Abstract
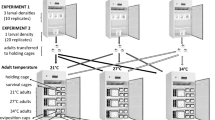
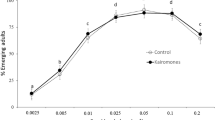
The evolutionary maintenance of inducible defences is governed by the costs and benefits of the defensive traits. The defensive traits should increase the chances of survival in the presence of predators, but be costly in their absence. The costs and benefits of inducible defensive traits can be influenced by environmental conditions, which subsequently affect the ability of prey to induce those defensive traits. We examine how temperature affects the costs of behavioural defences in larval mosquitoes, Aedes notoscriptus, which reduce activity in the presence of predator cues to limit detectability. The costs of reducing activity could either be exacerbated at warmer temperatures via increased metabolic demand, or ameliorated at warmer temperatures via accelerated development reducing exposure time. We compared life history traits of A. notoscriptus reared in control conditions to those exposed to predation cues as larvae at 18, 23 and 28 °C. Larvae reared in predation cues reduced activity, grew and developed slower and emerged later and smaller. While the reduction in activity increased with temperature, the negative effects on life history of A. notoscriptus were greatest at the coolest temperature. Our results show that the costs of inducible defences in A. notoscriptus are temperature dependent. This work suggests that variation in the thermal environment may have a strong influence on the dynamics of predator–prey interactions and the evolutionary maintenance of plasticity of defensive traits in natural populations.





Similar content being viewed by others
References
Alton LA, Wilson RS, Franklin CE (2010) Risk of predation enhances the lethal effects of UV-B in amphibians. Glob Change Biol 16:538–545
Anholt BR, Werner EE (1998) Predictable changes in predation mortality as a consequence of changes in food availability and predation risk. Evol Ecol 12:729–738
Armbruster P, Hutchinson RA (2002) Pupal mass and wing length as indicators of fecundity in Aedes albopictus and Aedes geniculatus (Diptera: Culicidae). J Med Entomol 39:699–704
Atkinson D (1996) Ectotherm life history responses to developmental temperature. In: Johnston IA, Bennett AF (eds) Animals and temperature: phenotypic and evolutionary adaptation. Cambridge University Press, Cambridge, pp 183–204
Barry MJ (1999) The effects of a pesticide on inducible phenotypic plasticity in Daphnia. Environ Pollut 104:217–224
Beketov MA, Liess M (2007) Predation risk perception and food scarcity induce alterations of life-cycle traits of the mosquito Culex pipiens. Ecol Entomol 32:405–410
Beklioglu M, Akkas SB, Ozcan HE, Bezirci G, Togan I (2010) Effects of 4-nonylphenol, fish predation and food availability on survival and life history traits of Daphnia magna straus. Ecotoxicology 19:901–910
Briegel H (1990) Metabolic relationship between female body size, reserves, and fecundity of Aedes aegypti. J Insect Physiol 36:165–172
Callahan HS, Maughan H, Steiner UK (2008) Phenotypic plasticity, costs of phenotypes, and costs of plasticity: toward an integrative view. Ann NY Acad Sci 1133:44–66
Crawley MJ (2007) The R book. Wiley, West Sussex
DeWitt TJ (1998) Costs and limits of phenotypic plasticity: tests with predator-induced morphology and life history in a freshwater snail. J Evol Biol 11:465–480
DeWitt TJ, Scheiner SM (2004) Phenotypic plasticity: functional and conceptual approaches. Oxford University Press, Oxford
Ferrari M, Messier F, Chivers D (2007) Variable predation risk and the dynamic nature of mosquito antipredator responses to chemical alarm cues. Chemoecology 17:223–229
Fraker ME (2008) The effect of hunger on the strength and duration of the antipredator behavioural response of green frog (Rana clamitans) tadpoles. Behav Ecol Sociobiol 62:1202–1205
Gillooly J, Brown J, West G, Savage V, Charnov E (2001) Effects of size and temperature on metabolic rate. Science 293:2248–2251
Gillooly J, Charnov E, West G, Savage V, Brown J (2002) Effects of size and temperature on development time. Science 417:70–73
Hammill E, Rogers A, Beckerman AP (2008) Costs, benefits and the evolution of inducible defences: a case study with Daphnia pulex. J Evol Biol 21:705–715
Havel JE, Dodson SI (1984) Chaoborus predation on typical and spined morphs of Daphnia pulex: behavioural observations. Limnol Oceanogr 29:487–494
Hazlett BA (2003) The effects of starvation on crayfish responses to alarm odour. Ethology 109:587–592
Hechtel LJ, Juliano SA (1997) Effects of a predator on prey metamorphosis: plastic responses by prey or selective mortality? Ecology 78:838–851
Horat P, Semlitsch RD (1994) Effects of predation risk and hunger on the behaviour of two species of tadpoles. Behav Ecol Sociobiol 34:393–401
Houston AI, McNamara JM, Hutchinson JMC (1993) General results concerning the trade-off between gaining energy and avoiding predation. Philos Trans R Soc 341:375–397
Juliano SA, Reminger L (1992) The relationship between vulnerability to predation and behavior of larval treehole mosquitos: geographic and ontogenic differences. Oikos 63:465–476
