Abstract
Individuals at high risk of developing pancreatic ductal adenocarcinoma are eligible for surveillance within research programs. These programs employ periodic imaging in the form of magnetic resonance imaging/magnetic resonance cholangiopancreatography or endoscopic ultrasound for the detection of early cancer or high-grade precursor lesions. This narrative review discusses the role of endoscopic ultrasound within these surveillance programs. It details its overall strengths and limitations, yield, burden on patients, and how it compares to magnetic resonance imaging. Finally, recommendations are given when and how to incorporate endoscopic ultrasound in the surveillance of high-risk individuals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An inherited genetic predisposition is presumed in around 10% of pancreatic ductal adenocarcinoma (PDAC) patients based on familial clustering [1]. In many families, the exact cause of the increased risk remains unidentified, but in around one-third this is explained by an inherited pathogenic variant of one of the known PDAC susceptibility genes, including LKB1/STK11, CDKN2A, BRCA1, BRCA2, PALB2, ATM, TP53, and MLH1/MSH2/MSH6. Even in the absence of a positive family history, such a pathogenic variant can be found in 3% of PDAC patients [2].
Surveillance of these so-called high-risk individuals is aimed at detecting neoplastic pancreatic lesions at the earliest possible stage: as a stage I cancer that is still limited to the pancreas or, preferably, as a high-grade precursor lesion which includes pancreatic intraepithelial neoplasia 3 (PanIN-3) lesions and intraductal papillary mucinous neoplasms (IPMNs) with high-grade dysplasia. To date, surveillance relies solely on imaging, as available biomarkers are insufficiently able to differentiate between low-grade and high-grade dysplasia, or between neoplastic and non-neoplastic abnormalities.
Imaging techniques unsuited for surveillance include computed tomography (CT), 18-fluorodeoxyglucose positron emission tomography (FDG-PET), and abdominal ultrasound. CT has a relatively low sensitivity for detecting subcentimeter lesions, and the cumulative radiation exposure renders it unfit for repeated imaging [3]. It is, however, still the modality of choice for staging of pancreatic cancers [4,5,6,7]. FDG-PET is useful for differentiating pancreatic cancer from mass-forming chronic pancreatitis and for detecting distant metastases [8, 9]. However, its diagnostic performance in detecting small lesions is debatable [10, 11]. Abdominal ultrasound has a low sensitivity for the detection of small pancreatic abnormalities and often visualizes the pancreas incompletely or unclearly, especially in individuals with a high body mass index, making it unsuitable for surveillance [4].
Better suited imaging modalities for surveillance are endoscopic ultrasound (EUS) and magnetic resonance imaging/magnetic resonance cholangiopancreatography (MRI/MRCP). The first attempts of surveillance in high-risk individuals employed EUS as the primary test, with additional endoscopic retrograde cholangiopancreatography (ERCP) and CT in case EUS detected abnormalities [12,13,14,15,16]. From 2009 onwards, surveillance programs started to incorporate MRI/MRCP as a primary test; either alternating or combining it with EUS. Since then, these prospective programs have demonstrated that both can detect high-grade precursor lesions and early cancers during surveillance of high-risk individuals [17,18,19,20].
It is still a matter of debate which imaging modality has the best performance to detect pancreatic lesions at the desired early stage. A high diagnostic accuracy is crucial as undertreatment quickly results in advanced cancer given the rapidly progressive nature of PDAC, while overtreatment of low-grade precursors or benign lesions poses high risks of morbidity and mortality associated with pancreatic surgery [21]. In the latest international Cancer of the Pancreas Surveillance (CAPS) consortium consensus guidelines, experts could not reach consensus on which imaging modality is superior [22]. Their conclusion was that both are suitable and surveillance can be performed using either one or both; a conclusion that has been adopted by guidelines of the ACG, ASGE, AGA, and ESGE [23,24,25,26].
The aim of the current review is to discuss the strengths and limitations of EUS in detecting pancreatic abnormalities during surveillance of high-risk individuals, such as the diagnostic yield and burden to patients. These characteristics will be compared to those of MRI/MRCP, and areas of insufficient knowledge will be identified. Finally, we give our recommendations when and how to implement EUS in a surveillance program.
Strengths
Diagnostic performance
The foremost strength of EUS is its ability to depict the pancreas from close proximity, resulting in optimal detection of (small) pancreatic abnormalities. Multiple studies have reported a high diagnostic accuracy for PDAC detection for EUS, with a sensitivity ranging between 81 and 100% and specificity ranging from 73 to 100%. It outperforms CT, which has a lower sensitivity (53–74%) and slightly lower specificity (53–94%) [27,28,29]. MRI has the same reported performance as CT [4]. For pancreatic lesions smaller than 3 cm, this superiority over CT and MRI is even clearer [29, 30]. An additional strength of EUS is its high sensitivity in detecting pancreatic neuroendocrine tumors of 80–92%, compared to 63% for CT and 66% for MRI [31, 32], although this is not the main objective of surveillance [22].
EUS may have difficulty in differentiating between neoplastic lesions and other hypoechoic abnormalities due to inflammatory masses, signs of previous inflammation due to chronic or acute pancreatitis, lipomatous parenchyma, or atrophy due to old age. In such cases, the diagnostic accuracy can be improved by performing additional contrast-enhancement EUS [33]. In a study in 277 patients with solid pancreatic lesions, the overall sensitivity and specificity of contrast-enhanced harmonic EUS was 95% and 89% for ductal adenocarcinomas and 79% and 99% for neuroendocrine tumors. In the subgroup of adenocarcinomas smaller than 2 cm, contrast-enhanced EUS outperformed CT, with a sensitivity of 91% and specificity of 94% [34]. This bears important clinical relevance, because the majority of small asymptomatic solid pancreatic lesions are not PDAC in both low-risk and high-risk populations [20, 35], and accurate stratification is essential to prevent unnecessary surgery. In the future, developments in artificial intelligence applied to EUS imaging may prove an additional means to improve its diagnostic accuracy.
Tissue acquisition
A significant advantage of EUS over MRI/MRCP is the possibility of tissue acquisition through fine-needle aspiration (FNA) or biopsy (FNB). As mentioned, the majority of small asymptomatic solid pancreatic lesions are not PDAC, and a histologic confirmation is a prerequisite before performing an oncological pancreatic resection. Multiple meta-analyses have demonstrated a high diagnostic accuracy of FNA to diagnose PDAC, with a pooled sensitivity ranging between 87% and 91%, and specificity of 94–96% [36, 37]. A more recent meta-analysis reported on the results of 18 randomized controlled trials comparing FNA to FNB. Both were shown to have a good diagnostic accuracy, ranging 78–83% for FNA, and 83–87% for FNB, with a small but significant advantage of FNB.
EUS-FNA may also detect PDACs that are not visible on other imaging modalities. In a retrospective study of 116 patients with clinical findings suggestive of PDAC (based on presentation, laboratory abnormalities, ductal dilation) but no mass on CT-scan, EUS identified a focal mass in 84 patients, of which 44 were a pancreatic ductal adenocarcinoma [38]. In this group, EUS-FNA had a diagnostic accuracy of 91%. In addition, there were 32 patients without a focal mass on CT and EUS, in whom EUS-FNA performed at a narrowing of the bile duct or pancreatic duct revealed six additional cancers. This particular observation deserves attention in the context of surveillance, as PDAC cases in high-risk individuals have been described in which a pancreatic duct dilation was the only preceding sign of malignancy [20, 39].
Pancreatic juice collection
An added utility of EUS is the possibility to collect pancreatic juice from within the duodenum after stimulation of its excretion by intravenous secretin injection. Pancreatic juice might be a valuable biomarker source as it is secreted directly by the ductal cells from which neoplasia originates, potentially containing more accurate markers than other biomaterials such as serum or feces. This may be beneficial specifically for high-risk individuals, as they already undergo repeated EUS in the course of surveillance. Multiple biomarkers in pancreatic juice, including protein biomarkers like S100P, microRNAs, and cell-free DNA mutations like KRAS, SMAD4 and TP53, are associated with PDAC [40,41,42,43,44,45,46,47,48]. If proven sufficiently predictive, such markers may provide earlier detection of neoplasia and allow for personalization, such as a tailored surveillance interval or more accurate selection for surgery. In addition, they may be able to differentiate between advanced neoplasia (high-grade dysplasia or PDAC) and low-grade precursor lesions [49], an area in which current imaging-based surveillance lacks [20, 39], but this remains to be determined.
Limitations
Interobserver variability
The biggest limitation of EUS is its operator-dependency. It takes relatively long to develop the core skills required to perform EUS [50]. When surveilling high-risk individuals, in which the smallest and earliest signs of potential neoplastic progression are of importance, even more extensive expertise is required. Even among experts, interobserver agreement of EUS images of high-risk individuals has been shown to be poor [51]. Additionally, studies have reported on individuals being selected for surgery because of a suspicious lesion after which histologic evaluation revealed mere low-grade dysplasia or even non-neoplastic lesions, although this is equally true for MRI [39]. There also might be a learning curve of the collective multidisciplinary team involved in a pancreatic cancer surveillance program. In the CAPS study, one of the largest ongoing prospective surveillance studies worldwide, patient selection for surgery improved over time, with less patients resected for low-grade dysplastic lesions [17]. These challenges have resulted in the international CAPS consortium to recommend surveillance only within dedicated multidisciplinary research programs in which extensive expertise can be built up, and that EUS should be used as a primary modality only if executed by expert endosonographers [22].
