Abstract
BRCA1 and BRCA2 play a central role in DNA repair and their germline pathogenic variants (gBRCA) confer a high risk for developing breast and ovarian cancer. Standard chemotherapy regimens for these cancers include DNA-damaging agents. We hypothesized that gBRCA carriers might be at higher risk of developing chemotherapy-related hematologic toxicity and therapy-related myeloid neoplasms (t-MN). We conducted a retrospective study of women newly diagnosed with invasive breast or ovarian cancer who were screened for gBRCA1/gBRCA2 at Geneva University Hospitals. All patients were treated with (neo-)adjuvant chemotherapy. We evaluated acute hematologic toxicities by analyzing the occurrence of febrile neutropenia and severe neutropenia (grade 4) at day 7–14 of the first cycle of chemotherapy and G-CSF use during the entire chemotherapy regimen. Characteristics of t-MN were collected. We reviewed medical records from 447 patients: 58 gBRCA1 and 40 gBRCA2 carriers and 349 non-carriers. gBRCA1 carriers were at higher risk of developing severe neutropenia (32% vs. 14.5%, p = 0.007; OR = 3.3, 95% CI [1.6-7], p = 0.001) and of requiring G-CSF for secondary prophylaxis (58.3% vs. 38.2%, p = 0.011; OR = 2.5, 95% CI [1.4–4.8], p = 0.004). gBRCA2 carriers did not show increased acute hematologic toxicities. t-MN were observed in 2 patients (1 gBRCA1 and one non-carrier). Our results suggested an increased acute hematologic toxicity upon exposure to chemotherapy for breast and ovarian cancer among gBRCA1 but not gBRCA2 carriers. A deeper characterization of t-MN is warranted with the recent development of PARP inhibitors in frontline therapy in gBRCA breast and ovarian cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
BRCA1 and BRCA2 are tumor suppressor genes playing a central role in the repair of DNA double-strand breaks through homologous recombination, a fundamental DNA repair process that maintains genome integrity during cell proliferation [1]. Carrying germline pathogenic variants in BRCA1 or BRCA2 (gBRCA) confer a high risk for developing breast or ovarian cancer throughout a patient’s life [2, 3]. The average cumulative breast cancer and ovarian cancer risks by age 70 in gBRCA1 carriers are estimated at 72% and 44%, respectively, and for gBRCA2 carriers, at 69% and 17% [3]. The reason why breast and ovary are the mainly affected organs by the increased risk of cancer remains unanswered. One explanation is hormonally driven: oxidative DNA damage occurring during each menstrual cycle needs efficient homologous recombination pathway repair and could be exacerbated in haploinsufficient BRCA1 cells [4,5,6].
Severe neutropenia and hematologic toxicities usually arise due to myelosuppressive chemotherapy [7]. Febrile neutropenia is defined as an absolute neutrophil count (or expected to fall below) < 0.5 × 109/L with a single temperature > 38.3 °C or a sustained temperature > 38.0 °C for more than one hour [8]. It confers 15% higher risk of mortality than in patients without febrile neutropenia [9, 10]. Primary prophylaxis for the prevention of febrile neutropenia is based on the expected risk of febrile neutropenia with the planned chemotherapy regimen, adjusted with age, comorbidities or any other factors increasing the risk of febrile neutropenia. Primary prophylaxis is not recommended if the overall risk of febrile neutropenia is estimated to be less than 20% [10].
All the cells of carriers of BRCA1 or BRCA2 germline pathogenic variants are haploinsufficient for the gene product involved (alteration of a single allele). In these patients, carcinogenesis implies a somatic loss of the second allele either by loss of heterozygosity or somatic alteration of the second allele [1, 11, 12]. Preclinical data support the hypothesis that non-tumoral cells, through haploinsufficiency, present genomic instability and are more sensitive to DNA-damaging agents [11, 13,14,15,16]. There are conflicting data on whether germline BRCA1/BRCA2 variants are associated with an increased incidence of developing febrile neutropenia. We previously reported that breast cancer patients with gBRCA1 have a higher incidence of febrile neutropenia and grade 4 neutropenia under chemotherapy [17]. Post-hoc subgroup analyses on randomized trials suggested that breast cancer patients carrying gBRCA [18], but not ovarian cancer patients [19, 20], showed a higher incidence of acute hematologic toxicities under taxanes. Long-term follow-up of gBRCA carriers treated with poly-(ADP-ribose) polymerase (PARP) inhibitors suggested an increased incidence of therapy-related myeloid neoplasms, i.e. myelodysplastic syndrome and acute myeloid leukemia in gBRCA carriers [21,22,23,24]. Moreover, these patients are also at a higher risk of developing anthracyclines-induced cardiotoxicity [25].
