeded 280°C and we confirmed combustion of the peat soil
Underground temperature at depth during combustion of 1 vol% soap-based firefighting agent solution in meso-scale fire extinguishing experiments. The underground temperature measurements began after removing the ash from the combustion of the soft fiberboard (approximately 40 min, because the experiments had started). We set thermocouples every 2 cm in the downward direction. After 80 min from the start of the temperature measurements, the peat soil at a depth of 2 cm had reached 300°C and we confirmed combustion
This result indicates that the underground peat in-depth spread rate was 1.2 cm/h. At depths of 8 and 10 cm, the maximum temperature reached only to 70°C; thus, pyrolysis did not occur. In accordance with Hayasaka et al., peat soil begins pyrolysis at > 275°C [22]. We therefore simulated peat fires because the underground temperature of the peat soil in this study exceeded 275°C.
Figure 8 shows the total quantity of a water or 1 vol% soap-based firefighting agent solution required to extinguish the fire. The peat fire reignited and burned for the sites treated with solely water and additional firefighting activities were required, resulting in an average spray density of 8.1 L/m2, after which there was no resumption of burning and the peat surface temperature remained < 50°C. In contrast, no additional firefighting activity was required for the sites treated with the 1 vol% soap-based firefighting agent solution and the average spray density was 4.7 L/m2.
Quantity of water required to extinguish the fire in the meso-scale fire extinguishing experiments. We carried out the initial firefighting activity 240 min after the start of combustion with a backpack-type tank until the peat surface temperature was < 100°C. We carried out additional firefighting activity against hot spots where the peat surface temperature exceeded 100°C. The quantity of water required to extinguish the fire was approximately 8.1 L/m2 for the water treatment and 4.7 L/m2 for the 1 vol% soap-based firefighting agent solution treatment
The results of the meso-scale fire extinguishing experiments verified the large-scale experimental parameters of using soft fiberboards as an igniter. We set the spray density of 3.0 L/m2 for the initial firefighting activity, and a standard of fire extinguishing peat surface temperature of 50°C to assess the fire extinguishing procedure. Santoso et al. also set the standard of fire extinguishing temperature to 50°C [25].
We calculated the spray density from the quantity of water used and the combustion area. We calculated the combustion area by measuring the burned portion and estimating the combustion area by combining polygons, such as squares and triangles. The results of meso-scale experiments (Table 1) indicate that the average spray density required to extinguish fires was 8.1 L/m2 for water and 4.7 L/m2 for the 1 vol% soap-based firefighting agent solution. The reason why we set the spray density to 3.0 L/m2 for the large-scale experiment was to avoid spraying excessive water. In other words, we set the conditions to < 4.7 L/m2, which was able to extinguish fires with a 1 vol% soap-based firefighting agent solution in the meso-scale experiments.
In the meso-scale experiments, we used 28 igniters in 1.5 m × 1.5 m plots (12 igniters/m2). In the large-scale experiments, there is a possibility that more combustion will occur than in the meso-scale experiments, thus we reduced the number of the igniters to 9 igniters/m2. We set the fire extinguishing temperature for initial firefighting at 100°C in the meso-scale experiments, but because we confirmed re-ignitions, we set the temperature to an even lower temperature of 50°C in the large-scale experiments.
3.2 Large-Scale Experiments
Prior to firefighting activity, the peat surface temperature was measured with a thermal imaging camera, as shown in Figure 9a and b. The peat surface temperature reached > 280°C, indicating that a peat fire had occurred. The standard for extinguishing temperature was 50°C based on the results of the meso-scale experiments. Santoso et al. set the standard of fire extinguishing temperature to ≤ 50°C instead of to 60°C because there was no reignition [25] and this result is congruent. We observed many areas of hot spots exceeding 150°C in the water treatment, whereas there were almost no hot spots exceeding 150°C in the 1 vol% soap-based firefighting agent solution, and hot spots < 70°C accounted for the majority (as shown in Figure 9c and d). We observed re-ignition in both the water and 1 vol% soap-based firefighting agent solution treatments; however, as indicated in Figure 10, the total spray density of the 1 vol% soap-based firefighting agent solution treatment (3.6 L/m2) was substantially lower than that of the water treatment (7.2 L/m2). Additionally, as indicated in Figure 11, the permeation time into the peat soil was 102 s for the water treatment, but only 10 s for the 1 vol% soap-based firefighting agent solution treatment. We therefore substantially increased the permeability of water by adding the soap-based firefighting agent.