Longitudinal comparison
An advantage of MRI/MRCP over EUS is the easier comparison of the findings to previous surveillance MRI/MRCPs. Although EUS images and videos can be stored for future reference, these are usually not accessible during the investigation. In addition, a direct comparison is often difficult, for example due to differences in scope positioning and signal quality. For cystic lesions especially, a direct comparison between visits is useful for determining growth. Fast cyst growth is a worrisome feature for developing malignancy in neoplastic cystic lesions within the general population [52], and has also been shown to be predictive of PDAC in high-risk individuals [17, 20]. Pancreatic cysts may even grow faster in high-risk individuals compared to the general population [53]. Although these findings need further confirmation, they put emphasis on a detailed assessment of size and growth of each cyst at each surveillance visit, and place MRI in a pole position in this respect.
Complication risk
Diagnostic non-interventional EUS has a very low risk of complications of less than 0.3% [54]. Potential complications include perforations of the esophagus or duodenum, aspiration, or bacteremia. Complications are more likely following FNA or FNB (< 3%), and most often include post-procedural pain, acute pancreatitis, hemorrhage, or fever and infectious complications [55,56,57]. The procedure-related mortality risk is estimated to be very low, at 0.02–0.19% [55, 56].
Needle-tract seeding after FNA/FNB of a pancreatic malignancy is a potential risk but rare [58]. This mainly concerns seeding into the gastric wall after a biopsy of the body or tail, as tumors in the head of the pancreas are usually biopsied through the duodenal wall, which is subsequently resected in case of malignancy. The possibility of needle-tract seeding has been substantiated by an increasing number of case reports, with a recent systematic review identifying 46 such published cases [59]. A large retrospective Japanese study, not yet published at the time of the systematic review, reported on 9300 patients with resected PDAC who had preoperatively undergone FNA or FNB, and found needle-tract seeding lesions after 0 of 4746 (0%) transduodenal tissue acquisitions, but after 38 of 4435 (0.86%) transgastric tissue acquisitions (P-value < 0.001), almost all (97.4%) of which were located in the gastric wall [60]. While relevant, it should be noted that this risk is still likely much lower compared to that of percutaneous biopsies [61]. In terms of clinical outcomes, multiple studies have shown no overall difference in survival or recurrence-free survival between PDAC patients who underwent pre-operative FNA or not [58, 62]. Within the group of patients in whom needle-tract seeding had taken place, the Japanese study did find longer survival (median 52 months versus 26 months) for patients who underwent resection of the needle-tract lesion compared to those who didn’t [60].
Patient burden
Multiple prospective programs have assessed the psychological impact of imaging-based surveillance on high-risk individuals [63,64,65,66,67,68,69,70,71,72]. Although study endpoints and used psychological instruments vary across study designs, overall, they report positive psychological outcomes, such as low degrees of cancer worries, anxiety, depression, and general distress [73], with participants having a lower perceived cancer risk than non-participating high-risk individuals [74]. Studies that evaluated long-term psychological outcomes all reported a decrease in cancer-related distress during surveillance [66, 69, 71, 74, 75].
A few studies assessed differences between the burden of EUS and MRI, and evaluated participants’ preferences. The only head-to-head comparisons come from the Dutch familial pancreatic cancer surveillance program, in which high-risk individuals underwent both EUS and MRI at each visit. In the first evaluation of 66 high-risk individuals, only 10% reported the procedures to be burdensome, without differences between EUS and MRI [63]. In a later study of 140 individuals, results were similar: 11% experienced EUS as burdensome, and 10% MRI. Of note, before their baseline visit, 34% had dreaded their first EUS compared to only 3% their MRI, but this dropped significantly after subsequent follow-up visits to equal levels as MRI (from 9% after the first EUS to 6% after the fourth EUS, and from 8% after the first MRI to 0% after the fourth MRI) [74]. When analyzing a subgroup of participants who had to undergo additional examinations after shortened intervals because of lesions of unknown relevance, or who underwent surgery, more participants preferred EUS over MRI than vice versa [70]. In addition to the results of this study, other EUS-based programs have reported an improvement in psychological outcomes at long-term follow-up, and that receiving a negative EUS or EUS-FNA test result improved quality of life [72].
Performance within surveillance of high-risk individuals
Chronic pancreatitis findings
In high-risk individuals, a high prevalence of findings similar to those found in early chronic pancreatitis, such as hyperechoic parenchymal foci, calcifications, hyperechoic pancreatic duct walls, or lobularity, has been reported since the earliest EUS-based surveillance programs were initiated [12, 15, 76, 77]. It is thought that these findings are associated with lobulocentric atrophy caused by PanIN lesions [12, 78], and hence, that their detection could be helpful in identifying patients with neoplastic progression. However, these features did not evolve in one study during a three-year follow-up [79], and patients who underwent resection for such features mostly harbored low-grade PanINs, which are common incidental findings with a low risk of neoplastic progression [16, 80, 81]. If having multiple EUS features of chronic pancreatitis is associated with an increased risk of PanIN-3 lesions, representing the clinically more relevant high-grade dysplasia, remains inconclusive [81].
Pancreatic cystic lesions
Pancreatic cystic lesions are already highly prevalent in the general population and, although direct comparisons have not been made, their prevalence seems to be even higher among high-risk individuals, including many IPMNs [17, 20, 53, 82,83,84,85,86]. The sensitivity of EUS to detect cystic lesions in high-risk individuals has been compared in a blinded fashion in one study by Harinck et al. They compared baseline EUS to baseline MRI/MRCP in 139 high-risk individuals, and found a sensitivity for cystic lesions of 39% for EUS and 90% for MRI [87]. In the same cohort, the diagnostic yield was revaluated (non-blinded) in 366 individuals after a mean follow-up of 63 months. Again, MRI/MRCP was more sensitive than EUS (83% versus 42%), but this difference was much smaller for cystic lesions ≥ 10 mm (92% versus 70%), and not different for main pancreatic duct dilation (60% versus 62%). Moreover, when looking specifically at high-risk features such as a solid component or mural nodule, EUS outperformed MRI/MRCP (100% versus 20%), although the number of cases was small [20]. Other surveillance studies have also reported detection rates for EUS and MRI, but none performed both tests at the same time during each visit, hampering the comparability of the results.
Similar to early chronic pancreatitis findings, the clinical relevance of the detection of IPMNs in high-risk individuals is unclear. Almost all IPMNs detected in high-risk individuals concern branch-duct IPMNs, which, in the general population, have a low risk of malignancy, and this risk is even lower if they remain stable during several years of follow-up [88, 89]. Also for high-risk individuals, IPMNs were initially deemed of little importance, because their hereditary risk was thought to be the result of a solid precursor pathway rather than a cystic one, based on pathological and genetical analyses of resected familial pancreatic cancers [90, 91]. However, in clinical studies, almost half of neoplastic progressors seem to have arisen from a cystic lesion [39], and fast cyst growth was shown to be a predictor for the development of PDAC in multiple surveillance cohorts [17, 20]. The actual malignancy risk of branch-duct IPMNs in high-risk individuals has not been established, and it remains uncertain if this is higher than in the general population. One study directly compared the growth rate and malignancy risk of IPMNs in 81 high-risk individuals to those in 442 individuals without familial risk, and found that IPMNs in high-risk individuals grew faster and were more likely to develop worrisome growth speeds (≥ 2.5 mm/year) [53]. PDAC risk seemed higher in pathogenic variant carriers (11%) compared to pathogenic-variant negative familial pancreatic cancer kindreds (0%) and individuals without familial risk (1%), although numbers were insufficient to demonstrate statistical significance. The malignancy risk of an IPMN in a pathogenic variant carrier was 23% in case of a growth rate of ≥ 2.5 mm/year, 30% for ≥ 5 mm/year and 60% for ≥ 10 mm/year, warranting more intensive surveillance or even surgical resection in selected cases. The international CAPS consortium consensus guidelines provide guidance on the management of cysts with worrisome features in high-risk individuals [22], which are largely based on the guidelines for sporadic IPMNs in the general population. However, correct selection for surgical resection of IPMNs in high-risk individuals remains challenging, as these selection criteria have been shown to require further scrutiny [92].
Advanced neoplastic lesions
While the relevance of presumed low-risk abnormalities like branch-duct IPMNs and chronic pancreatitis findings is still unclear, the most important question remains: which imaging test is best suited for reaching the primary goal of surveillance, namely the detection of high-grade dysplasia or PDAC confined to the pancreas? Table 1 lists all prospective surveillance programs that incorporate EUS and their rates of detection for neoplastic progressors overall, and early-stage neoplastic progressors in particular. The difficulty in drawing firm conclusions is that the number of cases is low in all studies, varying from zero to 36, and that almost no programs perform both EUS and MRI/MRCP at the same time. Thus, it cannot be established if EUS or MRI/MRCP is superior in detecting neoplastic progressors.
Three meta-analyses have attempted to combine these results. In 2015, Lu et al. analyzed nine surveillance cohorts and reported that EUS detected 64% of pancreatic cancers versus 43% for MRI, without specifying the detection rates for high-grade dysplasia or early PDAC [93]. In 2018, Signoretti et al. included 16 studies with 1588 high-risk individuals, in which a pooled prevalence of early-stage neoplastic progressors of 3.3% was found [94]. EUS detected more solid pancreatic lesions compared to MRI (5.2% versus 4.1%), but the pooled prevalence of pancreatic lesions considered a successful target of surveillance was similar (2.9% for EUS and 2.5% for MRI). In 2019, Corral et al. analyzed 19 studies comprising 1660 high-risk individuals, with an overall diagnostic yield of high-grade dysplasia or PDAC of 0.74 per 100 patient-years [77]. In this analysis, EUS detected more of such lesions (1.07 per 100 patient-years) than did MRI (0.41), but this difference did not reach statistical significance.