We hypothesized that gBRCA1/BRCA2 carriers developing breast or ovarian cancer might be at higher risk for developing chemotherapy-related acute hematologic toxicity and therapy-related myeloid neoplasms. If shown, such association could impact breast and ovarian cancer management among this particular subpopulation of patients.
Material and methods
Study design
We conducted a retrospective study of all women newly diagnosed with breast or ovarian cancer who underwent germline BRCA1/BRCA2 testing between December 1995 and December 2018 at the Unit of Oncogenetics and Cancer Prevention, Hôpitaux Universitaires de Genève. The Geneva Ethics Committee approved the research protocol (CCER 15–158). Deceased patients were included without consent, and living patients were included after written informed consent was obtained.
Inclusion and exclusion criteria
We identified eligible patients for our study from the database of the UOPC. Inclusion criteria were women newly diagnosed with breast and ovarian cancer who underwent BRCA1/BRCA2 germline testing and received first line of neoadjuvant or adjuvant chemotherapy. Exclusion criteria were primary prophylaxis with G-CSF, metastatic breast cancer and the absence of available clinical data/follow-up.
Data collection
All data were collected from medical records. Tumor characteristics and laboratory results were collected from pathology and laboratory reports. For ovarian cancer patients, we collected the following clinical data: age at diagnosis, type of chemotherapy regimen and timing (neoadjuvant or adjuvant), dates (beginning and end) of chemotherapy, number of cycles administered, tumor characteristics (FIGO stage, histotype and grade). For breast cancer patients, we collected the following clinical data: age at diagnosis, type of chemotherapy regimen and timing (neoadjuvant or adjuvant), dates (beginning and end) of chemotherapy, number of cycles administered, tumor characteristics (TNM stage, grade, estrogen/progesterone receptors and HER2 status).
Hematologic toxicities
Regarding acute hematologic toxicities, we collected hematologic values (neutrophil count, leukocyte count, hemoglobin and platelets) at baseline, i.e. before the first cycle of chemotherapy (C1) and 7–14 days after its administration. Hematological toxicity was graded according to the Common Terminology Criteria for Adverse Events version 5.0 [8], with agranulocytosis defined as absolute neutrophil count < 0.5 × 109/L. Febrile neutropenia was defined as absolute neutrophil count < 1 × 109/L and fever > 38.3 °C. We reported whether the patients received G-CSF to complete the entire chemotherapy treatment, dose reductions of chemotherapy and the occurrence of febrile neutropenia. Long-term hematologic toxicity such as therapy-related myeloid neoplasms, i.e. myelodysplastic syndrome and acute myeloid leukemia were collected.
Endpoints
The primary endpoint was the incidence of febrile neutropenia at day 7–14 of the first cycle of chemotherapy. The secondary outcomes were the incidence of grade 3–4 neutropenia, G-CSF use and chemotherapy dose reduction during the entire chemotherapy regimen.
Statistical analysis
Outcomes were compared in gBRCA1, gBRCA2 and non-carriers. Absolute and relative frequencies were calculated for categorical data, whereas median, minimum and maximum values were determined for continuous data. Categorical data were compared using Fisher’s exact test. Continuous variables were compared using the Mann Whitney U test. Acute chemotherapy-related hematological toxicity frequencies were compared pair by pair by BRCA1/BRCA2 status (gBRCA1 vs. non-carriers; gBRCA2 vs. non-carriers) and corresponding age-adjusted odds ratio with 95% confidence interval were calculated using multivariable logistic regression models. Details of missing data for each variable can be found in supplementary Table 1. A double-sided p value < 0.05 was considered significant. All analyses were performed with R software (version 4.1.0).