Comparison of the peat surface temperature before and after the initial firefighting activity in a large-scale fire extinguishing experiment. We measured the peat surface temperature before and after the initial firefighting activity. The peat surface temperature of the burning peat soil reached exceeded 280°C before initial firefighting activity (a, b). After initial firefighting activity, we observed many areas of hot spots as high as 150°C in water treatment (c), whereas there were almost hot spots < 70°C accounted for the majority in the 1 vol% soap-based firefighting agent solution (d)
Quantity of water required to extinguish the fire in a large-scale fire extinguishing experiment. We carried out initial firefighting activity 240 min after the start of combustion until the peat surface temperature was < 50°C. We carried out additional firefighting activities with a backpack-type tank against hot spots where the peat surface temperature reached 50°C. The quantity of water required to extinguish the fire was 7.2 L/m2 for the water treatment compared with 3.6 L/m2 for the 1 vol% soap-based firefighting agent solution treatment
The reason that the quantity of water required to extinguish the fire by using the 1 vol% soap-based firefighting agent solution was notably lower than the water treatment is directly related to the higher permeability of water into peat soil by using the former, which efficiently cooled the underground temperature. There was also a large quantity of emitted water vapor from the peat surface immediately after the initial firefighting activity, which greatly lowered the surface temperature.
The quantity of 1 vol% soap-based firefighting agent solution required for firefighting in the meso- and large-scale fire extinguishing experiments was 4.7 and 3.6 L/m2, respectively, whereas 8.1 and 7.2 L/m2 were required for the water-only treatment (Figure 12). The 1 vol% soap-based firefighting agent solution therefore halved the total quantity of water required for firefighting.
Quantity of water required to extinguish the fire in meso- and large-scale fire extinguishing experiments. The quantity of 1 vol% soap-based firefighting agent solution required for firefighting in the meso and large-scale fire extinguishing experiments was 4.7 and 3.6 L/m2, respectively; whereas 8.1 and 7.2 L/m2 were required for the water only treatment. The 1 vol% soap-based firefighting agent solution therefore halved the total quantity of required water for firefighting
Regarding the peat fire suppressions, research on the quantity of fire extinguishing water from rainfall [26] and research on foam sealing with a fire extinguishing agent [27] are also being conducted. Compared with the results of the study by Shaorun et al. which assumed fire extinguishing by rain, the quantity of water required to extinguish the fire in this study was substantially less. The reason is that fire extinguishing is greatly affected by the quantity of spraying per unit time. Therefore, the method of spraying a large quantity of water in a short time can reduce the total quantity of water required for extinguishing the fire. To further optimize the peat fire suppression method with soap-based firefighting agents, it is necessary to consider the quantity of spraying per unit time.
Furthermore, foam sealing can extinguish peat fires by shutting off the oxygen supply and by cooling. Extinguishing with foam sealing by soap-based firefighting agents might also be considered in the future.
3.3 Vegetation Recovery in the Large-Scale Experiment Sites
10 months after spraying, we observed plants growing steadily in both of the experimental sites, as indicated in Figure 13; and we noted no differences between the 1 vol% soap-based firefighting agent solution as well as water treatment sites.
Vegetation recovery in large-scale fire extinguishing experiment sites. 10 months after the large-scale fire extinguishing experiments, we photographed the experimental sites and compared the vegetation recovery between the 1 vol% soap-based firefighting agent solution and water-sprayed sites. Plants were steadily growing in both experimental sites
Soap-based firefighting agents are less toxic to aquatic organisms [28, 29] and do not affect rice germination or growth [30]. Additionally, because the soap-based firefighting agent is highly biodegradable [31], it is unlikely to remain in the environment and its long-term impact on plants as well as other organisms is low. The results from this study suggest no substantial difference between the 1 vol% soap-based firefighting agent solution and water sites after a period of 10 months.
