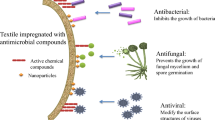
Several features of the preparation of an antimicrobial polymer composite material as a masterbatch filled with silver nanoparticles for producing items for various purposes are considered. The efficiency of the antibacterial activity of the obtained masterbatch was studied experimentally under laboratory conditions. The efficiency was determined as the yield of active antibacterial agent from the polymer (with a determination of the concentration and sizes of silver nanoparticles) in the aqueous phase and by scanning electron microscopy (SEM).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Much medical research on the generation of infection sites caused by the spread of colonies of bacteria, fungi, viruses, and other pathogenic organisms on polymer surfaces contacting people has stimulated the demand for antimicrobial polymers for various industrial sectors. The use of various antibacterial additives for polymers has reached a massive scale in the manufacturing of medical goods (wound dressings, catheters, medical textiles), food packaging (trays, extruded films, foam bottoms for meat and diary products), and everyday goods (trash bags, upholstery materials, wheelchair armrests, supermarket cart handles, telephone bodies). The demand for medical materials with long service lives has grown because of the recent worsening sanitary and epidemiological situation and the viral COVID-19 pandemic. Such materials can be fabricated from spunbond synthetic polymers as an alternative to the shortage of single-use medical masks, whites, and coveralls.
The optimal method for manufacturing polymers with bactericidal properties (lamination or impregnation with bioactive compositions) is incorporation of antimicrobial additives directly into the polymer matrix during final preparation of items. This method avoids additional manufacturing cycles, consumes less, and reduces lost time. Filled polymer composites (PCs) as masterbatches (superconcentrates of pigments or any other agents trapped in the polymer matrix) are already used to manufacture fibers, films or castables for dyeing and improving technological processes. Therefore, it was surmised that an active antimicrobial agent could be added to the same masterbatch with pigment or any other filler.
Special attention must be paid to the choice of antibacterial agent. It should be not only compatible with the other components of the masterbatch for dyeing polymer items but also efficient at low contents in the material without changing the structure of the already filled PC. Metal-containing compounds, e.g., silver, copper, tin, and their oxides, are known to be the most common antimicrobial agents [1, 2]. Use of silver nanoparticles (Ag NPs) has been proven to be highly efficacious [3]. Ag NPs are safe for humans and mammals, easily migrate to the surface of materials because of the extremely small size, and are not affected by UV radiation. Therefore, they can remain active for a prolonged time in the open air. Ag NPs are one of a few antibacterial agents that are active not only against fungi and bacteria, destroying their cell walls, but also viruses, e.g., HIV and herpes viruses [4, 5]. A distinguishing feature of metal-containing agents is the high efficacy at low concentrations, in contrast with biocidal chemical (polyphosphonates, isothiazolines, etc.) or plant compounds (betulin, propolis, etc.). One gram of Ag NPs is sufficient to provide antibacterial properties to 100 m2 of finished material [6].
The present work examined several features of the preparation of an antimicrobial polymer composite as a masterbatch filled with Ag NPs for the manufacture of polymer items for various purposes.
A PC as a masterbatch of POL PE 99991-AMS (sample No. 1) with Ag NP content 2% that was preliminarily ground into a powder containing a sufficient volume of the nanosized fraction was obtained from the industrial polymer company OOO Polistom and was compared to the pure polymer (sample No. 2). The antibacterial agent was mixed with the preliminarily powdered polymer matrix and an additional low-molecular-mass polyethylene dispersant in an LMX10-VS high-speed laboratory mixer. The PC modified by the antibacterial agent was manufactured on a granulation line including a Leistritz twin screw extruder for more uniform distribution of the filler. Linear low-density polyethylene SABIC 500026 (UAE) with a high melt flow index (MFI = 50 g/10 min at 90°C and 21.18 N load) [7] was selected as the matrix for creating the antibacterial PC. This also helped to distribute (disperse) more uniformly the nanofiller in the masterbatch. Furthermore, the polymer matrix was chosen for its low melting point of 120°C. Ag NPs are known to coalesce at high temperatures and rapidly agglomerate. Therefore, the PC was formulated at the lowest temperature possible, up to 140°C in all extruder zones.
The antibacterial properties of the PC were evaluated by the standard method in MUK 4.2.1890-04 for studying the sensitivity of microorganisms placed onto a solid growth medium, e.g., meat-peptone agar. The test subject was the Gram-positive spore-producing bacterium Bacillus subtilis. Samples of the masterbatch (sections of granules 8 mm in diameter with equal masses) were placed onto sterile Petri dishes with a previously inoculated zone of 1-d culture of the selected microorganism in growth medium. The dishes with the samples were incubated in a thermostat for 24 h at 37°C, after which the growth inhibition zones of the bacteria were determined. Figure 1 shows photographs of the samples.
Antimicrobial activity was determined by measuring the size of the growth inhibition zones of the bacteria and comparing them with the control values given in Table 1.
The antibacterial masterbatch was observed to be highly active as compared to the data for analogous masterbatches filled with antibacterial agents of different origins (plant or chemical) in 5-30% amounts [8, 9].
The biomodification also had a clearly pronounced effect of prolonged inhibition of the microorganism growth zone, i.e., persistent inactivation of the bacteria at room temperature (23°C) for the following 30 d.