While there is not yet convincing evidence that either test is superior, a trend is observed that EUS detects more solid lesions, lesions with high-grade dysplasia and PDAC. This is in line with our experiences within the Dutch familial pancreatic cancer surveillance study, in which EUS detected two early-stage PDACs that were not recognized on MRI/MRCP [20]. Several such cases have also been reported in the CAPS cohort of the Johns Hopkins hospital [3]. In addition, in our cohort we also found two cases in which both MRI/MRCP and EUS detected a cystic abnormality, but only EUS detected the high-risk features that led to surgery, revealing a T1 PDAC in both cases [20]. Figures 1 and 2 show EUS images illustrating these two latter cases. The superior ability of EUS to detect worrisome features and high-risk stigmata has been shown on a wider scale in a multicenter retrospective study by Tamburrino et al. They retrospectively analyzed 837 patients from the general population (without specific increased hereditary risk) undergoing surveillance of branch-duct IPMNs, and identified that EUS-based surveillance was independently associated with a higher detection rate of worrisome features and high-risk stigmata than MRI-based surveillance (HR 2.46, 95% CI 1.74–3.47), although the possibility of selection bias could not be excluded in this study [95].
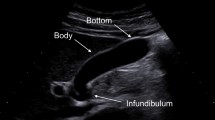
Endoscopic ultrasound images of a branch-duct intraductal papillary mucinous neoplasm (A) detected in the pancreatic tail of a 50-year old CDKN2A pathogenic variant carrier. It had grown from 15 to 23 mm in one year and developed a solid component that seemed hypovascular after contrast-enhancement (B). The lesion was visible on MRI/MRCP but not the solid component. After resection, histology revealed a T1cN1M0 intraductal papillary mucinous neoplasm-associated pancreatic ductal adenocarcinoma
Endoscopic ultrasound images of a 15 mm solid lesion (A) detected in the pancreatic head of a 54-year old patient with Peutz-Jeghers syndrome. The lesion had low uptake of contrast (not shown). On MRI/MRCP the lesion was dubiously present and could not be characterized. On CT it was not visible. At revaluation with endoscopic ultrasound two months later, the lesion was unchanged and fine-needle aspiration (B) was suggestive of malignancy. The lesion was resected and staged as a T1aN0M0 pancreatic ductal adenocarcinoma
Cost-effectiveness
Whether surveillance is cost-effective in high-risk individuals depends on the detection rate of high-grade dysplasia and PDAC and, therefore, on the degree of hereditary risk, which varies greatly between the different subgroups. A recent study estimated that for pancreatic cancer surveillance to be cost-effective, the lifetime PDAC risk needs to be at least 10% [96]. This highest-risk group includes carriers of a pathogenic CDKN2A variant and Peutz-Jeghers syndrome patients (lifetime risks estimated at 19% and 11–36%). For the other subgroups, including carriers of lower-risk pathogenic variants such as BRCA1, BRCA2, PALB2 and ATM, and pathogenic variant-negative familial pancreatic cancer kindreds, it is doubtful if their risk surpasses this threshold [20].
One meta-analysis from the USA compared the costs of EUS to MRI, and found that MRI was the most cost-effective strategy in high-risk individuals with a relative risk five to twenty times that of the general population, but in individuals with a relative risk of twenty or more, EUS was more cost-effective [97]. It should be noted that these results cannot be extrapolated to health care systems outside the USA.
Conclusions and recommendations
In summary, EUS is a test with a high diagnostic accuracy for detecting pancreatic abnormalities, and is the superior test specifically for lesions smaller than 3 cm. Additional advantages are the improved differentiation of lesions by the application of contrast enhancement, the possibility for tissue acquisition through FNA or FNB with high sensitivity and specificity, and the option to collect pancreatic juice for biomarker analysis. Complication rates are very low, especially for diagnostic EUS without tissue acquisition, and participants experience the burden of surveillance by EUS to be equally low as by MRI. EUS is inferior to MRI/MRCP in detecting any size pancreatic cysts, but this is not the case for cystic lesions larger than one centimeter or for pancreatic duct dilation, and it seems to outperform MRI/MRCP in detecting high-risk features such as solid components or nodules within cysts. While there is no hard evidence that one test has a better diagnostic accuracy for the primary targets of surveillance (high-grade dysplastic precursor lesions and early PDAC), currently available data show a trend favoring EUS. Based on these considerations it was decided in the Dutch familial pancreatic cancer surveillance study to stop performing both tests and to perform EUS as our standard diagnostic imaging modality, with MRI/MRCP performed only at the baseline visit and on indication during follow-up.
EUS is operator-dependent and has a relatively long learning curve that is even greater when used in the context of pancreatic cancer surveillance. Therefore, we recommend to use EUS as a primary surveillance modality only when performed by expert endosonographers, and within a dedicated research program which allows for the accumulation of experience. If these conditions are not met, then MRI/MRCP is a good alternative as a surveillance test. It needs to be stressed that to date, there is only ample evidence that pancreatic cancer surveillance leads to a true survival benefit. Only in pathogenic CDKN2A variant carriers, who are among the highest-risk group, has such data become available [98]. For all other risk groups, this remains to be proven and, therefore, surveillance is recommended to be performed only within research programs.
Data availability
No datasets were generated or analysed during the current study.
References
Hruban RH, Canto MI, Goggins M, Schulick R, Klein AP (2010) Update on familial pancreatic cancer. Adv Surg 44:293–311
Shindo K, Yu J, Suenaga M, Fesharakizadeh S, Cho C, Macgregor-Das A, Siddiqui A, Witmer PD, Tamura K, Song TJ, Navarro Almario JA, Brant A, Borges M, Ford M, Barkley T, He J, Weiss MJ, Wolfgang CL, Roberts NJ, Hruban RH, Klein AP, Goggins M (2017) Deleterious germline mutations in patients with apparently sporadic pancreatic adenocarcinoma. J Clin Oncol 35(30):3382–3390. https://doi.org/10.1200/jco.2017.72.3502
Canto MI, Hruban RH, Fishman EK, Kamel IR, Schulick R, Zhang Z, Topazian M, Takahashi N, Fletcher J, Petersen G, Klein AP, Axilbund J, Griffin C, Syngal S, Saltzman JR, Mortele KJ, Lee J, Tamm E, Vikram R, Bhosale P, Margolis D, Farrell J, Goggins M (2012) Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology 142(4):796–804 quiz e14-5. https://doi.org/10.1053/j.gastro.2012.01.005
Bipat S, Phoa SS, van Delden OM, Bossuyt PM, Gouma DJ, Lameris JS, Stoker J (2005) Ultrasonography, computed tomography and magnetic resonance imaging for diagnosis and determining resectability of pancreatic adenocarcinoma: a meta-analysis. J Comput Assist Tomogr 29(4):438–445
Nawaz H, Fan CY, Kloke J, Khalid A, McGrath K, Landsittel D, Papachristou GI (2013) Performance characteristics of endoscopic ultrasound in the staging of pancreatic cancer: a meta-analysis. JOP 14(5):484–497. https://doi.org/10.6092/1590-8577/1512
Tamburrino D, Riviere D, Yaghoobi M, Davidson BR, Gurusamy KS (2016) Diagnostic accuracy of different imaging modalities following computed tomography (CT) scanning for assessing the resectability with curative intent in pancreatic and periampullary cancer. Cochrane Database Syst Rev 9(9):CD011515. https://doi.org/10.1002/14651858.CD011515.pub2