The use of fire extinguishing agents in the United States is regulated by the Interagency Policy for Aerial and Ground Delivery of Wildland Fire Chemicals Near Waterways and Other Avoidance Areas, which was enacted in 2012. These policies serve as guidelines to avoid aerial dispersal of fire retardant or foams within 300 ft of canals and above ground dispersal of forest fire extinguishing in canals [32]. The main component of firefighting foam agents distributed in the United States is a synthetic surfactant, which is hypothesized to be highly toxic to aquatic organisms and plants [33]. Kawano et al. reported that soap-based firefighting agents are less toxic than synthetic surfactants in model biotope experiments and are comparable with water spraying [34]. The use of soap-based firefighting agents in Indonesia is promising because they are more effective than water in terms of extinguishing peat fires and have less of an impact on plants. The effects of fire extinguishing agents after spraying on peat soil [35] and rewetted dry soil as well as the subsequent possible increase in fire risk [36] have also been investigated on a laboratory scale. Dry porous peat cannot absorb and store water, and water is retained on the surface of peat. Therefore, when the rewetted peat burns, the water evaporates rapidly and the spread rate of smoldering is higher on rewetted peat than peat soil, which is already moist. However, soap-based firefighting agents with better penetrating power than by using solely water are more likely to be wetted, which might reduce the risk of water evaporation. We have not been able to verify soil surveys and re-ignition after spraying soap-based firefighting agents; thus, it might be necessary to carry out investigations on a field scale in the future.
4 Conclusion
We investigated the effectiveness of a soap-based firefighting agent in a series of peat fire experiments. We used soft fiberboards to ignite 1.5 m × 1.5 m and 7 m × 7 m experimental peat fires. The initial firefighting activity was at a water-spray density of 3.0 L/m2 on a field scale of 7 m × 7 m. After the initial firefighting activity, we observed many areas of hot spots as high as 150°C in water treatment, whereas there were almost hot spots < 70°C accounted for the majority in the 1 vol% soap-based firefighting agent solution. Approximately half the quantity of water-spray density was required to extinguish the peat fire by using the soap-based firefighting gent compared with firefighting by using only water treatment. 10 months after the experiments, vegetation had steadily recovered in both sites, implying no ecosystem damage because of the application of soap-based firefighting agent. Peat fires in Indonesia can therefore be extinguished by using soap-based firefighting agents as a promising alternative to the current fire extinguishing technique that uses solely water. Environmentally friendly soap-based firefighting agents can be used as a fire extinguishing medium to prevent damage caused by increasingly severe peat fires. The next challenge is to better understand peat fires and further improve soap-based firefighting agents for efficient firefighting responses to peat fires.
Data Availability
All relevant data are within the paper.
Code Availability
Not applicable.
References
Shiraishi T, Hirata R (2021) Estimation of carbon dioxide emissions from the megafires of Australia in 2019–2020. Sci Rep 11:8267. https://doi.org/10.1038/s41598-021-87721-x
Gatti LV, Basso LS, Miller JB, Gloor M, GattiDomingues L, Cassol HLG, Tejada G, Aragão LEOC, Nobre C, Peters W, Marani L, Arai E, Sanches AH, Corrêa SM, Anderson L, Von Randow C, Correia CSC, Crispim SP, Neves RAL (2021) Amazonia as a carbon source linked to deforestation and climate change. Nature 595:388–393. https://doi.org/10.1038/s41586-021-03629-6
Mansoor S, Farooq I, Mubashir Kachroo M, El Din Mahmoud A, Fawzy M, Mariana Popescu S, Alyemeni MN, Sonne C, Rinklebe J, Ahmad P (2002) Elevation in Wildfire frequencies with respect to the climate change. J Environ Manage 301:113769. https://doi.org/10.1016/j.jenvman.2021.113769
Braatz SM (1999) State of the world's forests 1999 The status of forest resources. Food and Agricalture Organization of the United Nations (FAO)