Because the masterbatch was a composite containing a filler in the polymer matrix, the amount of active Ag NPs directly involved in inhibition of the microorganisms had to be determined. For this, it was important to determine the concentration and size of the active Ag NPs released from the masterbatch granules into the water. This was studied using an SF-2000 spectrophotometer and a Photocor Mini particle-size analyzer supplied by the Technopark Slava Center for Collective Use (https://technopark-slava.ru). The aqueous phase was slightly colored after storing the masterbatch in water for 20 d. This indicated that the Ag NPs were actively released from the polymer granules of the antimicrobial masterbatch. Figure 2 shows the particle-size distribution of the Ag NPs released into the aqueous phase over the 20 d.
Plots of the dependence of optical density (D) of the aqueous phase of Ag NPs on wavelength and absorption spectra at these frequencies showed that the optical density increased over time from 2 min to 60 min, to 48 h, and then to 20 d. Therefore, NPs were constantly released into the aqueous phase during this time (Fig. 3).
Spectrophotometry and photon correlation spectroscopy proved that ~23 μg of the Ag NPs added to the PC composition were released over 20 d from the masterbatch. This amount did not exceed the threshold concentration for toxic effects of Ag NPs on living organisms and was within the limits of the allowed concentration, i.e., ≤500 μg/mL, according to the currently accepted GN 1.2.2633–10 (Hygienic standards of content of nanomaterials with high priority in environment). Nevertheless, research on the effects of NPs on living organisms is continuing [10, 11].
Figure 4 shows a kinetic curve constructed from the obtained data.
The results suggested that Ag NPs were extracted from the masterbatch only from the surface while the main part remained within the cylindrical granules. Therefore, it seemed advisable to reach the required effect by changing the concentration of the active ingredient in the final material by dosing the antibacterial masterbatch depending on the thickness of the item.
Transparent films of thickness 30 ± 5 μm based on LDPE with added antimicrobial masterbatch in amounts from 5 to 50% were produced to determine the distribution of Ag NPs in the finished items. Also, a film dyed by the masterbatch with a whitening pigment (titanium dioxide) and the antimicrobial Ag NPs mixed into it simultaneously was produced. It is noteworthy that the Ag NPs were rather evenly distributed throughout the whole area of the film. This was confirmed by photomicrographs obtained by scanning electron microscopy (SEM). The imaging used a QUANTA 650 FEG with an auto-emission cathode at the CCU, PMR, IPCE, RAS. The imaging parameters were secondary-electron detector, high vacuum, 5 kV potential, magnification (scale) from 80× to 50,000×. Figure 5 shows the results.
Ag NPs were observed to agglomerate into complexes on the film surface. This may have been related to the high-temperature method for fabricating the films (180°C) and indicated that the technological parameters for processing the antibacterial PC had to be carefully chosen. Films with high contents of the POL PE 99991-AMS antimicrobial masterbatch showed significant buildups of agglomerated particles. The film with the minimum dose of masterbatch had practically no agglomerations of Ag NPs. The NPs were uniformly positioned on the film surface and retained dimensions up to 100 nm.
References
A. L. Chulovskaya, E. V. Garasko, et. al., Usp. Khim. Khim. Tekhnol., XXVII, No. 3, 115-120 (2013).
A. D. Dmitrieva, et al., Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol., 58, No. 8, 67-70 (2015).
Ma Xiaole, et al., Mezhdunar. Stud. Nauchn. Vestn., No. 6 (2018); https://eduherald.ru/ru/article/view?id=19414 (accessed Jun. 12, 2022).
S. Qixiang, CN Pat. No. 104,415,090, “Production method of nano-silver antibacterial agent,” Mar. 18, 2015.
A. Mohammed Fayaz, et. al., Int. J. Nanomed., No. 7, 5007-5018 (2012); DOI: https://doi.org/10.2147/IJN.S34973.
Silver Nanoparticles – A Powerful Weapon Against Microbes; https://monib-health.com/ru/post/58-кaк-нaнo-cepeбpo-yбивaeт-бaктepии; (accessed Jun. 12, 2022).
C. Rauwendaal, Polymer Extrusion, Hanser Gardner Publ., Cincinnati, OH, 2001 [Russian translation, A. Ya. Malkin (ed.), Professiya, St. Petersburg, 2010, 762 pp].
T. V. Mirolyubova, L. V. Redina, et. al., “Innovative Development of Industrial Technology and Technologies,” in: Proceedings of a Scientific Conference of Young Researchers with International Participation [in Russian], Part 2, FGBOU VO RGU im. A. N. Kosygina, Moscow, 2021, pp. 219-223.
V. V. Kurenkov, et. al., Thermoplastic Materials and Functional Coatings: Proceedings of an All-Russian Scientific Technical Conference [in Russian], Moscow, Apr. 23, 2019, VIAM, Moscow, 2019, pp. 134-145; https://conf.viam.ru/sites/default/files/uploads/proceedings/1179.pdf.
V. A. Demin, et. al., Ross. Nanotekhnol., 10, No. 3-4, 103-109 (2015).
G. R. Tortella, O. Rubilar, et. al., J. Hazard. Mater., No. 12, 2-21 (2019); DOI: https://doi.org/10.1016/j.jhazmat.2019.121974.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimicheskie Volokna, No. 5, pp. 33-36, September—October, 2022.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mirolyubova, T.V., Redina, L.V., Chmutin, I.A. et al. Preparation of Polymer Composite Filled with Silver Nanoparticles and Investigation of its Antimicrobial Properties. Fibre Chem 54, 308–311 (2023). https://doi.org/10.1007/s10692-023-10397-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10692-023-10397-8