Abstract
Sex ratio theory has been very successful in predicting under which circumstances parents should bias their investment towards a particular offspring sex. However, most examples of adaptive sex ratio bias come from species with well-defined mating systems and sex determining mechanisms, while in many other groups there is still an on-going debate about the adaptive nature of sex allocation. Here we study the sex allocation in the mealybug Planococcus citri, a species in which it is currently unclear how females adjust their sex ratio, even though experiments have shown support for facultative sex ratio adjustment. Previous work has shown that the sex ratio females produce changes over the oviposition period, with males being overproduced early and late in the laying sequence. Here we investigate this complex pattern further, examining both the robustness of the pattern and possible explanations for it. We first show that this sex allocation behaviour is indeed consistent across lines from three geographical regions. Second, we test whether females produce sons first in order to synchronize reproductive maturation of her offspring, although our data provide little evidence for this adaptive explanation. Finally we test the age at which females are able to mate successfully and show that females are able to mate and store sperm before adult eclosion. Whilst early-male production may still function in promoting protandry in mealybugs, we discuss whether mechanistic constraints limit how female allocate sex across their lifetime.
Similar content being viewed by others
Introduction
The allocation of resources between male and female offspring constitutes an important life history decision for sexually reproducing organisms (West 2009). Under many circumstances equal investment in both offspring sexes is expected (Fisher 1930), and this is reflected in the appearance of genetic sex determining systems that lead to equal sex ratios (Bull 1983; Uller et al. 2007). However, there are numerous circumstances under which individuals are predicted, and have been observed, to bias their sex ratio towards a particular offspring sex (West 2009; West et al. 2000; West and Sheldon 2002). This is because parents are selected to bias the sex ratio of their offspring if one offspring sex is expected to have a higher reproductive value than the other sex (Hamilton 1967; Trivers and Willard 1973; West 2009). The differences in reproductive value of offspring that drive the evolution of adaptive sex allocation can be caused by a variety of factors. For example when offspring compete amongst each other, the offspring sex that suffers most from this competition will have a lower reproductive value (Clark 1978; Hamilton 1967). Alternatively, the condition of parents can affect the reproductive value of their offspring sexes differently if one sex suffers more from reduced investment by the parents (Trivers and Willard 1973). These factors can drive selection on parents to adjust their sex ratio to local conditions.
Utilising this well-developed theory base, sex allocation studies have provided much evidence of adaptive behaviour, especially in organisms with well-defined mating systems and mechanisms of sex determination, in particular the Hymenoptera (including fig wasps, parasitoid wasps and the social Hymenoptera: Bourke and Franks 1995; Godfray 1994; King 1993; West 2009; West et al. 2000). However, outside these groups, there is still an on-going debate over the adaptive nature of the observed patterns of sex allocation (West and Sheldon 2002; West et al. 2005). This is particularly true for systems where the mechanisms of sex determination and especially adaptive sex allocation are unknown (including mammals and birds, as well as many insects: Cockburn et al. 2002; Pike and Petrie 2003; West 2009). As such, when patterns fit with theoretical expectations, the limitations of our understanding of mechanism may be partly put to one side; however, when sex ratio patterns are complex or non-intuitive, the need for a more integrated understanding of sex allocation, putting the phenotype into the context of a species’ biology and mechanism of sex determination, becomes more obvious (West 2009). Only then can adaptive explanations be more fully tested, and the role of mechanistic constraints (for instance in terms of the role of the genetic system, or information-processing: West et al. 2005; Shuker and West 2004) be more fully understood.
In this paper we are interested in trying to explain the pattern of sex allocation observed in a species with an unusual type of sex determination in which the mechanism of facultative sex allocation is unknown. The mealybug Planococcus citri exhibits paternal genome elimination (PGE), where although both sexes develop from fertilized eggs and are diploid, males do not transmit their father’s chromosomes (Brown and Nelson-Rees 1961; Schrader 1921). Therefore, the method by which female mealybugs adjust their sex ratio is different from that used by females of haplodiploid species (the genetic system found in many species with adaptive sex allocation, such as the Hymenoptera), who can simply control egg fertilisation (Bull 1983). Despite this, there is evidence for facultative changes in sex allocation. For instance, we have recently tested predictions from local resource competition (LRC) and Trivers-Willard sex allocation theory (Ross et al. 2010a, 2011). To summarise this work, experiments manipulating rearing density and the likelihood of competition amongst related female offspring (i.e. local resource competition among daughters) did not produce the shift towards male-biased sex ratios predicted by LRC theory (Ross et al. 2010a). Instead, more male-biased sex ratios were produced when global rather than local (among kin) competition was increased, suggesting that female offspring suffer more from high population density, becoming of less reproductive value to mothers. On the other hand, when maternal condition was experimentally manipulated (through age at mating or food restriction), mothers in lower condition produced more female-biased sex ratios, perhaps because daughters are less reliant on maternal resources than sons (Ross et al. 2011).