van Lierop P, Lindquist E, Sathyapala S, Franceschini G (2015) Global forest area disturbance from fire, insect pests, diseases and severe weather events. For Ecol Manage 352(7):78–738. https://doi.org/10.1016/j.foreco.2015.06.010
Page SE, Siegert F, Rieley JO, Boehm H-D, Jaya A, Limin S (2002) The amount of carbon released from peat and forest fires in Indonesia during 1997. Nature 420:61–65. https://doi.org/10.1038/nature01131
Lohberger S, Stängel M, Atwood EC, Siegert F (2017) Spatial evaluation of Indonesia’s 2015 fire-affected area and estimated carbon emissions using Sentinel-1. Glob Change Biol 24(2):1–11. https://doi.org/10.1111/gcb.13841
Rieley JO, Gaston Sieffermann R, Page SE (1993) The origin, development, present status and importance of the lowland peat swamp forests of Borneo. Suo 43:241–244
Kunii O, Kanagawa S, Yajima I, Hisamatsu Y, Yamamura S, Amagai T, SachrulIsmail IT (2002) The 1997 haze disaster in Indonesia: its air quality and health effects. Arch Environ Health Int J 57(1):16–22. https://doi.org/10.1080/00039890209602912
The World Bank (2015) Indonesia economic quarterly, Reforming amid uncertainty; December
Honma T, Kaku K, Usup A, Hidayat A (2016) Detection and prediction systems of peat-forest fires in Central Kalimantan. In: Osaki M, Tsuji N (eds) Tropical Peatland Ecosystems. Springer, Japan, pp 397–406
Takahashi H, Jaya A, Limin SH (2016) Compact firefighting system for villages and water resources for firefighting in peatland area of Central Kalimantan. In: Osaki M, Tsuji N (eds) Tropical Peatland Ecosystems. Springer, Japan, pp 407–417
Lin S, Liu Y, Huang X (2021) How build a firebreak to stop smouldering peat fire: insights from a laboratory-Scale Study. Int J Wildland Fire 30(6):454–461. https://doi.org/10.1071/WF20155
Turetsky MR, Benscoter B, Page S, Rein G, van der Werf GR, Watts A (2015) Global vulnerability of peatlands to fire and carbon loss. Nature Geosci 8(1):11–14. https://doi.org/10.1038/ngeo2325
Huang X, Rein G (2014) Smouldering combustion of peat in wildfires: Inverse modelling of the drying and the thermal and oxidative decomposition kinetics. Combust Flame 161:1633–1644. https://doi.org/10.1016/j.combustflame.2013.12.013
Ramadhan ML, Palamba P, Imran FA, Kosasih EA, Nugroho YS (2017) Experimental study of the effect of water spray on the spread of smoldering in Indonesian peat fires. Fire Saf J 91:671–679. https://doi.org/10.1016/j.firesaf.2017.04.012
Mizuki H, Uezu K, Kawano T, Kadono T, Kobayashi M, Hatae S, Oba Y, Iwamoto S, Mitumune S, Nagatomo Y, Owari M, Umeki H, Yamaga K (2007) Novel environmental friendly soap-based fire-fighting agent. Environ Eng Manag J 17(6):403–408. https://doi.org/10.2525/ecb.54.75
Rawet D, Smith R, Kravainis G (1996) A comparison of water additives for mopping-up after forest fires. Int J Wildland Fire 6(1):37–43
Kawahara T, Hatae S, Kanyama T, Ishizaki Y, Uezu K (2016) Development of eco-friendly soap-based firefighting foam for forest fire. Environ Control Biol 54(1):75–78. https://doi.org/10.2525/ecb.54.75
Rivai M, Hambali E, Suryani A, Fitria R, Firmansyah S, Pradesi J (2017) Synthesis of palm oil fatty acid as foaming agent for firefighting application. IOP Conf Ser Earth Environ Sci 65(1):012047. https://doi.org/10.1088/1755-1315/65/1/012047
Subekti P, Hambali E, Suryani A, Suryadarma P (2017) Potential production of palm oil-based foaming agent as fire extinguisher of peatlands in Indonesia: literature review. IOP Conf Ser Earth Environ Sci 65(1):012038. https://doi.org/10.1088/1755-1315/65/1/012038
Hayasaka H, Takahashi H, Limin SH, Yulianti N, Usup A (2016) Peat Fire Occurrence. In: Osaki M, Tsuji N (eds) Tropical Peatland Ecosystems. Springer, Japan, pp 377–395
Osaki M, Setiadi B, Takahashi H, Evri M (2016) Peat land in Kalimantan. In: Osaki M, Tsuji N (eds) Tropical peatland ecosystems. Springer, Japan, pp 91–112
Moreno L, Jiménez M-E, Aguilera H, Jiménez P, de la Losa A (2011) The 2009 Smouldering Peat Fire in Las Tablas de Daimiel National Park (Spain). Fire Technol 47:519–538. https://doi.org/10.1007/S10694-010-0172-Y
Santoso MA, Cui W, Amin HMF, Christensen EG, Nugroho YS, Rein G (2021) Laboratory study on the suppression of smouldering peat wildfires: effects of flow rate and wetting agent. Int J Wildland Fire 30:378–390. https://doi.org/10.1071/WF20117