Rahman MIO, Chan BPH, Far PM, Mbuagbaw L, Thabane L, Yaghoobi M (2020) Endoscopic ultrasound versus computed tomography in determining the resectability of pancreatic cancer: a diagnostic test accuracy meta-analysis. Saudi J Gastroenterol 26(3):113–119. https://doi.org/10.4103/sjg.SJG_39_20
Rijkers AP, Valkema R, Duivenvoorden HJ, van Eijck CHJ (2014) Usefulness of F-18-fluorodeoxyglucose positron emission tomography to confirm suspected pancreatic cancer: a meta-analysis. Eur J Surg Oncol (EJSO) 40(7):794–804. https://doi.org/10.1016/j.ejso.2014.03.016
Santhosh S, Mittal BR, Bhasin D, Srinivasan R, Rana S, Das A, Nada R, Bhattacharya A, Gupta R, Kapoor R (2013) Role of (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in the characterization of pancreatic masses: experience from tropics. J Gastroenterol Hepatol 28(2):255–261. https://doi.org/10.1111/jgh.12068
Seo S, Doi R, Machimoto T, Kami K, Masui T, Hatano E, Ogawa K, Higashi T, Uemoto S (2008) Contribution of 18F-fluorodeoxyglucose positron emission tomography to the diagnosis of early pancreatic carcinoma. J Hepatobiliary Pancreat Surg 15(6):634–639. https://doi.org/10.1007/s00534-007-1339-x
Matsumoto I, Shirakawa S, Shinzeki M, Asari S, Goto T, Ajiki T, Fukumoto T, Kitajima K, Ku Y (2013) 18-Fluorodeoxyglucose positron emission tomography does not aid in diagnosis of pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol 11(6):712–718. https://doi.org/10.1016/j.cgh.2012.12.033
Brentnall TA, Bronner MP, Byrd DR, Haggitt RC, Kimmey MB (1999) Early diagnosis and treatment of pancreatic dysplasia in patients with a family history of pancreatic cancer. Ann Intern Med 131(4):247–255
Rulyak SJ, Brentnall TA (2001) Inherited pancreatic cancer: surveillance and treatment strategies for affected families. Pancreatology 1(5):477–485. https://doi.org/10.1159/000055851
Kimmey MB, Bronner MP, Byrd DR, Brentnall TA (2002) Screening and surveillance for hereditary pancreatic cancer. Gastrointest Endosc 56(4 Suppl):S82–S86
Canto MI, Goggins M, Yeo CJ, Griffin C, Axilbund JE, Brune K, Ali SZ, Jagannath S, Petersen GM, Fishman EK, Piantadosi S, Giardiello FM, Hruban RH (2004) Screening for pancreatic neoplasia in high-risk individuals: an EUS-based approach. Clin Gastroenterol Hepatol 2(7):606–621
Canto MI, Goggins M, Hruban RH, Petersen GM, Giardiello FM, Yeo C, Fishman EK, Brune K, Axilbund J, Griffin C, Ali S, Richman J, Jagannath S, Kantsevoy SV, Kalloo AN (2006) Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol 4(6):766–781 quiz 665. https://doi.org/10.1016/j.cgh.2006.02.005
Canto MI, Almario JA, Schulick RD, Yeo CJ, Klein A, Blackford A, Shin EJ, Sanyal A, Yenokyan G, Lennon AM, Kamel IR, Fishman EK, Wolfgang C, Weiss M, Hruban RH, Goggins M (2018) Risk of neoplastic progression in individuals at high risk for pancreatic Cancer Undergoing Long-Term Surveillance. Gastroenterology 155(3):740–751e2. https://doi.org/10.1053/j.gastro.2018.05.035
Dbouk M, Katona BW, Brand RE, Chak A, Syngal S, Farrell JJ, Kastrinos F, Stoffel EM, Blackford AL, Rustgi AK, Dudley B, Lee LS, Chhoda A, Kwon R, Ginsberg GG, Klein AP, Kamel I, Hruban RH, He J, Shin EJ, Lennon AM, Canto MI, Goggins M (2022) The Multicenter Cancer of pancreas Screening Study: Impact on Stage and Survival. J Clin Oncol 40(28):3257–3266. https://doi.org/10.1200/JCO.22.00298
Klatte DCF, Boekestijn B, Wasser M, Feshtali Shahbazi S, Ibrahim IS, Mieog JSD, Luelmo SAC, Morreau H, Potjer TP, Inderson A, Boonstra JJ, Dekker FW, Vasen HFA, van Hooft JE, Bonsing BA, van Leerdam ME (2022) Pancreatic Cancer surveillance in carriers of a germline CDKN2A pathogenic variant: yield and outcomes of a 20-Year prospective Follow-Up. J Clin Oncol 40(28):3267–3277. https://doi.org/10.1200/JCO.22.00194
Overbeek KA, Levink IJM, Koopmann BDM, Harinck F, Konings I, Ausems M, Wagner A, Fockens P, van Eijck CH, Groot Koerkamp B, Busch ORC, Besselink MG, Bastiaansen BAJ, van Driel L, Erler NS, Vleggaar FP, Poley JW, Cahen DL, van Hooft JE, Bruno MJ (2022) Dutch Familial Pancreatic Cancer Surveillance Study G Long-term yield of pancreatic cancer surveillance in high-risk individuals. Gut 71(6):1152–1160. https://doi.org/10.1136/gutjnl-2020-323611
van Rijssen LB, Koerkamp BG, Zwart MJ, Bonsing BA, Bosscha K, van Dam RM, van Eijck CH, Gerhards MF, van der Harst E, de Hingh IH, de Jong KP, Kazemier G, Klaase J, van Laarhoven CJ, Molenaar IQ, Patijn GA, Rupert CG, van Santvoort HC, Scheepers JJ, van der Schelling GP, Busch OR, Besselink MG (2017) Nationwide prospective audit of pancreatic surgery: design, accuracy, and outcomes of the Dutch pancreatic Cancer audit. HPB (Oxford) 19(10):919–926. https://doi.org/10.1016/j.hpb.2017.06.010
Goggins M, Overbeek KA, Brand R, Syngal S, Del Chiaro M, Bartsch DK, Bassi C, Carrato A, Farrell J, Fishman EK, Fockens P, Gress TM, van Hooft JE, Hruban RH, Kastrinos F, Klein A, Lennon AM, Lucas A, Park W, Rustgi A, Simeone D, Stoffel E, Vasen HFA, Cahen DL, Canto MI, Bruno M (2020) Management of patients with increased risk for familial pancreatic cancer: updated recommendations from the International Cancer of the pancreas Screening (CAPS) Consortium. Gut 69(1):7–17. https://doi.org/10.1136/gutjnl-2019-319352. International Cancer of the Pancreas Screening c
Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW (2015) ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol 110(2):223–262 quiz 263. https://doi.org/10.1038/ajg.2014.435
Sawhney MS, Calderwood AH, Thosani NC, Rebbeck TR, Wani S, Canto MI, Fishman DS, Golan T, Hidalgo M, Kwon RS, Riegert-Johnson DL, Sahani DV, Stoffel EM, Vollmer CM Jr., Qumseya BJ, Prepared by ASOPC (2022) ASGE guideline on screening for pancreatic cancer in individuals with genetic susceptibility: summary and recommendations. Gastrointest Endosc 95(5):817–826. https://doi.org/10.1016/j.gie.2021.12.001
Aslanian HR, Lee JH, Canto MI (2020) AGA clinical practice update on Pancreas Cancer Screening in High-Risk individuals. Expert Rev Gastroenterol 159(1):358–362. https://doi.org/10.1053/j.gastro.2020.03.088
Saftoiu A, Hassan C, Areia M, Bhutani MS, Bisschops R, Bories E, Cazacu IM, Dekker E, Deprez PH, Pereira SP, Senore C, Capocaccia R, Antonelli G, van Hooft J, Messmann H, Siersema PD, Dinis-Ribeiro M, Ponchon T (2020) Role of gastrointestinal endoscopy in the screening of digestive tract cancers in Europe: European Society of Gastrointestinal Endoscopy (ESGE) position Statement. Endoscopy 52(4):293–304. https://doi.org/10.1055/a-1104-5245
Tang S, Huang G, Liu J, Liu T, Treven L, Song S, Zhang C, Pan L, Zhang T (2011) Usefulness of 18F-FDG PET, combined FDG-PET/CT and EUS in diagnosing primary pancreatic carcinoma: a meta-analysis. Eur J Radiol 78(1):142–150. https://doi.org/10.1016/j.ejrad.2009.09.026
DeWitt J, Devereaux B, Chriswell M, McGreevy K, Howard T, Imperiale TF, Ciaccia D, Lane KA, Maglinte D, Kopecky K, LeBlanc J, McHenry L, Madura J, Aisen A, Cramer H, Cummings O, Sherman S (2004) Comparison of endoscopic ultrasonography and multidetector computed tomography for detecting and staging pancreatic cancer. Ann Intern Med 141(10):753–763. https://doi.org/10.7326/0003-4819-141-10-200411160-00006
Dewitt J, Devereaux BM, Lehman GA, Sherman S, Imperiale TF (2006) Comparison of endoscopic ultrasound and computed tomography for the preoperative evaluation of pancreatic cancer: a systematic review. Clin Gastroenterol Hepatol 4(6):717–725 quiz 664. https://doi.org/10.1016/j.cgh.2006.02.020
Muller MF, Meyenberger C, Bertschinger P, Schaer R, Marincek B (1994) Pancreatic tumors: evaluation with endoscopic US, CT, and MR imaging. Radiology 190(3):745–751. https://doi.org/10.1148/radiology.190.3.8115622