These experiments suggested that sex allocation is influenced by more than one type of sex ratio selection (Ross et al. 2011; see also Wild and West 2007 and Pryke et al. 2011 for further examples and discussion). However, these experiments have also shown a noticeable change in sex allocation across the oviposition period, with male biased sex ratios being produced early and late in the oviposition period, although the (historical) data are more equivocal (Nelson-Rees 1960; Ross et al. 2010a; Varndell and Godfray 1996). Our work has also suggested that this temporal variation can give the impression of adaptive allocation (Ross et al. 2011). We therefore would like to investigate why females change their sex ratio during the oviposition period and how this affects their ability to facultatively change their sex ratio. Given that we know rather little about how sex is determined or allocated, here we test whether these temporal patterns are (1) consistent across a sample of geographic populations, increasing our confidence in their robustness; and (2) associated with male and female development such that reproductive maturity of the sexes is synchronised.
We present the results from two experiments. The first experiment explores variation in sex allocation patterns between lines from three geographic regions to see if the pattern of sex ratio change over the oviposition period is consistent. In the second experiment, we consider whether early male production is associated with when males and females become reproductively mature. If males and females take different lengths of time to mature to adulthood, then synchronising male and female maturity may involve producing the longer-developing sex first (in this case males). This may be particularly important in species such as P. citri where mating can occur amongst kin, so that one’s offspring have mating partners. Alternatively, early male production may be associated with so-called protandry, where males mature first enhancing their competitive access to mates (Thornhill and Alcock 1983; Morbey and Ydenberg 2001). We compared the age at which males and females are able to mate successfully, predicting that males are produced earlier in order to either synchronise reproductive maturity or promote protandry.
Methods
Study organism
The citrus mealybug P. citri (Hemiptera: Coccoidea) is a cosmopolitan species that feeds on wide range of host plants and causes economic damage to both crops and ornamental plants (Ben-Dov et al. 2011). P. citri reproduces sexually and there is a strong dimorphism between the sexes both in life history and behaviour (Ross et al. 2010a). While the sexes are indistinguishable as nymphs, males undergo a form of metamorphosis after the second instar and adult males are winged, while the females do not undergo metamorphosis and grow much larger than the males (Sutherland 1932). Additionally, males do not feed after their second instar and adult males lack functional mouthparts, while females continue feeding until they die. This results in a large difference in lifespan between the sexes, with males only living up to 3 days after eclosion while females can live several weeks after becoming reproductively mature (Nelson-Rees 1960). Adult females are almost completely sedentary and the highly mobile crawlers (first instar nymphs) are assumed to be the main agent of dispersal (Gullan and Kosztarab 1997).
Lines used
All specimens used were cultured on sprouting potatoes (cultivar Desiree) in plastic food store boxes covered with fine mesh. For both experiments we used mealybugs from three different geographical areas: Portugal (collected 3 months before the start of our experiments from 3 different citrus orchards), Israel (long-term lab population) and the UK (long-term lab population). We created 6 isofemale lines for each area by allowing a single egg-laying female (P) to reproduce for three days. So each line consisted of siblings, although there is a possibility that some females might have mated with multiple males. 15 lines produced life offspring (F1), although three lines were lost before the start of the second experiment (see Table 1).
We allowed free mating among our F1. On the first day of ovipositing, we isolated the newly matured female in a test tube. Eggs (F2) were removed and preserved each day from the start of ovipositing until the end (experiment 1) to test for variation between the lines in sex allocation behaviour.
For the second experiment, we took three mated females from the original isofemale lines (F1) and allowed them to lay eggs (F2). We isolated males as soon as they made their pupae to ensure virgin females, and we isolated the females as soon we they could be safely moved. Isolated males from same or different isolines respectively were later used to test mating age in the females.