Lin S, Cheung YK, Xiao Y, Huang X (2020) Can rain suppress smoldering peat fire? Sci Total Environ 727:138468. https://doi.org/10.1016/j.scitotenv.2020.138468
Ratnasari NG, Dianti A, Palamba P, Ramadhan ML, Prayogo G, Pamitran AS, Nugroho YS (2018) Laboratory scale experimental study of foam suppression on smouldering combustion of a tropical peat. J Phys Conf Ser. https://doi.org/10.1088/1742-6596/1107/5/052003
Goto K, Lin C, Kadono T, Hirono M, Uezu K, Kawano T (2007) Eco-Toxicity of soap component (sodium oleate) and a synthetic detergent cocktail using green paramecia assayed in natural water samples from East Asia. Environ Eng Manag J 17(6):377–383
Kawano T, Lin C, Kadono T, Uezu K (2007) Ecological risk assessment of fire-fighting chemicals using medaka fish (Oryzias latipes) in different water conditions. ITE Lett Batt New Technol Med 8(3):C48
Kawano T, Kadono T, Matsuoka N, Tamura T, Uezu K (2002) Development of soap-based fire-fighting agents less toxic to germinating rice (Oryza sativa L.) Seeds. New Technol Med 8(5):C38
Mizuki H, Toyomura M, Uezu K, Yasui H, Kawano T, Akiba I, Kawahara T, Hatae S, Sakamoto N, Akiyama M, Mizota C, Umeki H, Yamaga K (2010) Microbial degradation of a soap-based fire-fighting agent in activated sludge. Environ Eng Manag J 20(2):109–113
https://www.fs.fed.us/rm/fire/wfcs/Interagency_Policy_Aerial-Ground_%20Delivery_112912_508.pdf
Kalabokidis KD (2000) Effects of wildfire suppression chemicals on people and the environment—a review. Global NEST J 2(2):129–137. https://doi.org/10.30955/gnj.000144
Kawano T, Otsuka K, Kadono T, Inokuchi R, Ishizaki Y, Dewancker B, Uezu K (2014) Eco-toxicological evaluation of fire-fighting foams in small-sized aquatic and semi-aquatic biotopes. Adv Mater Res 875–877:699–707. https://doi.org/10.4028/www.scientific.net/AMR.875-877.699
Blake D, Katie Lu, Horwitz P, Boyce M (2012) Fire suppression and burnt sediments: effects on the water chemistry of fire-affected wetlands. Int J Wildland Fire 21(5):557–561. https://doi.org/10.1071/WF10125
Dianti A, Ratnasari NG, Palamba P, Nugroho Y (2018) Effect of rewetting on smouldering combustion of a tropical peat. E3S Web Conf. https://doi.org/10.1051/e3sconf/20186702042
Acknowledgements
This work was supported by the Japan International Cooperation Agency (JICA) Partnership Program entitled “Enhancing Fire-Fighting Techniques against Peat-Land and Forest Fires in Balikpapan, Indonesia.” We thank Esther Posner, PhD, and Michael Scott Long, PhD, for editing a draft of this manuscript.
Funding
This work was supported by the Japan International Cooperation Agency (JICA) partnership program.
Author information
Authors and Affiliations
Contributions
NF, TKan, KU, TKaw: Preparation for fire extinguishing experiments and fire extinguishing activities. TKan: Writing—original draft. KU, Taw: Writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
A soap-based firefighting agent is sold by Shabondama Soap Co., Ltd. The funder (Shabondama Soap Co., Ltd.) provided support in the form of salaries for T. Kawahara and T. Kanyama.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10694_2023_1381_MOESM1_ESM.tif
Supplementary file1 (TIF 27586 KB) SI. 1 Underground temperature at depth during combustion of water site (one set) in meso-scale fire extinguishing experiments
10694_2023_1381_MOESM2_ESM.tif
Supplementary file2 (TIF 26009 KB) SI. 2 Underground temperature at depth during combustion of water site (two sets) in meso-scale fire extinguishing experiments
10694_2023_1381_MOESM3_ESM.tif
Supplementary file3 (TIF 24174 KB) SI. 3 Underground temperature at depth during combustion of 1 vol% soap-based firefighting agent solution site (two sets) in meso-scale fire extinguishing experiments
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kanyama, T., Fukuda, N., Uezu, K. et al. Field Experimental Investigations on the Performance of an Environmentally Friendly Soap-Based Firefighting Agent on Indonesian Peat Fire. Fire Technol 59, 1007–1025 (2023). https://doi.org/10.1007/s10694-023-01381-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10694-023-01381-z