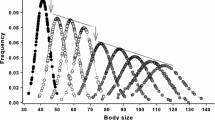
Abstract
Although evidence that reptiles exhibit indeterminate growth remains equivocal and based on inadequate data, the assumption that they do is still widely accepted as a general trait of reptiles. We examined patterns of variation in adult growth using long-term mark-recapture data on 13 populations of 9 species representing 3 families of freshwater turtles located in South Carolina, Michigan, and Arizona in the USA and in Ontario, Canada. Across 13 study populations, growth rates of all adults and only those that grew averaged 1.5 and 1.9 mm/yr respectively. Sources of variation in growth rates included species, population, sex, age, and latitude. Most adults of both sexes with recapture intervals greater than 10 years grew, but across all populations an average of 19 % of individuals did not grow (some with recapture intervals up to 30 years). For known-age adults of three species, the highest growth rates occurred during the 10 years following sexual maturity, and the proportions of non-growing individuals increased with age. Growth rates of adults were on average 92 % lower than those of juveniles. Based on linear relationships of clutch size and body size of females at average juvenile and adult growth rates it would take 0.7 (0.2–1.2) years and 8.6 (min–max = 2.3–18.5) years, respectively, to grow enough to increase clutch size by one egg. The majority of within population variation in adult body size in 3 species appeared to be a combination of differences in ages at maturity and juvenile and early adult growth, rather than indeterminate growth. The results from our study populations indicate that increases in body size (and associated reproductive output) that results from indeterminate growth are not substantial enough to represent a major factor in the evolution of life histories in general or the evolution of longevity and aging specifically.

Similar content being viewed by others
References
Andrews RM (1982) Patterns of growth in reptiles. In: Gans C, Pough FH (eds) Biology of the reptilia, vol 13. Cornell University, Ithaca
Baudisch A (2005) Hamilton’s indicators of the force of selection. Proc Nat Acad Sci USA 102:8263–8268
Bellairs A (1970) The Life of Reptiles. Volume II. The Universe Natural History Series. Universe Books, New York
Bjorndal KA (1980) Demography of the breeding population of the green turtle Chelonia mydas, at Tortuguero, Costa Rica. Copeia 3:525–530
Brooks RJ, Shilton CM, Brown GP et al (1992) Body size, age distribution, and reproduction in a northern population of Wood Turtles (Clemmys insculpta). Can J Zool 70:462–469
Buhlmann KA, Congdon JD, Gibbons JW et al (2009) Ecology of chicken turtles (Deirochelys reticularia) in a seasonal wetland ecosystem: exploiting resource and refuge environments. Herpetologica 65:39–53
Carr A, Goodman D (1970) Ecological implications of size and growth in Chelonia. Copeia 1970:783–786
Charnov EL, Berrigan D (1991) Evolution of life history parameters in animals with indeterminate growth, particularly fish. Evol Ecol 5:63–68
Charnov EL, Turner TF, Winemiller KO (2001) Reproductive constraints and the evolution of life histories with indeterminate growth. Proc Natl Acad Sci USA 98(16):9460–9464
Congdon JD, Gibbons JW (1985) Egg components and reproductive characteristics of turtles: relationships to body size. Herpetologica 41:194–205
Congdon JD, Gibbons JW (1990) The evolution of turtle life histories. In: Gibbons JW (ed) Life History and Ecology of the Slider Turtle. Smithsonian Institution Press, Washington, DC
Congdon JD, van Loben Sels RC (1991) Growth and body size in Blanding’s turtles (Emydoidea blandingii): relationships to reproduction. Can J Zool 69:239–245
Congdon JD, van Loben Sels RC (1993) Reproductive characteristics and body size: relationships with attainment of sexual maturity and age in Blanding’s turtles (Emydoidea blandingii). J Evol Biol 6:547–557
Congdon JD, Nagle RD, Kinney OM et al (2001) Hypotheses of aging in a long-lived vertebrate, Blanding’s turtle (Emydoidea blandingii). Exp Geront 36(2001):813–827
Congdon JD, Nagle RD, Kinney OM et al (2003) Testing hypotheses of aging in long-lived painted turtles (Chrysemys picta). Exp Gerontol 38:765–772
Dunham AE, Gibbons JW (1990) Growth of the slider turtle. In: Gibbons JW (ed) Life History and Ecology of the Slider Turtle. Smithsonian Institution Press, Washington, DC
Ernst CH, Barbour RW (1972) Turtles of the United States. University Press of Kentucky, Kentucky
Flower SS (1944) Persistent growth in the tortoise, Testudo graeca. Proc Zool Soc London 114:451–455
Frazer NB, Gibbons JW, Greene JL (1991a) Growth, survivorship and longevity of Painted Turtles Chrysemys picta in a southwestern Michigan marsh. Am Midl Nat 125:245–258
Frazer NB, Gibbons JW, Greene JL (1991b) Life history and demography of the common mud turtle Kinosternon subrubrum in South Carolina, USA. Ecology 72:2218–2231
Gibbons JW (1983) Reproductive characteristics and ecology of the mud turtle, Kinosternon subrubrum (Lacépède). Herpetologica 39:254–271
Gibbons JW (1990) Turtle studies at SREL: a research perspective. In: Gibbons JW (ed) Life History and Ecology of the Slider Turtle. Smithsonian Institution Press, Washington, DC
Goode J (1967) Freshwater Tortoises of Australia and New Guinea (in the Family Chelidae). Lansdowne Press, Melbourne
Halliday TR, Verrell PA (1988) Body size and age in amphibians and reptiles. J Herpetol 22:253–265