Experiment 1: Primary sex ratios
The goal of this experiment was to partition variation in sex ratio within and between lines and to test if the pattern with which females adjust their sex ratio over time is consistent across lines. To this end, the primary sex ratio was measured for the entire oviposition period of approximately 10 females from 15 isofemale lines from three geographic locations. In order to obtain virgin females for mating, 3rd instar females were isolated from their isofemale line boxes 20–25 days after hatching, and males were also isolated at this time. After isolation, females were kept in large glass tubes and provided with a potato sprout, while males were kept in small tubes without food (as they do not feed after their second instar). Ideally, 10 females and 15 males per isofemale line were separated, but due to low male and female availability in some lines, 1–9 females per line (see Table 1) and 3–17 males per line were separated.
When the males reached maturity, they were added to the tube of a female of the same isofemale line (their full-sib) in order to inseminate her. Successfully mated (egg-laying) females were used for the rest of the experiment (see Table 1). Unfortunately the methods used were not sufficient to ensure female virginity of all females as females were found able to mate very prematurely (see experiment 2) and therefore were already fertilized before they were isolated. However as these females were still mated by a full-sib we chose to include them in the analysis and corrected for the possible effect of early mating by including mating age as a covariate in the analysis.
After females were mated they were checked daily for signs of oviposition. Once females commenced oviposition, eggs were collected daily from the start of oviposition until the female died. Collected eggs were fixed in Carnoys fluid (4:3:1, Chloroform:Ethanol:Glacial acetic acid) for four days and subsequently stored in 90% ethanol. Primary sex ratios (presented throughout as proportion of offspring that are male) from oviposition days 1, 3, 6, 9 and 12 were later counted under a fluorescent microscope by staining the egg DNA with DAPI dissolved in phosphate buffered saline (PBS) (ratio: 1:1,000). This way, male eggs can easily be identified by their heterochromatized chromosomes (see Ross et al. 2010a for more details on staining methods).
Experiment 2: Development time
An important goal of this study was to see if the sexual dichronism (i.e. males being produced first) could be an adaptation to the difference in development time between the sexes. In order to test this we first of all wanted to see if males do indeed take longer to develop. However just comparing development times of males and females might not be sufficient as earlier studies have suggested that females might be able to mate prematurely and store sperm. Therefore in this experiment we compared the minimum age at which females can be successfully fertilized with male eclosion times. In order to do so we measured the development times and mortality rates of males and females from different isofemale lines to determine variation within and between lines. We also considered how development time and mortality of males and females was influenced by the density and sex ratio of the population in which they were raised. Twelve of the isofemale lines from experiment 1 were used in this experiment (see Table 1 for the lines used and sample sizes) and the experiment was performed in two blocks, which was controlled for in the analysis. For each line, three boxes were set up containing three randomly selected, egg-laying founding females without their clutch. Females were allowed to oviposit for exactly one day and were then removed, leaving their clutch behind. This way the exact age of all offspring is known and all males and females uses in the experiment were the same age. Boxes were checked every day so males could be isolated into glass tubes on the very day they started spinning their cocoon. Females were isolated when the first male in their box pupated. All males and females from a box were counted in order to gain secondary sex ratio and density data.
For males, the following data were collected: age at start of pupation and age at reproductive maturity (defined as one day after emergence from the pupa). For females, we collected data on mating age (ability to mate successfully, obtained by mating random females of the same line over a range of ten days and determining a posteriori if the mating was successful (eggs) or not (no eggs)) and age at the start of oviposition (for females that were mated successfully). Females were preferentially mated to males from their own line but when these where not available they were mated to a randomly selected male from a different line.
Data analysis
Data analysis was performed using R (R Development Core Team 2010). All data were analysed using general and generalized linear mixed effects models. For the analysis of general mixed effect models the R package nlme (Pinheiro et al. 2007) was used. For the analysis of generalized mixed effect models (GLMM) we used the package MCMCglmm (Hadfield 2010a): this package analyses mixed models in a Bayesian framework while allowing a range of possible error-structures.