Kesavaraju B, Juliano SA (2004) Differential behavioral responses to water-borne cues to predation in two container-dwelling mosquitoes. Ann Entomol Soc 97:194–201
Kishida O, Trussell GC, Nishimura K (2007) Geographic variation in a predator-induced defense and its genetic basis. Ecology 88:1948–1954
Kraft P, Wilson RS, Franklin CE (2005) Phenotypic plasticity as a defence strategy in tadpoles of Limnodynastes peronii: induction cues, costs and benefits. Austral Ecol 30:558–563
Laurila A, Pakkasmaa S, Merila J (2006) Population divergence in growth rate and antipredator defences in Rana arvalis. Oecologia 147:585–595
Laurila A, Lindgren B, Laugen AT (2008) Antipredator defenses along a latitudinal gradient in Rana temporaria. Ecology 89:1399–1413
Lima SL (1998) Non-lethal effects in the ecology of predator-prey interactions: what are the ecological effects of anti-predator decision-making? Bioscience 48:25–34
Merila J, Laurila A, Pahkala M, Rasanen K, Laugen AT (2000) Adaptive phenotypic plasticity in timing of metamorphosis in the common frog Rana temporaria. Ecoscience 7:18–24
Nilsson PA, Bronmark C, Pettersson LB (1995) Benefits of a predator-induced morphology in crucian carp. Oecologia 104:291–296
Pestana JLT, Loureiro S, Baird DJ, Soares AMVM (2010) Pesticide exposure and inducible antipredator responses in the zooplankton grazer, Daphnia magna straus. Chemosphere 78:241–248
Ponlawat A, Harrington LC (2007) Age and body size influence male sperm capacity of the dengue vector Aedes aegypti (Diptera: Culicidae). J Med Entomol 44:422–426
R Development Core Team (2008) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-Q, URL: http://www.R-project.org
Relyea RA, Mills N (2001) Predator-induced stress makes the pesticide carbaryl more deadly to gray treefrog tadpoles (Hyla versicolor). Proc Natl Acad Sci USA 98:2491–2496
Savage V, Gillooly J, Brown J, West G, Charnov E (2004) Effects of body size and temperature on population growth. Am Nat 163:429–441
Shoeppner NM, Relyea RA (2005) Damage, digestion, and defence: the roles of alarm cues and kairomones for inducing prey defences. Ecol Lett 8:505–512
Sih A (1992) Prey uncertainty and the balancing of antipredator and feeding needs. Am Nat 139:1052–1069
Skelly DK, Werner EE (1990) Behavioral and life-historical responses of larval American toads to an odonate predator. Ecology 71:2313–2322
Smith-Gill SJ, Berven KA (1979) Predicting amphibian metamorphosis. Am Nat 113:563–585
Steiner UK, Van Buskirk J (2009) Predator-induced changes in metabolism cannot explain the growth/predation risk tradeoff. PLoS One 4:e6160
Stoks R, De Block M, Van De Meutter F, Johansson F (2005a) Predation cost of rapid growth: behavioural coupling and physiological decoupling. J Anim Ecol 74:708–715
Stoks R, De Block M, McPeek MA (2005b) Alternative growth and energy storage responses to mortality threats in damselflies. Ecol Lett 8:1307–1316
Tollrian R, Harvell CD (1999) The ecology and evolution of inducible defenses: current ideas. In: Tollrian T, Harvell CD (eds) The ecology and evolution of inducible defenses. Princeton University Press, Princeton, pp p306–p321
Turner AM (1996) Freshwater snails alter habitat use in response to predation. Anim Behav 51:747–756
Turner AM (1997) Contrasting short-term and long-term effects of predation risk on consumer habitat use and resources. Behav Ecol 8:120–125
van Uitregt VO, Hurst TP, Wilson RS (2012) Reduced size and starvation resistance in adult mosquitoes, Aedes notoscriptus, exposed to predation cues as larvae. J Anim Ecol 81:108–115
Weetman D, Atkinson D (2002) Antipredator reaction norms for life history traits in Daphnia pulex: dependence on temperature and food. Oikos 98:299–307
Werner EE, Anholt BR (1993) Ecological consequences of the trade-off between growth and mortality-rates mediated by foraging activity. Am Nat 142:242–272
Wilson RS, Kraft PG, Van Damme R (2005) Predator-specific changes in morphology and swimming performance of anuran larvae. Funct Ecol 19:238–244
Acknowledgments
We thank Leslie Alton, Ben Barth, Candice Bywater and Sean Fitzgibbon for assistance in the laboratory and Kay Marshall for assistance with colonisation of mosquitoes. All experiments were done in accordance with the guidelines of The University of Queensland Animal Ethics and Welfare Committee (Permit number: SBS/287/09/ARC) and Medical Research Ethics committee (Approval number: 2009001078). Fish were collected in accordance with the Queensland Government Fisheries Act 1994 (Permit number: 95992). This project was partially funded by a research grant from the Ecological Society of Australia. VU was supported by Australian Research Council Linkage Grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van Uitregt, V.O., Hurst, T.P. & Wilson, R.S. Greater costs of inducible behavioural defences at cooler temperatures in larvae of the mosquito, Aedes notoscriptus . Evol Ecol 27, 13–26 (2013). https://doi.org/10.1007/s10682-012-9576-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-012-9576-0