Kann PH (2018) Is endoscopic ultrasonography more sensitive than magnetic resonance imaging in detecting and localizing pancreatic neuroendocrine tumors? Rev Endocr Metab Disord 19(2):133–137. https://doi.org/10.1007/s11154-018-9464-1
Khashab MA, Yong E, Lennon AM, Shin EJ, Amateau S, Hruban RH, Olino K, Giday S, Fishman EK, Wolfgang CL, Edil BH, Makary M, Canto MI (2011) EUS is still superior to multidetector computerized tomography for detection of pancreatic neuroendocrine tumors. Gastrointest Endosc 73(4):691–696. https://doi.org/10.1016/j.gie.2010.08.030
Fusaroli P, Spada A, Mancino MG, Caletti G (2010) Contrast harmonic echo-endoscopic ultrasound improves accuracy in diagnosis of solid pancreatic masses. Clin Gastroenterol Hepatol 8(7):629–634. https://doi.org/10.1016/j.cgh.2010.04.012. e1-2
Kitano M, Kudo M, Yamao K, Takagi T, Sakamoto H, Komaki T, Kamata K, Imai H, Chiba Y, Okada M, Murakami T, Takeyama Y (2012) Characterization of small solid tumors in the pancreas: the value of contrast-enhanced harmonic endoscopic ultrasonography. Am J Gastroenterol 107(2):303–310. https://doi.org/10.1038/ajg.2011.354
Dietrich CF, Sahai AV, D’Onofrio M, Will U, Arcidiacono PG, Petrone MC, Hocke M, Braden B, Burmester E, Moller K, Saftoiu A, Ignee A, Cui XW, Iordache S, Potthoff A, Iglesias-Garcia J, Fusaroli P, Dong Y, Jenssen C (2016) Differential diagnosis of small solid pancreatic lesions. Gastrointest Endosc 84(6):933–940. https://doi.org/10.1016/j.gie.2016.04.034
Puli SR, Bechtold ML, Buxbaum JL, Eloubeidi MA (2013) How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass? A meta-analysis and systematic review. Pancreas 42(1):20–26. https://doi.org/10.1097/MPA.0b013e3182546e79
Chen G, Liu S, Zhao Y, Dai M, Zhang T (2013) Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for pancreatic cancer: a meta-analysis. Pancreatology 13(3):298–304. https://doi.org/10.1016/j.pan.2013.01.013
Wang W, Shpaner A, Krishna SG, Ross WA, Bhutani MS, Tamm EP, Raju GS, Xiao L, Wolff RA, Fleming JB, Lee JH (2013) Use of EUS-FNA in diagnosing pancreatic neoplasm without a definitive mass on CT. Gastrointest Endosc 78(1):73–80. https://doi.org/10.1016/j.gie.2013.01.040
Overbeek KA, Goggins MG, Dbouk M, Levink IJM, Koopmann BDM, Chuidian M, Konings I, Paiella S, Earl J, Fockens P, Gress TM, Ausems M, Poley JW, Thosani NC, Half E, Lachter J, Stoffel EM, Kwon RS, Stoita A, Kastrinos F, Lucas AL, Syngal S, Brand RE, Chak A, Carrato A, Vleggaar FP, Bartsch DK, van Hooft JE, Cahen DL, Canto MI, Bruno MJ, International Cancer of the Pancreas Screening C (2022) Timeline of Development of Pancreatic Cancer and implications for successful early detection in high-risk individuals. Gastroenterology 162(3):772–785. e4
Yu J, Sadakari Y, Shindo K, Suenaga M, Brant A, Almario JAN, Borges M, Barkley T, Fesharakizadeh S, Ford M, Hruban RH, Shin EJ, Lennon AM, Canto MI, Goggins M (2017) Digital next-generation sequencing identifies low-abundance mutations in pancreatic juice samples collected from the duodenum of patients with pancreatic cancer and intraductal papillary mucinous neoplasms. Gut 66(9):1677–1687. https://doi.org/10.1136/gutjnl-2015-311166
Eshleman JR, Norris AL, Sadakari Y, Debeljak M, Borges M, Harrington C, Lin E, Brant A, Barkley T, Almario JA, Topazian M, Farrell J, Syngal S, Lee JH, Yu J, Hruban RH, Kanda M, Canto MI, Goggins M (2015) KRAS and guanine nucleotide-binding protein mutations in pancreatic juice collected from the duodenum of patients at high risk for neoplasia undergoing endoscopic ultrasound. Clin Gastroenterol Hepatol 13(5):963–9e4. https://doi.org/10.1016/j.cgh.2014.11.028
Kanda M, Sadakari Y, Borges M, Topazian M, Farrell J, Syngal S, Lee J, Kamel I, Lennon AM, Knight S, Fujiwara S, Hruban RH, Canto MI, Goggins M (2013) Mutant TP53 in duodenal samples of pancreatic juice from patients with pancreatic cancer or high-grade dysplasia. Clin Gastroenterol Hepatol 11(6):719–30e5. https://doi.org/10.1016/j.cgh.2012.11.016
Lu X, Xu T, Qian J, Wen X, Wu D (2002) Detecting K-ras and p53 gene mutation from stool and pancreatic juice for diagnosis of early pancreatic cancer. Chin Med J (Engl) 115(11):1632–1636
Nesteruk K, Levink IJM, de Vries E, Visser IJ, Peppelenbosch MP, Cahen DL, Fuhler GM, Bruno MJ (2022) Extracellular vesicle-derived microRNAs in pancreatic juice as biomarkers for detection of pancreatic ductal adenocarcinoma. Pancreatology 22(5):626–635. https://doi.org/10.1016/j.pan.2022.04.010
Levink IJM, Visser IJ, Koopmann BDM, van Driel L, Poley JW, Cahen DL, Bruno MJ, Fuhler GM (2022) Protein biomarkers in pancreatic juice and serum for identification of pancreatic cancer. Gastrointest Endosc 96(5):801–813e2. https://doi.org/10.1016/j.gie.2022.04.1342
Matsunaga T, Ohtsuka T, Asano K, Kimura H, Ohuchida K, Kitada H, Ideno N, Mori Y, Tokunaga S, Oda Y, Guha S, Raimondo M, Nakamura M, Tanaka M (2017) S100P in Duodenal Fluid is a useful diagnostic marker for pancreatic ductal adenocarcinoma. Pancreas 46(10):1288–1295. https://doi.org/10.1097/mpa.0000000000000940
Sadakari Y, Ohtsuka T, Ohuchida K, Tsutsumi K, Takahata S, Nakamura M, Mizumoto K, Tanaka M (2010) MicroRNA expression analyses in preoperative pancreatic juice samples of pancreatic ductal adenocarcinoma. JOP 11(6):587–592
Nakamura S, Sadakari Y, Ohtsuka T, Okayama T, Nakashima Y, Gotoh Y, Saeki K, Mori Y, Nakata K, Miyasaka Y, Onishi H, Oda Y, Goggins M, Nakamura M (2019) Pancreatic juice exosomal MicroRNAs as biomarkers for detection of pancreatic ductal adenocarcinoma. Ann Surg Oncol 26(7):2104–2111. https://doi.org/10.1245/s10434-019-07269-z
Suenaga M, Yu J, Shindo K, Tamura K, Almario JAN, Zaykoski CM, Witmer PD, Fesharakizadeh S, Borges M, Lennon AM, Shin EJ, Canto MI, Goggins MG (2018) Pancreatic juice mutation concentrations can help predict the grade of dysplasia in patients undergoing pancreatic surveillance. Clin Cancer Res https://doi.org/10.1158/1078?0432.ccr-17-2463
Wani S, Han S, Simon V, Hall M, Early D, Aagaard E, Abidi WM, Banerjee S, Baron TH, Bartel M, Bowman E, Brauer BC, Buscaglia JM, Carlin L, Chak A, Chatrath H, Choudhary A, Confer B, Cote GA, Das KK, DiMaio CJ, Dries AM, Edmundowicz SA, El Chafic AH, El Hajj I, Ellert S, Ferreira J, Gamboa A, Gan IS, Gangarosa L, Gannavarapu B, Gordon SR, Guda NM, Hammad HT, Harris C, Jalaj S, Jowell P, Kenshil S, Klapman J, Kochman ML, Komanduri S, Lang G, Lee LS, Loren DE, Lukens FJ, Mullady D, Muthusamy RV, Nett AS, Olyaee MS, Pakseresht K, Perera P, Pfau P, Piraka C, Poneros JM, Rastogi A, Razzak A, Riff B, Saligram S, Scheiman JM, Schuster I, Shah RJ, Sharma R, Spaete JP, Singh A, Sohail M, Sreenarasimhaiah J, Stevens T, Tabibian JH, Tzimas D, Uppal DS, Urayama S, Vitterbo D, Wang AY, Wassef W, Yachimski P, Zepeda-Gomez S, Zuchelli T, Keswani RN (2019) Setting minimum standards for training in EUS and ERCP: results from a prospective multicenter study evaluating learning curves and competence among advanced endoscopy trainees. Gastrointest Endosc 89(6):1160–1168 e9. https://doi.org/10.1016/j.gie.2019.01.030
Topazian M, Enders F, Kimmey M, Brand R, Chak A, Clain J, Cunningham J, Eloubeidi M, Gerdes H, Gress F, Jagannath S, Kantsevoy S, LeBlanc JK, Levy M, Lightdale C, Romagnuolo J, Saltzman JR, Savides T, Wiersema M, Woodward T, Petersen G, Canto M (2007) Interobserver agreement for EUS findings in familial pancreatic-cancer kindreds. Gastrointest Endosc 66(1):62–67. https://doi.org/10.1016/j.gie.2006.09.018
(2018) European evidence-based guidelines on pancreatic cystic neoplasms. Gut 67(5):789–804. https://doi.org/10.1136/gutjnl-2018-316027