The data on sex ratio, mortality and mating success were analyzed using a GLMM with quasi-binomial error structure, to avoid effects of over-dispersion. Data on development times for both sexes were analysed using a GLM with a normal error structure (and log transformation to normalize the residuals if necessary). In order to avoid pseudo-replication, the sex ratio data were analyzed with Female ID and line as random effects and country of origin as a fixed effect. All continuous covariates included in the analyses were centered using the scale function in R. In all analyses of the second experiment line, box and tube (for female development) were fitted as random effects (with box and tube nested in line) and experimental block as a fixed effect. For the analysis of female mortality and mating success we used the number of surviving/successfully mated female per tube and used a binomial error structure, while for male mortality we also fitted a binomial model using the proportion of surviving males per box. For the Bayesian analysis using MCMCglmm, each model was run for a million iterations with a burn-in time of 200,000. Flat non-informative parameter expanded priors with a uniform low degree of belief across all parameters values were used for the random effects (Hadfield 2010b). We compared the model outcomes using different degree of belief parameters and the resulting models gave qualitatively (and quantitatively) similar answers Additionally, for each model in MCMCglmm, convergence of the chain was tested by using an autocorrelation statistic. For the fixed effects estimated using MCMCglmm we report the probability that the estimate is larger than zero and refer to this as pMCMC (this can be interpreted as a Bayesian equivalent of a p value (Hadfield 2010b)). For the random effects, we report the percentage of variance explain by each factor. For the random effects estimated with MCMCglmm we also give the 95% credibility interval for these estimates while for the analysis using LME we test the significance of the random effects by comparing models with and without the random effect with a likelihood test. A wide credibility interval (including a very small lower bound, less than 0.001 for instance) suggests limited or low power to estimate the random effect; to put in analogous frequentist terms, this can be interpreted as not significantly different to zero.
Results
Experiment 1: Sex ratio variation
Oviposition day had a strong effect on sex ratio (Fig. 1b, c, d) and the relationship was polynomial rather than linear as both the linear and quadratic effects of day were significant (see Table 2). When a female started ovipositing, she laid a highly male biased clutch (day 1: 0.85), which quickly decreased (day 3: 0.54) towards a very female bias (day 6: 0.23, day 9: 0.28). Around the tenth day after ovipositing, sex ratios started becoming less female biased again (day12: 0.32, day15: 0.47) (see Fig. 1b, c, d). In order to test if this pattern was consistent across the lines, we estimated how much of the variation in sex ratio can be explained by line effects (Fig. 1a). We estimated this in two different ways (see methods). A simple mixed model with arcsin square root transformation estimates that line explains 4.88% of the variation in sex ratio and this effect was marginally significant (LR6,7 = 3.93, p = 0.048). However the estimate of line effect with a Bayesian GLMM gives an estimation of 0.19% (CI 95%: 0.00096–22.15%) of the total variance, suggesting the variance explained was not significantly different to zero. So although there is some suggestion that the total sex ratio produced differed slightly between lines (Fig. 1), the pattern with which sex ratio changes over time is similar between the lines (see Fig. 1b–d), with in all cases males being produced first. We also tested for differences in sex ratio between the countries of origin and found that there are no significantly differences in sex allocation (Portugal: 0.45 ± 0.0098 s.e., Israel: 0.51 ± 0.0094 s.e., UK: 0.57 ± 0.0098, difference Portugal-Israel: pMCMC = 0.384, difference Portugal-UK: pMCMC = 0.254, see Table 2).
One factor that did affect the pattern of sex allocation however was the day on which females were mated. There was a significant interaction between mating day and oviposition day (pMCMC = 0.012, Table 2), with females that were mated later changing sex ratios throughout their oviposition period at a steeper slope than females that were mated early. Mating day did not change the total sex ratio produced however (pMCMC = 0.106, Table 2) and neither did it change the overproduction of male offspring at the beginning and end of the oviposition period.
Experiment 2: Development time and mortality
We first present results exploring male development and then female development, before evaluating the potential for the synchronisation of male and female reproductive maturity or protandry.
Male development
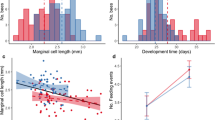
Total male development time—the number of days from hatching until reproductive maturity—did not differ between lines (LR8,7 = 0.35, p = 0.554, Fig. 2a) and explains only 4.55% of the variance. Male development was instead influenced by environmental factors. Density in the box the males were raised in influenced their development time, with males developing faster at higher densities (F1,20 = 18.79, p = 0.0003, Fig. 2b). In addition, rearing box explained a significant proportion of the variance in development time (box: 14.08% of the total variance, LR10,9 = 16.42, p < 0.0001) and males from the two experimental blocks also differed in their development time (F1,20 = 9.98, p = 0.005, although the difference was small: block 1: 25.0 days, block 2: 24.9). Overall, the average time taken for males to reach adulthood was 25.0 days (±2.0 SD), with a minimum time of 21 days and a maximum of 31 days (Table 3).