Hamilton WD (1966) The moulding of senescence by natural selection. J. Theoret Biol 12:12–45
Heatwole H (1976) Reptile Ecology. University of Queensland Press, Brisbane
Heino M, Kaitala V (1999) Evolution of resource allocation between growth and reproduction in animals with indeterminate growth. J Evol Biol 12:423–429
Jokela J (1997) Optimal energy allocation tactics and indeterminate growth: life-history evolution of long-lived bivalves. In: Streit B, Stadler T, Lively CM (eds) Evolutionary Ecology of Freshwater Animals: Concepts and Case Studies. Birkhauser Verlag Basel, Switzerland
Karkach AS (2006) Trajectories and models of individual growth. Demog Res 15:347–400. doi:10.4054/DemRes.2006.15.12
Kingsolver JG, Diamond SE (2011) Phenotypic selection in natural populations: what limits directional selection? Am Nat 177:346–357
Kingsolver JG, Pfennig DW (2004) Individual-level selection as a cause of Cope’s rule of phyletic size increase. Evolution 58:1608–1612
Kirkpatrick M (1984) Demographic models based on size, not age, for organisms with indeterminate growth. Ecology 65:1874–1884
Kirkwood T, Rose M (1991) Evolution of senescence: late survival sacrificed for reproduction. Philosophical Trans Biol Sci 332:15–24
Kozlowski J (1996) Optimal allocation of resources explains interspecific life-history patterns in animals with indeterminate growth. Proc Biol Sci 263:559–566
Legler JM (1960) Natural history of the ornate box turtle, Terrapene ornata ornata Agassiz. Univ Kansas Publ Mus Nat Hist 11:527–669
Lika K, Kooijman SALM (2003) Life history implications of allocation to growth versus reproduction in dynamic energy budgets. Bull Math Biol 65:809–834
Lincoln RJ, Boxshall GA, Clark PF (1982) A dictionary of ecology, evolution and systematics. Cambridge University Press, Cambridge
Lovich JE, Ernst CH, McBreen JF (1990) Growth, maturity, and sexual dimorphism in the wood turtle, Clemmys insculpta. Can J Zool 68:672–677
Lovich JE, Ernst CH, Herman DW (1998) Geographic variation in growth and sexual size dimorphism of bog turtles (Clemmys muhlenbergii). Am Midl Nat 139:69–78
Medawar P (1952) An Unsolved Problem of Biology. HK Lewis, London
Oliver JA (1955) The natural history of North American amphibians and reptiles. D. Van Nostrand Company, Inc, Princeton, NJ
Olsson M, Shine R (1996) Does reproductive success increase with age or with size in species with indeterminate growth? A case study using sand lizards (Lacerta agilis). Oecologia 105:175–178
Pappas MJ, Brecke BJ, Congdon JD (2000) The Blanding’s turtle of Weaver Dunes, Minnesota. Chelon Conserv Biol 3:557–568
Plummer MV (1977) Reproduction and growth in the turtle Trionyx muticus. Copeia 1977:440–447
Pough HF, Andrews RM, Cadle JE, Crump ML, Savitzky AH, Wells KD (2003) Herpetology, 3rd edn. Prentice Hall, Upper Saddle River, NJ
Samson J (2003) The life history strategy of a northern population of midland painted turtles, Chrysemys picta marginata. M.Sc. thesis, Department of Zoology, University of Guelph, ON, Canada
Sebens KP (1987) The ecology of indeterminate growth in animals. Ann Rev Ecol Syst 18:371–407
Sparkman AS, Arnold J, Bronikowski AM (2007) An empirical test of evolutionary theories for reproductive senescence and reproductive effort in the garter snake Thamnophis elegans. Proc R Soc Lond B 274:943–950
Stearns SC (1992) The Evolution of Life Histories. Oxford University Press, Oxford
Tinkle DW (1979) Long-term field studies. Bioscience 29:717
Vitt LJ, Caldwell JP (2009) Herpetology: an introductory biology of amphibians and reptiles. Academic Press, Burlington, MA
Williams GC (1957) Pleiotropy natural selection evolution of senescence. Evol 11:398–411
Williams GC (1966) Adaptation and Natural Selection. Princeton University Press, Princeton
Woodward HN, Horner JR, Farlow JO (2011) Osteohistological evidence for determinate growth in the American alligator. J Herpetol 45:339–342
Zug GR, Vitt LJ, Caldwell JL (2001) Herpetology: an Introductory Biology of Amphibians and Reptiles, 2nd edn. Academic Press, San Diego
Acknowledgments
Roy Nagle and Owen Kinney, Richard van Loben Sels, and Todd Quinter provided many years of field assistance and continuity to the ESGR study. Judy Greene, Peggy Burkman, and Ruth Estes provided invaluable assistance with managing the extensive data sets from SREL and the ESGR. We thank Tony Tucker, Judy Greene, and Mike Dorcas for providing data on Malaclemys terrapin. R. G. Farmer provided assistance with analyses of growth rates. National Science Foundation grants supported some of the long-term research conducted by J.W.G. (DEB-79-04758) and J.D.C. (DEB-74-070631, DEB-79-06301, BSR-84-00861 and BSR-90-19771). The last half of the E. S. George Reserve study was primarily funded by J. Congdon and N. Dickson. Manuscript preparation was aided by the Environmental Remediation Sciences Division of the Office of Biological and Environmental Research, U.S. Department of Energy through the Financial Assistant Award no. DE-FC09-96SR18546 to the University of Georgia Research Foundation. Previous drafts of the manuscript were improved by comments from N. Dickson, M. Pappas, and L Vitt.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Congdon, J.D., Gibbons, J.W., Brooks, R.J. et al. Indeterminate growth in long-lived freshwater turtles as a component of individual fitness. Evol Ecol 27, 445–459 (2013). https://doi.org/10.1007/s10682-012-9595-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-012-9595-x