Overbeek KA, Koopmann BDM, Levink IJM, Tacelli M, Erler NS, Arcidiacono PG, Ausems MGE, Wagner A, van Eijck CH, Groot Koerkamp B, Busch OR, Besselink MG, van der Vlugt M, van Driel L, Fockens P, Vleggaar FP, Poley JW, Capurso G, Cahen DL, Bruno MJ Dutch familial pancreatic Cancer surveillance study work g (2023) intraductal papillary mucinous neoplasms in High-Risk individuals: incidence, growth rate, and Malignancy Risk. Clin Gastroenterol Hepatol https://doi.org/10.1016/j.cgh.2023.03.035
Jenssen C, Alvarez-Sanchez MV, Napoleon B, Faiss S (2012) Diagnostic endoscopic ultrasonography: assessment of safety and prevention of complications. World J Gastroenterol 18(34):4659–4676. https://doi.org/10.3748/wjg.v18.i34.4659
Wang KX, Ben QW, Jin ZD, Du YQ, Zou DW, Liao Z, Li ZS (2011) Assessment of morbidity and mortality associated with EUS-guided FNA: a systematic review. Gastrointest Endosc 73(2):283–290. https://doi.org/10.1016/j.gie.2010.10.045
Zhu H, Jiang F, Zhu J, Du Y, Jin Z, Li Z (2017) Assessment of morbidity and mortality associated with endoscopic ultrasound-guided fine-needle aspiration for pancreatic cystic lesions: a systematic review and meta-analysis. Dig Endosc 29(6):667–675. https://doi.org/10.1111/den.12851
Tian G, Ye Z, Zhao Q, Jiang T (2020) Complication incidence of EUS-guided pancreas biopsy: a systematic review and meta-analysis of 11 thousand population from 78 cohort studies. Asian J Surg 43(11):1049–1055. https://doi.org/10.1016/j.asjsur.2019.12.011
Gao RY, Wu BH, Shen XY, Peng TL, Li DF, Wei C, Yu ZC, Luo MH, Xiong F, Wang LS, Yao J (2020) Overlooked risk for needle tract seeding following endoscopic ultrasound-guided minimally invasive tissue acquisition. World J Gastroenterol 26(40):6182–6194. https://doi.org/10.3748/wjg.v26.i40.6182
Archibugi L, Ponz de Leon Pisani R, Petrone MC, Balzano G, Falconi M, Doglioni C, Capurso G, Arcidiacono PG (2022) Needle-Tract Seeding of Pancreatic Cancer after EUS-FNA: A Systematic Review of Case Reports and Discussion of Management. Cancers (Basel) 14(24)10.3390/cancers14246130
Kitano M, Yoshida M, Ashida R, Kita E, Katanuma A, Itoi T, Mikata R, Nishikawa K, Matsubayashi H, Takayama Y, Kato H, Takenaka M, Ueki T, Kawashima Y, Nakai Y, Hashimoto S, Shigekawa M, Nebiki H, Tsumura H, Okabe Y, Ryozawa S, Harada Y, Mitoro A, Sasaki T, Yasuda H, Miura N, Ikemoto T, Ozawa E, Shioji K, Yamaguchi A, Okuzono T, Moriyama I, Hisai H, Fujita K, Goto T, Shirahata N, Iwata Y, Okabe Y, Hara K, Hashimoto Y, Kuwatani M, Isayama H, Fujimori N, Masamune A, Hatamaru K, Shimokawa T, Okazaki K, Takeyama Y, Yamaue H, Committee of Clinical Research JPS (2022) Needle tract seeding after endoscopic ultrasound-guided tissue acquisition of pancreatic tumors: a nationwide survey in Japan. Dig Endosc. https://doi.org/10.1111/den.14346
Micames C, Jowell PS, White R, Paulson E, Nelson R, Morse M, Hurwitz H, Pappas T, Tyler D, McGrath K (2003) Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc 58(5):690–695. https://doi.org/10.1016/s0016-5107(03)02009-1
Yane K, Kuwatani M, Yoshida M, Goto T, Matsumoto R, Ihara H, Okuda T, Taya Y, Ehira N, Kudo T, Adachi T, Eto K, Onodera M, Sano I, Nojima M, Katanuma A (2020) Non-negligible rate of needle tract seeding after endoscopic ultrasound-guided fine-needle aspiration for patients undergoing distal pancreatectomy for pancreatic cancer. Dig Endosc 32(5):801–811. https://doi.org/10.1111/den.13615
Harinck F, Nagtegaal T, Kluijt I, Aalfs C, Smets E, Poley JW, Wagner A, van Hooft J, Fockens P, Bruno M, Bleiker EM (2011) Feasibility of a pancreatic cancer surveillance program from a psychological point of view. Genet Med 13(12):1015–1024. https://doi.org/10.1097/GIM.0b013e31822934f5
Konings I, Sidharta G, Harinck F, Aalfs C, Poley JW, Smets E, Wagner A, Fockens P, Van Rens A, Van Hooft J, Bruno M, Bleiker E (2014) Repeated pancreatic surveillance in high risk individuals for pancreatic cancer: the psychological burden. United Eur Gastroenterol J 2(1):A75
Paiella S, Marinelli V, Secchettin E, Mazzi MA, Ferretto F, Casolino R, Bassi C, Salvia R (2020) The emotional impact of surveillance programs for pancreatic cancer on high-risk individuals: a prospective analysis. Psychooncology. https://doi.org/10.1002/pon.5370
O’Neill RS, Meiser B, Emmanuel S, Williams DB, Stoita A (2019) Long-term positive psychological outcomes in an Australian pancreatic cancer screening program. Fam Cancer. https://doi.org/10.1007/s10689-019-00147-3
Maheu C, Vodermaier A, Rothenmund H, Gallinger S, Ardiles P, Semotiuk K, Holter S, Thayalan S, Esplen MJ (2010) Pancreatic cancer risk counselling and screening: impact on perceived risk and psychological functioning. Fam Cancer 9(4):617–624. https://doi.org/10.1007/s10689-010-9354-5
Konings IC, Harinck F, Kuenen MA, Kieffer JM, Aalfs CM, Poley JW, Smets EM, Wagner A, Van Rens A, Vleggaar FP, Ausems MG, Fockens P, Van Hooft JE, Bruno MJ, Bleiker EM (2016) Factors associated with cancer worries in individuals participating in annual pancreatic cancer surveillance. Gastroenterology 150(4):S362–S363
Franke FS, Matthai E, Slater EP, Schicker C, Kruse J, Bartsch DK (2018) German National Case Collection for familial pancreatic Cancer (FaPaCa) - acceptance and psychological aspects of a pancreatic cancer screening program. Hered Cancer Clin Pract 16:17. https://doi.org/10.1186/s13053-018-0100-6
Overbeek KA, Cahen DL, Kamps A, Konings I, Harinck F, Kuenen MA, Koerkamp BG, Besselink MG, van Eijck CH, Wagner A, Ausems MGE, van der Vlugt M, Fockens P, Vleggaar FP, Poley JW, van Hooft JE, Bleiker EMA, Bruno MJ, Dutch Familial Pancreatic Cancer Surveillance Study G (2020) Patient-reported burden of intensified surveillance and surgery in high-risk individuals under pancreatic cancer surveillance. Fam Cancer 19(3):247–258. https://doi.org/10.1007/s10689-020-00171-8
Hart SL, Torbit LA, Crangle CJ, Esplen MJ, Holter S, Semotiuk K, Borgida A, Ardiles P, Rothenmund H, Gallinger S (2012) Moderators of cancer-related distress and worry after a pancreatic cancer genetic counseling and screening intervention. Psychooncology 21(12):1324–1330. https://doi.org/10.1002/pon.2026
Cazacu IM, Luzuriaga Chavez AA, Mendoza TR, Qiao W, Singh BS, Bokhari RH, Saftoiu A, Lee JH, Weston B, Stroehlein JR, Kim MP, MH GK, Maitra A, McAllister F, Bhutani MS (2020) Quality of life impact of EUS in patients at risk for developing pancreatic cancer. Endosc Ultrasound 9(1):53–58. https://doi.org/10.4103/eus.eus_56_19
Cazacu IM, Luzuriaga Chavez AA, Saftoiu A, Bhutani MS (2019) Psychological impact of pancreatic cancer screening by EUS or magnetic resonance imaging in high-risk individuals: a systematic review. Endosc Ultrasound 8(1):17–24. https://doi.org/10.4103/eus.eus_25_18
Konings IC, Sidharta GN, Harinck F, Aalfs CM, Poley JW, Kieffer JM, Kuenen MA, Smets EM, Wagner A, van Hooft JE, van Rens A, Fockens P, Bruno MJ, Bleiker EM (2016) Repeated participation in pancreatic cancer surveillance by high-risk individuals imposes low psychological burden. Psychooncology 25(8):971–978. https://doi.org/10.1002/pon.4047
Konings IC, Harinck F, Kuenen MA, Sidharta GN, Kieffer JM, Aalfs CM, Poley JW, Smets EM, Wagner A, van Rens A, Vleggaar FP, Ausems MG, Fockens P, van Hooft JE, Bruno MJ, Bleiker EM (2017) Factors associated with cancer worries in individuals participating in annual pancreatic cancer surveillance. Fam Cancer 16(1):143–151. https://doi.org/10.1007/s10689-016-9930-4
Thiruvengadam SS, Chuang J, Huang R, Girotra M, Park WG (2019) Chronic pancreatitis changes in high-risk individuals for pancreatic ductal adenocarcinoma. Gastrointest Endosc 89(4):842–851e1. https://doi.org/10.1016/j.gie.2018.08.029