Results
Characteristics of the study cohort
We reviewed the medical records of 1078 patients, 472 of whom met the inclusion criteria of our study. Among them, 25 women were excluded from the analysis due to lack of information regarding clinical data (supplementary Fig. 1). In total, 447 patients were included for analysis: 304 had breast cancer and 140 had ovarian cancer patients. Fifty-eight (13%) were identified with gBRCA1, 40 (9%) with gBRCA2 and 349 (78%) were non-carriers. Among breast cancer patients, 32 (10%) were gBRCA1, and 26 (8.6%) were gBRCA2 carriers. Among 140 ovarian cancer patients, 26 (18.6%) were gBRCA1 carriers and 13 (9.3%) were gBRCA2 carriers. No differences in age at diagnosis were observed according to BRCA1/2 genotype, except for gBRCA1 ovarian cancer patients being younger than non-carriers, as expected. Patients’ demographics, clinical and treatment characteristics are summarized in Table 1. Missing data are listed in Supplementary Table 1.
Tumor characteristics and treatment
Among 304 breast cancer patients, 93 (30%) had triple-negative breast cancer and 22 (68%) of gBRCA1 breast cancer patients developed triple-negative breast cancer. Among these breast cancer patients 221 were previously described [17], and we added 86 new patients (9 gBRCA1, 5 gBRCA2 and 72 non-carriers). The large majority of the patients received doublet chemotherapy that included at least one DNA damaging agent: either platinum and taxane (94% of ovarian cancer patients) or cyclophosphamide and anthracyclines (88% of breast cancer patients; Table 1).
Acute hematologic toxicities
Overall, 19/447 (4%) experienced a febrile neutropenia event after the first cycle of chemotherapy: 5/58 gBRCA1 (8.6%; p = 0.16), 1/40 gBRCA2 (2.5%) and 13/349 non-carriers (3.7%). The incidence of severe neutropenia (grade 4) after the first cycle was more frequent among gBRCA1 (32.6%, p = 0.007). Most gBRCA1 (58.3%; p = 0.011) needed secondary prophylaxis with G-CSF to complete their neoadjuvant or adjuvant chemotherapy, but this was not the case for gBRCA2 carriers and non-carriers (Table 2). Overall, gBRCA1 but not gBRCA2 carriers were at higher risk of developing grade 3–4 neutropenia and requiring G-CSF to complete their adjuvant or neoadjuvant chemotherapy (Table 3).
Furthermore, we observed that gBRCA1 breast cancer patients, but not ovarian cancer ones, were at risk for developing acute hematologic toxicities (supplementary Tables 2 and 3).
Therapy-related myeloid neoplasms
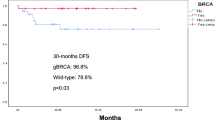
After a median follow-up of 8 years in breast cancer cohort and 5 years in ovarian cancer cohort, we observed 2 cases of therapy-related myeloid neoplasms: one gBRCA1 ovarian cancer patient who received chemotherapy and PARP inhibitor and one non-carrier breast cancer patient upon exposure to chemotherapy. The clinical and genomic characteristics of therapy-related myeloid neoplasms are described in Table 4.
Discussion
In the current study, we report that gBRCA1 carriers but not gBRCA2 carriers are at high risk of developing grade 3–4 neutropenia and are more likely to need secondary prophylaxis with G-CSF to complete their neoadjuvant or adjuvant chemotherapy. Increased risk of developing acute hematologic toxicities was observed only in breast cancer patients.
Our study was based on a biological hypothesis: we questioned whether the haploinsufficiency of the non-cancerous cells (here neutrophils) of women carrying gBRCA would induce greater sensitivity to DNA damage [13, 14]. This might be manifested by an increased incidence of acute hematologic toxicities upon exposure to myelosuppressive treatments such as chemotherapy.