a Male development times per line. The blue bars shows the average time (in days) between hatching and pupation, while the purple bars shows the average time (in days) between pupation and eclosion. Error bars show the standard error. b The relationship between population density and male development time. Plot shows the development times of the males (circles show the time between hatching and pupation, while the triangles show the time between pupation and eclosion) plotted against the density of the box they were raised in. (Color figure online)
Male mortality during pupation was low, around 6%, and line only explained 0.59% of the variance, with a very large credibility interval (CI 95%: 0.0004–86.81%). Male mortality was also not influenced by environmental factors experienced in the rearing box (for density, sex ratio and their interaction, all pMCMC > 0.2) and the difference in male mortality between the two experimental blocks was not significant (pMCMC = 0.120, 8% for the first block and 4% for the second).
Female development and age at mating
We analysed female development time as the time between hatching and the start of oviposition. Total development time was 33.69 days (±3.60 SD). Female development did not differ between lines (LR10,9 < 0.0001, p > 0.99), but the box a female was raised in explained a significant portion of the variance (Box: 19.48%, LR12,11 = 6.96, p = 0.008). Additionally there was a marginally non-significant effect of the density in the rearing box, with females developing faster at higher densities (F1,14 = 3.76, p = 0.073). There was also a difference between the two experimental blocks (block 1: 32.97 ± 2.66 SD, block 2: 34.61 ± 4.35 SD, F1,14 = 10.01, p = 0.007).
In order to test the age at which females were able to mate we introduced males at different ages. The fertilization success for females of the different lines is shown in Fig. 3. Line only explained about 2.92% of the variation in fertilization success (CI 95%: 0.00001–37.00%). On average, females mated successfully at the age of 25.3 days (±2.2 SD), although some would mate after 21 days (see Fig. 4a). Mating age had a significant effect on the age at which females started ovipositing (F1,66 = 8.56, p = 0.005), because females could only start reproducing once mated. However, the ages at which females were mated also affected the time between mating and oviposition. Females take an average of 8.36 days (±3.91 SD) to start producing offspring, but females that were mated young took significantly longer (F1,66 = 21.82, p < 0.0001, see Fig. 5). This suggests that females are able to mate prematurely and store sperm until their oocytes have developed. There was also a marginal difference in the time between mating and the start of oviposition between the different countries (Portugal: 9.00 ± 3.90 SD, Israel: 7.28 ± 3.56 SD, UK: 10.17 ± 4.09 SD, F2,9 = 3.66, p = 0.069). Finally, increasing density in the rearing box reduced the time between mating and oviposition (F1,14 = 14.92, p = 0.002, r = −0.016).
Females experienced considerable mortality from the time they were isolated, with only 45% of females surviving until adulthood. We tested for factors affecting this mortality. Neither the density nor the sex ratio in the box in which the females developed affected their later survival (for both pMCMC > 0.1), and box only explained 0.38% (CI 95%: 0.00003–51.64%) of the variation in mortality rate. The mortality rates for different lines are shown in Fig. 3. Line did not explain any of the variance in mortality 0.40% (CI 95%: 0.0002–71.10%, Fig. 3). There was also no difference in mortality rates of females between the two blocks (pMCMC = 0.136).
Synchronised reproductive maturity
From this experiment there is no evidence that males are produced early to increase reproductive synchrony across broods. We re-iterate that all individuals in this experiment came from eggs collected within a 24 h period. In 16 out of 30 boxes that produced both viable males and females, some females could mate before some males could. This was revealed by taking the age of the first male to reach maturity and of the first female to mate successfully. In some boxes, males were mature 4 days before the first female could mate, while in another, some females were mature for 8 days already before the first male matured. Averaging across all replicates, there was no difference (0.0 days ± 2.6 SD) between first male and first female ability to mate: 23.5 days for both. This means that if males are produced first (as in Fig. 1), they would mature first, rather than synchronously with females.