Corral JE, Mareth KF, Riegert-Johnson DL, Das A, Wallace MB (2019) Diagnostic yield from screening asymptomatic individuals at high risk for pancreatic Cancer: a Meta-analysis of Cohort studies. Clin Gastroenterol Hepatol 17(1):41–53. https://doi.org/10.1016/j.cgh.2018.04.065
Brune K, Abe T, Canto M, O’Malley L, Klein AP, Maitra A, Volkan Adsay N, Fishman EK, Cameron JL, Yeo CJ, Kern SE, Goggins M, Hruban RH (2006) Multifocal neoplastic precursor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. Am J Surg Pathol 30(9):1067–1076. pas.0000213265.84725.0b
Konings I, Cahen DL, Harinck F, Fockens P, van Hooft JE, Poley JW, Bruno MJ (2018) Evolution of features of chronic pancreatitis during endoscopic ultrasound-based surveillance of individuals at high risk for pancreatic cancer. Endosc Int Open 6(5):E541–E548. https://doi.org/10.1055/a-0574-2396
Basturk O, Hong SM, Wood LD, Adsay NV, Albores-Saavedra J, Biankin AV, Brosens LA, Fukushima N, Goggins M, Hruban RH, Kato Y, Klimstra DS, Kloppel G, Krasinskas A, Longnecker DS, Matthaei H, Offerhaus GJ, Shimizu M, Takaori K, Terris B, Yachida S, Esposito I, Furukawa T, Baltimore Consensus M (2015) A revised classification system and recommendations from the Baltimore Consensus Meeting for neoplastic precursor lesions in the pancreas. Am J Surg Pathol 39(12):1730–1741. https://doi.org/10.1097/PAS.0000000000000533
LeBlanc JK, Chen JH, Al-Haddad M, Luz L, McHenry L, Sherman S, Juan M, Dewitt J (2014) Can endoscopic ultrasound predict pancreatic intraepithelial neoplasia lesions in chronic pancreatitis? A retrospective study of pathologic correlation. Pancreas 43(6):849–854. https://doi.org/10.1097/MPA.0000000000000142
Bartsch DK, Matthai E, Mintziras I, Bauer C, Figiel J, Sina-Boemers M, Gress TM, Langer P, Slater EP (2021) The German National Case Collection for familial pancreatic carcinoma (FaPaCa)-Knowledge gained in 20 years. Dtsch Arztebl Int 118(10):163–168. https://doi.org/10.3238/arztebl.m2021.0004
Sheel ARG, Harrison S, Sarantitis I, Nicholson JA, Hanna T, Grocock C, Raraty M, Ramesh J, Farooq A, Costello E, Jackson R, Chapman M, Smith A, Carter R, McKay C, Hamady Z, Aithal GP, Mountford R, Ghaneh P, Hammel P, Lerch MM, Halloran C, Pereira SP, Greenhalf W (2019) Identification of cystic lesions by secondary screening of familial pancreatic Cancer (FPC) Kindreds is not Associated with the stratified risk of Cancer. Am J Gastroenterol 114(1):155–164. https://doi.org/10.1038/s41395-018-0395-y
Potjer TP, Schot I, Langer P, Heverhagen JT, Wasser MN, Slater EP, Kloppel G, Morreau HM, Bonsing BA, de Vos Tot Nederveen, Cappel WH, Bargello M, Gress TM, Vasen HF, Bartsch DK (2013) Variation in precursor lesions of pancreatic cancer among high-risk groups. Clin Cancer Res 19(2):442–449. https://doi.org/10.1158/1078?0432.ccr-12-2730
Paiella S, Capurso G, Carrara S, Secchettin E, Casciani F, Frigerio I, Zerbi A, Archibugi L, Bonifacio C, Malleo G, Cavestro GM, Barile M, Larghi A, Assisi D, Fantin A, Milanetto AC, Fabbri C, Casadei R, Donato G, Sassatelli R, De Marchi G, Di Matteo FM, Arcangeli V, Panzuto F, Puzzono M, Buono AD, Pezzilli R, Salvia R, Rizzatti G, Casadio M, Franco M, Butturini G, Pasquali C, Coluccio C, Ricci C, Cicchese N, Sereni G, de Pretis N, Stigliano S, Rudnas B, Marasco M, Lionetto G, Arcidiacono PG, Terrin M, Crovetto A, Mannucci A, Laghi L, Bassi C, Falconi M (2023) Outcomes of a 3-year prospective surveillance in individuals at high-risk for pancreatic cancer. Am J Gastroenterol. https://doi.org/10.14309/ajg.0000000000002546
Shah I, Silva-Santisteban A, Germansky KA, Trindade A, Raphael KL, Kushnir V, Pawa R, Mishra G, Anastasiou J, Inamdar S, Tharian B, Bilal M, Sawhney MS (2023) Pancreatic Cancer screening for At-Risk individuals (pancreas scan study): yield, Harms, and outcomes from a prospective Multicenter Study. Am J Gastroenterol 118(9):1664–1670. https://doi.org/10.14309/ajg.0000000000002314
Harinck F, Konings IC, Kluijt I, Poley JW, van Hooft JE, van Dullemen HM, Nio CY, Krak NC, Hermans JJ, Aalfs CM, Wagner A, Sijmons RH, Biermann K, van Eijck CH, Gouma DJ, Dijkgraaf MG, Fockens P, Bruno MJ Individuals Drgopcsih-r (2015) a multicentre comparative prospective blinded analysis of EUS and MRI for screening of pancreatic cancer in high-risk individuals. Gut https://doi.org/10.1136/gutjnl-2014-308008
Marchegiani G, Pollini T, Burelli A, Han Y, Jung HS, Kwon W, Rocha Castellanos DM, Crippa S, Belfiori G, Arcidiacono PG, Capurso G, Apadula L, Zaccari P, Noia JL, Gorris M, Busch O, Ponweera A, Mann K, Demir IE, Phillip V, Ahmad N, Hackert T, Heckler M, Lennon AM, Afghani E, Vallicella D, Dall’Olio T, Nepi A, Vollmer CM, Friess H, Ghaneh P, Besselink M, Falconi M, Bassi C, Goh BK, Jang JY, Castillo F-D, Salvia C (2023) R Surveillance for Presumed BD-IPMN of the Pancreas: Stability, Size, and Age Identify Targets for Discontinuation. Gastroenterology 165(4):1016–1024 e5. https://doi.org/10.1053/j.gastro.2023.06.022
Overbeek KA, van Leeuwen N, Tacelli M, Anwar MS, Yousaf MN, Chhoda A, Arcidiacono PG, Gonda TA, Wallace MB, Capurso G, Farrell JJ, Cahen DL, Bruno MJ (2022) International external validation of a stratification tool to identify branch-duct intraductal papillary mucinous neoplasms at lowest risk of progression. United Eur Gastroenterol J 10(2):169–178. https://doi.org/10.1002/ueg2.12207
Harinck F, Boersma F, Konings I, Fockens P, Van Hooft J, Dijkgraaf M, Dinjens W, Biermann K, Bruno M (2014) Clinicopathological characteristics of pancreatic resection specimens of inherited/familial versus sporadic pancreatic ductal adenocarcinoma. United Eur Gastroenterol J 2(1):A74–A75
Roberts NJ, Norris AL, Petersen GM, Bondy ML, Brand R, Gallinger S, Kurtz RC, Olson SH, Rustgi AK, Schwartz AG, Stoffel E, Syngal S, Zogopoulos G, Ali SZ, Axilbund J, Chaffee KG, Chen YC, Cote ML, Childs EJ, Douville C, Goes FS, Herman JM, Iacobuzio-Donahue C, Kramer M, Makohon-Moore A, McCombie RW, McMahon KW, Niknafs N, Parla J, Pirooznia M, Potash JB, Rhim AD, Smith AL, Wang Y, Wolfgang CL, Wood LD, Zandi PP, Goggins M, Karchin R, Eshleman JR, Papadopoulos N, Kinzler KW, Vogelstein B, Hruban RH, Klein AP (2016) Whole genome sequencing defines the genetic heterogeneity of familial pancreatic Cancer. Cancer Discov 6(2):166–175. https://doi.org/10.1158/2159-8290.cd-15-0402
Dbouk M, Brewer Gutierrez OI, Lennon AM, Chuidian M, Shin EJ, Kamel IR, Fishman EK, He J, Burkhart RA, Wolfgang CL, Hruban RH, Goggins MG, Canto MI (2021) Guidelines on management of pancreatic cysts detected in high-risk individuals: an evaluation of the 2017 Fukuoka guidelines and the 2020 International Cancer of the pancreas Screening (CAPS) consortium statements. Pancreatology. https://doi.org/10.1016/j.pan.2021.01.017
Lu C, Xu CF, Wan XY, Zhu HT, Yu CH, Li YM (2015) Screening for pancreatic cancer in familial high-risk individuals: a systematic review. World J Gastroenterol 21(28):8678–8686. https://doi.org/10.3748/wjg.v21.i28.8678
Signoretti M, Bruno MJ, Zerboni G, Poley JW, Delle Fave G, Capurso G (2018) Results of surveillance in individuals at high-risk of pancreatic cancer: a systematic review and meta-analysis. United Eur Gastroenterol J 6(4):489–499. https://doi.org/10.1177/2050640617752182
Tamburrino D, de Pretis N, Perez-Cuadrado-Robles E, Uribarri-Gonzalez L, Ateeb Z, Belfiori G, Maisonneuve P, Capurso G, Vanella G, Petrone MC, Arcidiacono PG, Vaalavuo Y, Frulloni L, Dominguez-Munoz JE, Deprez PH, Falconi M, Del Chiaro M, Crippa S, Laukkarinen J (2022) Identification of patients with branch-duct intraductal papillary mucinous neoplasm and very low risk of cancer: multicentre study. Br J Surg 109(7):617–622. https://doi.org/10.1093/bjs/znac103