Febrile neutropenia is a life-threatening consequence of chemotherapy. It increases mortality risk by 15% compared to patients with the same treatment. In our cohort of breast and ovarian cancer, 4.3% (19/447) of the patients developed febrile neutropenia, but this frequency increased to 8.6% among gBRCA1 carriers. Furthermore, we found that the majority (58.3%) of gBRCA1 carriers needed secondary prophylaxis with G-CSF to complete their adjuvant or neoadjuvant chemotherapy, while this was less the case for gBRCA2 carriers and non-carriers.
Recently, post-hoc subgroup analyses of several randomized trials addressed whether gBRCA carriers are at higher risk for acute hematologic toxicities. The largest study in breast cancer patients was reported from the German Breast Group, which pooled several randomized trials’ data. They included only patients with triple-negative breast cancer (n = 1’171), of whom 210 were gBRCA. They found that gBRCA carriers (84% were in fact gBRCA1) were at high risk for developing acute hematologic toxicities if they received taxanes [18]. One limitation of the GBG analyses is that almost 40% of the patients received primary G-CSF prophylaxis. Another post-hoc subgroup analysis in the randomized phase III trial BROCADE3 investigating the combination of PARP inhibitor veliparib with carboplatin/paclitaxel in advanced breast cancer stage among gBRCA carriers found that anemia and thrombocytopenia were more frequent among gBRCA1 than gBRCA2 carriers [26].
For ovarian cancer, two post-hoc subgroup analyses from randomized phase III trials evaluating platinum/taxane doublet therapy combined with PARP inhibitor veliparib were recently published [19, 20]. Both studies did not show any increase of hematologic toxicities among gBRCA carriers, compared to non-carriers in the chemotherapy arm or chemotherapy and PARPi combination arm [20]. These observations are consistent with our subgroup analysis ovarian vs. breast cancer, where only gBRCA1 breast cancer carriers were at risk for developing acute hematologic toxicities. This finding is intriguing since ovarian cancer patients receive platinum as frontline chemotherapy. A plausible explanation is that most breast cancer patients received 2 DNA damage agents: alkylating agent cyclophosphamide that induces DNA inter-strand crosslinks lesions, similarly to platinum [27], and a topoisomerase II inhibitor anthracycline.
Besides acute hematologic toxicity such as febrile neutropenia, it will be important to investigate whether gBRCA carriers are at higher risk for developing t-MN such as myelodysplastic syndrome and acute myeloid leukemia. tMN are rare but life-threatening events. With the recent approval of PARP inhibitors as frontline maintenance therapy in ovarian and breast cancer patients with gBRCA variants [28,29,30], this question becomes particularly important in these curable cancers [31, 32]. In breast cancer patients, the risk of t-MN is highest in older women who received anthracyclines-based chemotherapy [33, 34]. Few reports suggested an increased incidence of t-MN among gBRCA carriers [35, 36]. However, these reports were not case-control studies and were limited in their follow-up. Few case reports from the first trials investigating PARP inhibitors in ovarian cacer patients suggested that t-MN could be a delayed adverse event [22]. In the SOLO2 trial that included only ovarian cancer patients carrying gBRCA, t-MN occurred in 8% of patients receiving olaparib and 4% of those receiving placebo [21], raising concerns on the safety of long-term use of PARPi in gBRCA carriers. Consistently, a retrospective case-control analysis of ovarian cancer patients enrolled in the ARIEL2 and ARIEL3 trials suggested an increased incidence of tMN among patients carrying pathogenic variants in genes involved in homologous recombination pathway (BRCA1, BRCA2, RAD51C and RAD51D) [24]. A recent systematic review and safety meta-analysis of 28 randomized controlled trials comparing PARP inhibitors to placebo reported an increased risk (two to three-fold) for tMN in cancer patients treated with PARP inhibitors. Most cases (85%) were reported in ovarian cancer trials, likely due to the longest follow-up in completed trials in this disease (2–6 years). However, this meta-analysis did not find a significantly increased risk of t-MN among gBRCA carriers.
Genomic studies brought new insights into the pathogenesis of t-MN. They support a model where cytotoxic therapy does not directly induce tMN. Rather, clonal hematopoiesis precedes cancer therapy [37,38,39]. DNA damaging agents such as platinum and topo-isomerase II inhibitors preferentially select clones enriched in mutated DNA damage response genes (TP53, PPM1D and CHEK2)[38] that expand and transform into t-MN, and this holds true for PARP inhibitors [39]. Indeed, it was shown that clonal hematopoiesis preceded t-MN and expanded in ovarian cancer patients treated with PARP inhibitors [24]. These t-MN harbor, similarly to those arising with chemotherapy, pathogenic variants in DNA damage response genes and are characterized by complex karyotypes [39].