Discussion
The extent to which genetic and sex determination systems influence sex allocation is an important component of trying to unravel the role of natural selection in shaping variation in sex ratios across species (Shuker et al. 2009). Here we have examined an unexplained temporal shift in sex ratio in the mealybug P. citri, a species with a paternal genome elimination (PGE) genetic system. Our survey of replicate populations from three geographic regions has confirmed a rather consistent pattern of temporal variation in sex allocation, suggesting that this temporal pattern is a widespread phenomenon in P. citri. All females produced a roughly equal sex ratio overall and females from all lines produced male offspring first, before switching to a female biased sex ratio and the subsequently producing increasingly more males until they died. Given that our data confirm that this is a pattern that needs explaining, why might females shift back and forth between male- and female-biased sex ratios?
We hypothesized that this pattern of sex allocation behaviour, with males being produced first, might have been an adaptation to ensure that male and female offspring become reproductively mature at the same time to ensure mating opportunities. However, we found that this is probably an unlikely explanation as males and females, when kept under similar environmental conditions, become reproductively mature at roughly the same age (i.e. males do not take longer to develop and do not need to be produced before females in order to synchronise reproductive maturity within a brood). Furthermore, we also found that females are able to mate and store sperm prior to their final moult, while previous experiments have shown that females are able mate successfully up to more than a month after they have become mature (Nelson-Rees 1960; Ross et al. 2010a). Therefore, as the window of female mating opportunity is so large, it seems unlikely that an adaptation to ensure synchronizing male and female maturation is necessary. However, even when males do not take longer to develop, it might still be beneficial for them to become reproductively mature slightly earlier than the females. Early-male emergence to enhance success in competitive mate-searching as a mating strategy, referred to as protandry, has been observed in many species across a wide taxonomic range (Morbey and Ydenberg 2001; Thornhill and Alcock 1983), including in species in which mating (and mating competition) among kin is common (Moynihan and Shuker 2011). Protandry might therefore explain why female produce sons first. The problem here is that this explanation does not explain the overproduction of sons late in life.
If this pattern of sex allocation is not adaptive in itself, to what extent does it also affect how females facultatively adjust their overall sex ratio with changes in the environment? For instance, previous experiments have suggested that females can adjust their sex ratio with respect to a variety of environmental factors (Nelson-Rees 1960; Ross et al. 2010a, 2011). Our best adaptive interpretation to date is that the reproductive value of sons and daughters varies both with maternal condition as envisaged by Trivers-Willard sex allocation (with females in good condition producing more sons) and also with extent of global resource competition (with high population density favouring sons). However, these factors (for example food restriction and high temperature, Ross et al. 2011), can often also affect maternal lifespan. In these cases, it is hard to distinguish if females adaptively alter their sex ratio, or if these factors affect lifespan and thereby curtail the schedule of male and female production, skewing sex ratios (as suggested by Ross et al. 2011). Therefore the patterns of facultative sex allocation we have been trying to explain may potentially be a non-adaptive side effect of the temporal order of sex allocation in P. citri.
Is the temporal pattern of sex allocation in P. citri associated with how sex is determined and controlled (or not) by females? Temporal patterns in sex allocation have been shown in other insects, including haplodiploid Hymenoptera which have the ability to very precisely adjust their sex ratio. In parasitoid wasps that adjust their sex allocation to avoid competition between male siblings, male production early in the laying sequence has been suggested to help increase the precision of adjustment (Chow and Mackauer 1996; Waage and Lane 1984). The more eggs a female is able to lay, the more likely her male offspring will experience local mate competition (LMC), and as a result of the laying sequence the more female biased her sex ratio will be, reducing the effects of LMC and increasing her fitness. As such, this pattern of allocating sex provides a mechanism for producing adaptive sex ratios in these species. However this explanation might not hold for P. citri, as the sex-sequence observed here is more complex than that described for parasitoids and the temporal pattern might instead be a constraint rather than a mechanism for precise sex ratio adjustment.
The sex determination mechanism in species with PGE is still almost completely unknown, but one possibility is that females add some type of maternal effect protein to those eggs destined to becoming male and that this causes the deactivation of the paternal genome in theses eggs (Buglia et al. 2009; Ross et al. 2010b). If this is indeed the way that sex is determined, females might not be able to precisely alter the concentration of this substance in individual eggs but rather change the amount produced over time. This hypothesis is supported by the observation of a gradual change in sex ratios over time, both under the standard laboratory conditions tested in the current study, but also under different environmental conditions (Ross et al. 2010a, 2011). However, it does not sit well with the observed changes in sex ratio (independent of female age) observed in response to mealybug density (Ross et al. 2010a). The timing of sex allocation is the obvious unknown here; to fit both sets of observations, some form of female product added to eggs to influence the sex of the offspring needs to be added soon enough before oviposition to allow facultative responses to factors such as adult crowding, but perhaps the same pool of that product has to vary over the female’s lifespan to generate the patterns we have seen here. It is also not clear why a female-derived protein that made eggs male would start at high levels, be reduced (leading to female biased sex ratios) and then increase again near the end of a female’s life. Thus, although the way that females change their sex ratio over time might constrain the precision with which females can adjust the sex ratio of her offspring, much remains unclear about how this constraint arises at the mechanistic level and is then manifested at the phenotypic level.