Ibrahim IS, Vasen HFA, Wasser M, Feshtali S, Bonsing BA, Morreau H, de Inderson A Vos Tot Nederveen Cappel WH, van den Hout WB (2023) Cost-effectiveness of pancreas surveillance: The CDKN2A-p16-Leiden cohort. United European Gastroenterol J 11(2):163–170. https://doi.org/10.1002/ueg2.12360
Corral JE, Das A, Bruno MJ, Wallace MB (2019) Cost-effectiveness of pancreatic Cancer surveillance in high-risk individuals: an economic analysis. Pancreas 48(4):526–536
Klatte DCF, Boekestijn B, Onnekink AM, Dekker FW, van der Geest LG, Wasser M, Feshtali S, Mieog JSD, Luelmo SAC, Morreau H, Potjer TP, Inderson A, Boonstra JJ, Vasen HFA, van Hooft JE, Bonsing BA, van Leerdam ME (2023) Dutch Pancreatic Cancer G Surveillance for Pancreatic Cancer in High-Risk Individuals Leads to Improved Outcomes: A Propensity Score-Matched Analysis. Gastroenterology 164(7):1223–1231 e4. https://doi.org/10.1053/j.gastro.2023.02.032
Langer P, Kann PH, Fendrich V, Habbe N, Schneider M, Sina M, Slater EP, Heverhagen JT, Gress TM, Rothmund M, Bartsch DK (2009) Five years of prospective screening of high-risk individuals from families with familial pancreatic cancer. Gut 58(10):1410–1418. https://doi.org/10.1136/gut.2008.171611
Schneider R, Slater EP, Sina M, Habbe N, Fendrich V, Matthai E, Langer P, Bartsch DK (2011) German national case collection for familial pancreatic cancer (FaPaCa): ten years experience. Fam Cancer 10(2):323–330. https://doi.org/10.1007/s10689-010-9414-x
Bartsch DK, Slater EP, Carrato A, Ibrahim IS, Guillen-Ponce C, Vasen HF, Matthai E, Earl J, Jendryschek FS, Figiel J, Steinkamp M, Ramaswamy A, Vazquez-Sequeiros E, Munoz-Beltran M, Montans J, Mocci E, Bonsing BA, Wasser M, Kloppel G, Langer P, Fendrich V, Gress TM (2016) Refinement of screening for familial pancreatic cancer. Gut 65(8):1314–1321. https://doi.org/10.1136/gutjnl-2015-311098
Kluijt I, Cats A, Fockens P, Nio Y, Gouma DJ, Bruno MJ (2009) Atypical familial presentation of FAMMM syndrome with a high incidence of pancreatic cancer: case finding of asymptomatic individuals by EUS surveillance. J Clin Gastroenterol 43(9):853–857. https://doi.org/10.1097/MCG.0b013e3181981123
Poley JW, Kluijt I, Gouma DJ, Harinck F, Wagner A, Aalfs C, van Eijck CH, Cats A, Kuipers EJ, Nio Y, Fockens P, Bruno MJ (2009) The yield of first-time endoscopic ultrasonography in screening individuals at a high risk of developing pancreatic cancer. Am J Gastroenterol 104(9):2175–2181. https://doi.org/10.1038/ajg.2009.276
Verna EC, Hwang C, Stevens PD, Rotterdam H, Stavropoulos SN, Sy CD, Prince MA, Chung WK, Fine RL, Chabot JA, Frucht H (2010) Pancreatic cancer screening in a prospective cohort of high-risk patients: a comprehensive strategy of imaging and genetics. Clin Cancer Res 16(20):5028–5037. https://doi.org/10.1158/1078?0432.ccr-09-3209
Vasen H, Ibrahim I, Ponce CG, Slater EP, Matthai E, Carrato A, Earl J, Robbers K, van Mil AM, Potjer T, Bonsing BA, de Vos Tot Nederveen, Cappel WH, Bergman W, Wasser M, Morreau H, Kloppel G, Schicker C, Steinkamp M, Figiel J, Esposito I, Mocci E, Vazquez-Sequeiros E, Sanjuanbenito A, Munoz-Beltran M, Montans J, Langer P, Fendrich V, Bartsch DK (2016) Benefit of Surveillance for Pancreatic Cancer in High-Risk individuals: outcome of long-term prospective Follow-Up studies from three European Expert centers. J Clin Oncol 34(17):2010–2019 JCO.2015.64.0730 [pii] 10.1200/JCO.2015.64.0730
Al-Sukhni W, Borgida A, Rothenmund H, Holter S, Semotiuk K, Grant R, Wilson S, Moore M, Narod S, Jhaveri K, Haider MA, Gallinger S (2012) Screening for pancreatic cancer in a high-risk cohort: an eight-year experience. J Gastrointest Surg 16(4):771–783. https://doi.org/10.1007/s11605-011-1781-6
Sud A, Wham D, Catalano M, Guda NM (2014) Promising outcomes of screening for pancreatic cancer by genetic testing and endoscopic ultrasound. Pancreas 43(3):458–461. https://doi.org/10.1097/mpa.0000000000000052
Mocci E, Guillen-Ponce C, Earl J, Marquez M, Solera J, Salazar-Lopez MT, Calcedo-Arnaiz C, Vazquez-Sequeiros E, Montans J, Munoz-Beltran M, Vicente-Bartulos A, Gonzalez-Gordaliza C, Sanjuanbenito A, Guerrero C, Mendia E, Lisa E, Lobo E, Martinez JC, Real FX, Malats N, Carrato A (2015) PanGen-Fam: Spanish registry of hereditary pancreatic cancer. Eur J Cancer 51(14):1911–1917. https://doi.org/10.1016/j.ejca.2015.07.004
Joergensen MT, Gerdes AM, Sorensen J, Schaffalitzky de Muckadell O, Mortensen MB (2016) Is screening for pancreatic cancer in high-risk groups cost-effective? - experience from a Danish national screening program. Pancreatology 16(4):584–592. https://doi.org/10.1016/j.pan.2016.03.013
DaVee T, Coronel E, Papafragkakis C, Thaiudom S, Lanke G, Chakinala RC, Nogueras Gonzalez GM, Bhutani MS, Ross WA, Weston BR, Lee JH (2018) Pancreatic cancer screening in high-risk individuals with germline genetic mutations. Gastrointest Endosc. https://doi.org/10.1016/j.gie.2017.12.019
Lachter J, Rosenberg C, Hananiya T, Khamaysi I, Klein A, Yassin K, Half E (2018) Screening to Detect Precursor Lesions of Pancreatic Adenocarcinoma in High-risk Individuals: A Single-center Experience. Rambam Maimonides Med J 9(4)10.5041/rmmj.10353
Gangi A, Malafa M, Klapman J (2018) Endoscopic ultrasound-based pancreatic Cancer screening of high-risk individuals: a prospective observational trial. Pancreas 47(5):586–591. https://doi.org/10.1097/mpa.0000000000001038
McNamara GPJ, Ali KN, Vyas S, Huynh T, Nyland M, Almanza D, Laronga C, Klapman J, Permuth JB (2019) Characteristics and clinical outcomes of individuals at high risk for pancreatic Cancer: a descriptive analysis from a Comprehensive Cancer Center. Gastrointest Disord (Basel) 1(1):106–119. https://doi.org/10.3390/gidisord1010008
Paiella S, Capurso G, Cavestro GM, Butturini G, Pezzilli R, Salvia R, Signoretti M, Crippa S, Carrara S, Frigerio I, Bassi C, Falconi M, Iannicelli E, Giardino A, Mannucci A, Laghi A, Laghi L, Frulloni L, Zerbi A, Italian Association for the Study of the P (2018) Results of first-round of Surveillance in individuals at high-risk of pancreatic Cancer from the AISP (Italian Association for the study of the Pancreas) Registry. Am J Gastroenterol. https://doi.org/10.1038/s41395-018-0414-z
Bar-Mashiah A, Aronson A, Naparst M, DiMaio CJ, Lucas AL (2020) Elevated hemoglobin A1c is associated with the presence of pancreatic cysts in a high-risk pancreatic surveillance program. BMC Gastroenterol 20(1):161. https://doi.org/10.1186/s12876-020-01308-w
Raff JP, Cook B, Jafri FN, Boxer N, Maldonado J, Hopkins U, Roayaie S, Noyer C (2022) Successful pancreatic Cancer screening among individuals at elevated risk using endoscopic ultrasound and magnetic resonance imaging: a Community Hospital Experience. Pancreas 51(10):1345–1351. https://doi.org/10.1097/MPA.0000000000002182
Llach J, Aguilera P, Sanchez A, Gines A, Fernandez-Esparrach G, Soy G, Sendino O, Vaquero E, Carballal S, Ausania F, Ayuso JR, Darnell A, Pellise M, Castellvi-Bel S, Puig S, Balaguer F, Moreira L (2023) Pancreatic Cancer Surveillance in Carriers of a Germline Pathogenic Variant in CDKN2A. Cancers (Basel) 15(6)10.3390/cancers15061690
Funding
No funds, grants, or other support was received.
Author information
Authors and Affiliations
Contributions
K.O. performed the literature search and wrote the first draft of the manuscript. It was critically revised by D.C. and M.B. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent
This narrative review did not include the study of human subjects.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Overbeek, K.A., Cahen, D.L. & Bruno, M.J. The role of endoscopic ultrasound in the detection of pancreatic lesions in high-risk individuals. Familial Cancer (2024). https://doi.org/10.1007/s10689-024-00380-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10689-024-00380-5