Our study has several limitations. It is a retrospective monocentric study with a limited number of patients diagnosed over 15 years. The hematological data collected on days 7–14 do not always reflect toxicity. All the patients included met the criteria for germline genetic screening and this population is, therefore, not representative of all patients with breast or ovarian cancer. Additionally, genetic testing techniques have recently changed from Sanger sequencing to next-generation sequencing. Patient records included in our study were not all located in the same establishment. Chemotherapy regimens were not homogeneous, as we included both breast and ovarian cancer patients. This variability in the chemotherapy regimen is an important bias for the ovarian occurrence of hematologic toxicity because it reduces the dose intensity.
Nevertheless, our observations were consistent with recent post-hoc subgroup analyses from randomized trials in breast and ovarian cancer patients. Further investigation of tMN occurrence is warranted with the recent approval of PARPi in frontline maintenance therapy in curable cancers. Biobanks of prospective and longitudinal samples collected during PARPi trials are unique resources to investigate whether exposure to PARPi shapes clonal hematopoiesis toward t-MN [24, 40], and whether this effect may be more frequent among gBRCA1 or gBRCA2 carriers.
Data availability
Data might be made available upon request and approval by Geneva ethics committee.
References
Roy R, Chun J, Powell SN (2012) BRCA1 and BRCA2: different roles in a common pathway of genome protection. Nat Rev Cancer 12(1):68–78
Mavaddat N, Peock S, Frost D, Ellis S, Platte R, Fineberg E et al (2013) Cancer risks for BRCA1 and BRCA2 mutation carriers: results from prospective analysis of EMBRACE. J Natl Cancer Inst 105(11):812–822
Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos-Blom MJ et al (2017) Risks of breast, ovarian, and contralateral breast Cancer for BRCA1 and BRCA2 mutation carriers. JAMA 317(23):2402–2416
Savage KI, Matchett KB, Barros EM, Cooper KM, Irwin GW, Gorski JJ et al (2014) BRCA1 deficiency exacerbates estrogen-induced DNA damage and genomic instability. Cancer Res 74(10):2773–2784
Sasanuma H, Tsuda M, Morimoto S, Saha LK, Rahman MM, Kiyooka Y et al (2018) BRCA1 ensures genome integrity by eliminating estrogen-induced pathological topoisomerase II-DNA complexes. Proc Natl Acad Sci U S A 115(45):E10642–E51
Song L, Tang Z, Peng C, Yang Y, Guo C, Wang D et al (2020) Cell type-specific genotoxicity in estrogen-exposed ovarian and fallopian epithelium. BMC Cancer 20(1):1020
Lyman GH, Abella E, Pettengell R (2014) Risk factors for febrile neutropenia among patients with cancer receiving chemotherapy: a systematic review. Crit Rev Oncol Hematol 90(3):190–199
NCI. Common Terminology Criteria for Adverse Events V4.0 2010 [Available from: https://evs.nci.nih.gov/ftp1/CTCAE/About.html
Pizzo PA (1993) Management of fever in patients with cancer and treatment-induced neutropenia. N Engl J Med 328(18):1323–1332
Aapro MS, Bohlius J, Cameron DA, Dal Lago L, Donnelly JP, Kearney N et al (2011) 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 47(1):8–32
Deshpande M, Paniza T, Jalloul N, Nanjangud G, Twarowski J, Koren A et al (2022) Error-prone repair of stalled replication forks drives mutagenesis and loss of heterozygosity in haploinsufficient BRCA1 cells.Mol Cell.