We found little evidence for population-level (and thereby genetic) variation in patterns of sex ratio or life-history. Sex ratio is well understood at a theoretical level, and to a large extent at an empirical level (West 2009), but much less so at a genetic level (Pannebakker et al. 2008). We know rather little about the underlying genetics of both sex ratio itself, as well as variation in the ability of individuals to adjust it. A lack of genetic variation in sex ratio is not unheard of although there is a clear genetic component in the sex allocation behaviour of some species, for example the haplodiploid parasitoid wasp Nasonia vitripennis (reviewed in Pannebakker et al. 2008; Pannebakker et al. 2011). The present experiment shows that if there is a genetic basis for sex ratio variation in P. citri, this effect may well be small.
In this paper we studied a pattern of temporal variation in sex ratio in P. citri. We showed that this pattern was remarkably consistent across three geographic population, but were unable to find an adaptive explanation. Nonetheless our results, combined with those from earlier studies, suggest that this pattern may constrain facultative sex allocation, especially if it is a consequence of sex determination and the mechanism of sex allocation by females. This work therefore highlights the need for mechanistic and functional aspects of behaviour to be studied in concert.
References
Ben-Dov Y, Miller DR, Gibson GAP (2011) ScaleNet, economic importance. http://www.sel.barc.usda.gov/SCALENET/economic.htm
Bourke AFG, Franks NR (1995) Social evolution in ants. Princeton University Press, Princeton
Brown SW, Nelson-Rees WA (1961) Radiation analysis of a lecanoid genetic system. Genetics 46:983–1006
Buglia GL, Dionisi D, Ferraro M (2009) The amount of heterochromatic proteins in the egg is correlated with sex determination in Planococcus citri (Homoptera, Coccoidea). Chromosoma 118:737–746
Bull JJ (1983) The evolution of sex determining mechanisms. Benjamin Cummings, Menlo Park
Chow A, Mackauer M (1996) Sequential allocation of offspring sexes in the hyperparasitoid wasp, Dendrocerus carpenteri. Anim Behav 51:859–870
Clark AB (1978) Sex ratio and local resource competition in a prosimian primate. Science 201:163–165
Cockburn A, Legge S, Double MC (2002) Sex ration in birds and mammals: can the hypotheses be disentangled? In: Hardy ICW (ed) Sex ratios: concepts and research methods. Cambridge University Press, Cambridge
Fisher RA (1930) The genetical theory of natural selection. Clarendon Press, Oxford
Godfray HCJ (1994) Parasitoids. Behavioural and evolutionary ecology. Princeton University Press, Princeton
Gullan PJ, Kosztarab M (1997) Adaptations in scale insects. Annu Rev Entomol 42:23–50
Hadfield JD (2010a) MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J Stat Softw 33:1–22
Hadfield JD (2010b) MCMCglmm coursenotes
Hamilton WD (1967) Extraordinary sex ratios. Science 156:477–488
King BH (1993) Sex ratio manipulation by parasitic wasps. In: Wrensch DL, Ebbert MA (eds) Evolution and diversity of sex ratio in insects and mites. Chapman & Hall, New York, pp 418–441
Morbey YE, Ydenberg RC (2001) Protandrous arrival timing to breeding areas: a review. Ecol Lett 4:663–673
Moynihan AM, Shuker DM (2011) Sexual selection on male development time in the parasitoid wasp Nasonia vitripennis. J Evol Biol 24:2002–2013
Nelson-Rees WA (1960) A study of sex predetermination in the mealy bug Planococcus citri (Risso). J Exp Zool 144:111–137
Pannebakker BA, Halligan DL, Reynolds KT, Ballantyne GA, Shuker DM, Barton NH, West SA (2008) Effects of spontaneous mutation accumulation on sex ratio traits in a parasitoid wasp. Evolution 62:1921–1935
Pannebakker BA, Watt R, Knott SA, West SA, Shuker DM (2011) The quantitative genetic basis of sex ratio variation in Nasonia vitripennis: a QTL study. J Evol Biol 24:12–22. doi:10.1111/J.1420-9101.2010.02129.X