Maxwell KN, Wubbenhorst B, Wenz BM, De Sloover D, Pluta J, Emery L et al (2017) BRCA locus-specific loss of heterozygosity in germline BRCA1 and BRCA2 carriers. Nat Commun 8(1):319
Mgbemena VE, Signer RAJ, Wijayatunge R, Laxson T, Morrison SJ, Ross TS (2017) Distinct Brca1 mutations differentially reduce hematopoietic stem cell function. Cell Rep 18(4):947–960
Sedic M, Skibinski A, Brown N, Gallardo M, Mulligan P, Martinez P et al (2015) Haploinsufficiency for BRCA1 leads to cell-type-specific genomic instability and premature senescence. Nat Commun 6:7505
Pathania S, Bade S, Le Guillou M, Burke K, Reed R, Bowman-Colin C et al (2014) BRCA1 haploinsufficiency for replication stress suppression in primary cells. Nat Commun 5:5496
Konishi H, Mohseni M, Tamaki A, Garay JP, Croessmann S, Karnan S et al (2011) Mutation of a single allele of the cancer susceptibility gene BRCA1 leads to genomic instability in human breast epithelial cells. Proc Natl Acad Sci U S A 108(43):17773–17778
Friedlaender A, Vuilleumier A, Viassolo V, Ayme A, De Talhouet S, Combes JD et al (2019) BRCA1/BRCA2 germline mutations and chemotherapy-related hematological toxicity in breast cancer patients. Breast Cancer Res Treat 174(3):775–783
Furlanetto J, Mobus V, Schneeweiss A, Rhiem K, Tesch H, Blohmer JU et al (2021) Germline BRCA1/2 mutations and severe haematological toxicities in patients with breast cancer treated with neoadjuvant chemotherapy. Eur J Cancer 145:44–52
Gillen J, Miller A, Bell-McGuinn KM, Schilder RJ, Walker JL, Mathews CA et al (2021) Post hoc analyses of GOG 9923: does BRCA status affect toxicities?: an NRG oncology study. Gynecol Oncol 161(2):512–515
Aghajanian C, Swisher EM, Okamoto A, Steffensen KD, Bookman MA, Fleming GF et al (2022) Impact of veliparib, paclitaxel dosing regimen, and germline BRCA status on the primary treatment of serous ovarian cancer - an ancillary data analysis of the VELIA trial. Gynecol Oncol 164(2):278–287
Poveda A, Floquet A, Ledermann JA, Asher R, Penson RT, Oza AM et al (2021) Olaparib tablets as maintenance therapy in patients with platinum-sensitive relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a final analysis of a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 22(5):620–631
Morice PM, Leary A, Dolladille C, Chretien B, Poulain L, Gonzalez-Martin A et al (2021) Myelodysplastic syndrome and acute myeloid leukaemia in patients treated with PARP inhibitors: a safety meta-analysis of randomised controlled trials and a retrospective study of the WHO pharmacovigilance database. Lancet Haematol 8(2):e122–e34
Mirza MR, Benigno B, Dorum A, Mahner S, Bessette P, Barcelo IB et al (2020) Long-term safety in patients with recurrent ovarian cancer treated with niraparib versus placebo: results from the phase III ENGOT-OV16/NOVA trial. Gynecol Oncol 159(2):442–448
Kwan TT, Oza AM, Tinker AV, Ray-Coquard I, Oaknin A, Aghajanian C et al (2021) Preexisting TP53-Variant clonal hematopoiesis and risk of secondary myeloid neoplasms in patients with high-grade ovarian Cancer treated with Rucaparib. JAMA Oncol 7(12):1772–1781
Incorvaia L, Fiorino A, Gori S, Cinieri S, Curigliano G, Toss A et al (eds) (2022) Anthracycline-related cardiotoxicity in breast cancer patients carrying mutational signature of homologous recombination deficiency (HRD). ESMO annual meeting; ; Paris, France
Ayoub JP, Wildiers H, Friedlander M, Arun BK, Han HS, Puhalla S et al (2021) Safety and efficacy of veliparib plus carboplatin/paclitaxel in patients with HER2-negative metastatic or locally advanced breast cancer: subgroup analyses by germline BRCA1/2 mutations and hormone receptor status from the phase-3 BROCADE3 trial. Ther Adv Med Oncol 13:17588359211059601
Deans AJ, West SC (2011) DNA interstrand crosslink repair and cancer. Nat Rev Cancer 11(7):467–480
Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M et al (2018) Maintenance Olaparib in patients with newly diagnosed Advanced Ovarian Cancer. N Engl J Med 379(26):2495–2505
Tutt ANJ, Garber JE, Kaufman B, Viale G, Fumagalli D, Rastogi P et al (2021) Adjuvant olaparib for patients with BRCA1- or BRCA2-Mutated breast Cancer. N Engl J Med 384(25):2394–2405
Ray-Coquard I, Pautier P, Pignata S, Perol D, Gonzalez-Martin A, Berger R et al (2019) Olaparib plus Bevacizumab as First-Line maintenance in Ovarian Cancer. N Engl J Med 381(25):2416–2428
DiSilvestro P, Banerjee S, Colombo N, Scambia G, Kim BG, Oaknin A et al (2022) Overall survival with maintenance olaparib at a 7-Year Follow-Up in patients with newly diagnosed Advanced Ovarian Cancer and a BRCA mutation: the SOLO1/GOG 3004 Trial.J Clin Oncol. :JCO2201549
Geyer CE Jr, Garber JE, Gelber RD, Yothers G, Taboada M, Ross L et al (2022) Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high risk, early breast cancer.Ann Oncol.