Pike TW, Petrie M (2003) Potential mechanisms of avian sex manipulation. Biol Rev 78:553–574
Pinheiro J, Bates D, DebRoy S, Sarkar D, The R Core team (2007) nlme: linear and nonlinear mixed effects models
Pryke SR, Rollins LA, Griffith SC (2011) Context-dependent sex allocation: constraints on the expression and evolution of maternal effects. Evolution 65:2792–2799
R Development Core Team (2010) R: a language and environment for statistical computing: by RDC Team) R Foundation for Statistical Computing, Vienna, Austria
Ross L, Langenhof MBW, Pen I, Beukeboom LW, West SA, Shuker DM (2010a) Sex allocation in a species with paternal genome elimination: clarifying the role of crowding and female age in the mealybug Planococcus citri. Evol Ecol Res 12:89–104
Ross L, Pen I, Shuker DM (2010b) Genomic conflict in scale insects: the causes and consequences of bizarre genetic systems. Biol Rev 85:807–828
Ross L, Dealey EJ, Beukeboom LW, Shuker DM (2011) Temperature, age of mating and starvation determine the role of maternal effects on sex allocation in the mealybug Planococcus citri. Behav Ecol Sociobiol 65:909–919. doi:10.1007/S00265-010-1091-0
Schrader F (1921) The chromosomes of Pseudococcus nipae. Biol Bull 40:259–270
Shuker DM, West SA (2004) Information constraints and the precision of adaptation: sex ratio manipulation in wasps. Proc Natl Acad Sci USA 101:10363–10367
Shuker DM, Moynihan AM, Ross L (2009) Sexual conflict, sex allocation and the genetic system. Biol Lett 5:682–685
Sutherland JRG (1932) Some observations on the common mealy bug Pseudococcus citri (Risso). Quebec Soc Prot Plants Ann Rpts 23–24(1930–1932):105–118
Thornhill R, Alcock J (1983) The evolution of insect mating systems. Harvard University Press, Harvard
Trivers RL, Willard DE (1973) Natural selection of parental ability to vary sex-ratio of offspring. Science 179:90–92
Uller T, Pen I, Wapstra E, Beukeboom L, Komdeur J (2007) The evolution of sex ratios and sex-determining systems. Trends Ecol Evol 22:292–297. doi:10.1016/j.tree.2007.03.008
Varndell NP, Godfray HCJ (1996) Facultative adjustment of the sex ratio in an insect (Planococcus citri, Pseudococcidae) with paternal genome loss. Evolution 50:2100–2105
Waage JK, Lane JA (1984) The reproductive strategy of a parasitic wasp. 2. Sex allocation and local mate competition in Trichogramma-evanescens. J Anim Ecol 53:417–426
West SA (2009) Sex allocation. Princeton University Press (Monographs in Population Biology Series), Princeton
West SA, Sheldon BC (2002) Constraints in the evolution of sex ratio adjustment. Science 295:1685–1688
West SA, Herre EA, Sheldon BC (2000) The benefits of allocating sex. Science 290:288–290
West SA, Shuker DM, Sheldon BC (2005) Sex-ratio adjustment when relatives interact: a test of constraints on adaptation. Evolution 59:1211–1228
Wild G, West SA (2007) A sex allocation theory for vertebrates: combining local resource competition and condition-dependent allocation. Am Nat 170:E112–E128
Acknowledgments
We would very much like to thank Mike Copland, José Carlos Franco and Zvi Mendel for providing the specimens used in this study and for sharing their knowledge of mealybug biology and rearing methods. We like to thank Jarrod Hadfield for providing advice on the statistical procedure. John Hunt also provided extremely useful comments on an earlier version of the manuscript. We were supported by the Natural Environment Research Council, the University of Groningen and the University of Edinburgh Development Trust.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Ross, L., Langenhof, M.B.W., Pen, I. et al. Temporal variation in sex allocation in the mealybug Planococcus citri: adaptation, constraint, or both?. Evol Ecol 26, 1481–1496 (2012). https://doi.org/10.1007/s10682-012-9561-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-012-9561-7