Rosenstock AS, Niu J, Giordano SH, Zhao H, Wolff AC, Chavez-MacGregor M (2018) Acute myeloid leukemia and myelodysplastic syndrome after adjuvant chemotherapy: a population-based study among older breast cancer patients. Cancer 124(5):899–906
Freedman RA, Seisler DK, Foster JC, Sloan JA, Lafky JM, Kimmick GG et al (2017) Risk of acute myeloid leukemia and myelodysplastic syndrome among older women receiving anthracycline-based adjuvant chemotherapy for breast cancer on Modern Cooperative Group trials (Alliance A151511). Breast Cancer Res Treat 161(2):363–373
Iqbal J, Nussenzweig A, Lubinski J, Byrski T, Eisen A, Bordeleau L et al (2016) The incidence of leukaemia in women with BRCA1 and BRCA2 mutations: an international prospective cohort study. Br J Cancer 114(10):1160–1164
Churpek JE, Marquez R, Neistadt B, Claussen K, Lee MK, Churpek MM et al (2016) Inherited mutations in cancer susceptibility genes are common among survivors of breast cancer who develop therapy-related leukemia. Cancer 122(2):304–311
Wong TN, Ramsingh G, Young AL, Miller CA, Touma W, Welch JS et al (2015) Role of TP53 mutations in the origin and evolution of therapy-related acute myeloid leukaemia. Nature 518(7540):552–555
Bolton KL, Ptashkin RN, Gao T, Braunstein L, Devlin SM, Kelly D et al (2020) Cancer therapy shapes the fitness landscape of clonal hematopoiesis. Nat Genet 52(11):1219–1226
Martin JE, Khalife-Hachem S, Grinda T, Kfoury M, Garciaz S, Pasquier F et al (2021) Therapy-related myeloid neoplasms following treatment with PARP inhibitors: new molecular insights. Ann Oncol 32(8):1046–1048
Lin KK, Harrell MI, Oza AM, Oaknin A, Ray-Coquard I, Tinker AV et al (2019) BRCA reversion mutations in circulating tumor DNA predict primary and Acquired Resistance to the PARP inhibitor Rucaparib in High-Grade Ovarian Carcinoma. Cancer Discov 9(2):210–219
Acknowledgements
We thank all the patients who agreed to participate to this study. We thank Dr (A) Hugli, Dr M. Forni, Dr (B) Exquis, Dr (C) De Pree, Dr C. Irle, Dr L. Waelchli and Prof. A.-P. Sappino for providing clinical data.
Funding
Open access funding provided by University of Geneva
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hu-Heimgartner, K., Lang, N., Ayme, A. et al. Hematologic toxicities of chemotherapy in breast and ovarian cancer patients carrying BRCA1/BRCA2 germline pathogenic variants. A single center experience and review of the literature. Familial Cancer 22, 283–289 (2023). https://doi.org/10.1007/s10689-023-00331-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10689-023-00331-6