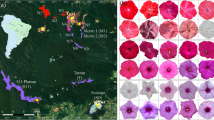
Abstract
Speciation requires the evolution of reproductive barriers to achieve isolation between species. In this paper, we examine the role of two major pre-zygotic barriers in reducing the chance of F1 hybrid formation between two pairs of Narcissus species. Field experiments were performed over 5 years in eight natural populations to determine whether flowering phenology and pollinator fidelity could act as reproductive isolation barriers in Narcissus. Our results show that reproductive isolation due to flowering phenology is highly variable and asymmetric. In some populations, pollinator fidelity was so strong that the quantification of reproductive isolation was complete and a strong negative correlation was found between the strength of this barrier and the abundance of hybrids. Nevertheless, the degree of pollinator fidelity was quite variable among populations indicating that reproductive isolation varies geographically but very consistent across years indicating that plant-pollinator interactions are well established. In fact, the finding that hybrid formation between these species occurs only in sites where pollinator fidelity is incomplete suggests that hybrid formation also varies geographically and that divergent evolutionary outcomes may occur in different sympatric populations of Narcissus.


Similar content being viewed by others
References
Aldridge G, Campbell DR (2007) Variation in pollinator preference between two Ipomopsis contact sites that differ in hybridization rate. Evolution 61:99–110
Alonso C (2005) Pollination success across an elevation and sex ratio gradient in gynodioecious Daphne laureola. Am J Bot 92:1264–1269
Bosch M, Waser NM (1999) Effect of local density on pollination and reproduction in Delphinium nuttalianum and Aconitum columbianum (Ranunculaceae). Am J Bot 86:871–879
Buerkle CA, Morris RJ, Asmussen MA, Rieseberg LH (2000) The likelihood of homoploid hybrid speciation. Heredity 84:441–451
Burke JM, Bulger MR, Wesselingh RA, Arnold ML (2000) Frequency and spatial patterning of clonal reproduction in Louisiana Iris hybrid populations. Evolution 54:137–144
Carney SE, Gardner KA, Rieseberg LH (2000) Evolutionary changes over the fifty-year history of a hybrid population of sunflowers (Helianthus). Evolution 54:462–474
Castro S, Münzbergová Z, Raabová J, Loureiro J (2011) Breeding barriers at a diploid–hexaploid contact zone in Aster amellus. Evo Ecol 25:795–814
Chari J, Wilson P (2001) Factors limiting hybridization between Penstemon spectabilis and Penstemon centranthifolius. Can J Bot 79:1439–1448
Cozzolino S, D’Emerico S, Widmer A (2004) Evidence for reproductive isolate selection in Mediterranean orchids: karyotype differences compensate for the lack of pollinator specificity. Proc R Soc Lond B Biol Sci 271:259–262
Dobzhansky T (1937) Genetics and the origin of species. Columbia University Press, New York
Docker MF, Dale A, Heath DD (2003) Erosion of interspecific reproductive barriers resulting from hatchery supplementation of rainbow trout sympatric with cutthroat trout. Mol Ecol 12:3515–3521
Egan SP, Funk DJ (2009) Ecologically dependent postmating isolation between sympatric host forms of Neochlamisus bebbianae leaf beetles. Proc Natl Acad Sci USA 106:19426–19431
Ellis AG, Johnson SD (1999) Do pollinators determine hybridization patterns in sympatric Satyrium (Orchidaceae) species? Plant Syst Evol 219:137–150
Emms SK, Arnold ML (2000) Site-to-site differences in pollinator visitation patterns in a Louisiana Iris hybrid zone. Oikos 91:568–578
Fenster CB, Dudash MR (2001) Spatiotemporal variation in the role of hummingbirds as pollinators of Silene virginica (Caryophyllaceae). Ecology 82:844–851
Fernandes A (1968) Keys to the identification of native and naturalized taxa of the genus Narcissus L. Daffodil Tulip Year Book 59:37–66
Grant V (1949) Pollination systems as isolating mechanisms in angiosperms. Evolution 3:82–97
Grant V (1992) Floral isolation between ornithophilous and sphingophilous species of Ipomopsis and Aquilegia. Proc Natl Acad Sci USA 89:11828–11831
Grant PR, Grant BR (2002) Unpredictable evolution in a 30-year study of Darwin’s finches. Science 296:707–711
Hanks GR (2002) Narcissus and daffodil: the genus Narcissus. Taylor and Francis, London
Herrera CM, Castellanos MC, Medrano M (2006) Geographical context of floral evolution: towards an improved research programme in floral diversification. In: Harder DH, Barrett SCH (eds) Ecology and evolution of flowers. New York, Oxford, pp 278–294
Husband BC, Sabara HA (2003) Reproductive isolation between autotetraploids and their diploid progenitors in fireweed, Chamerion angustifolium. New Phytol 161:703–713
Jacquemyn H, Brys R, Cammue B, Honnay O, Lievens B (2011) Mycorrhizal associations and reproductive isolation in three closely related Orchis species. Ann Bot 107:347–356
Jennersten O, Nilsson SG (1993) Insect flower visitation frequency and seed production in relation to patch size of Viscaria vulgaris (Caryophyllaceae). Oikos 68:283–292
Jersáková J, Castro S, Sonk N, Milchreit K, Schödelbauerová I, Tolasch T, Dötterl S (2010) Absence of pollinator-mediated premating barriers in mixed-ploidy populations of Gymnadenia conopsea s.l. (Orchidaceae). Evo Ecol 24:1199–1218
Kay KM (2006) Reproductive isolation between two closely related hummingbird-pollinated neotropical gingers. Evolution 60:538–552
Kearns CA, Inouye DW, Waser NM (1998) Endangered mutualisms: the conservation of plant-pollinator interactions. Annu Rev Ecol Syst 29:83–112
Keller B, Wolinska J, Manca M, Spaak P (2008) Spatial, environmental and anthropogenic effects on the taxon composition of hybridizing Daphnia. Phil Trans R Soc Lond B 363:2943–2952
Kevan P (2002) Flowers, pollination, and the associated diversity of flies. Biodiversity 3:16–18
Lamont BB, He T, Enright NJ, Krauss SL, Miller BP (2003) Anthropogenic disturbance promotes hybridization between Banksia species by altering their biology. J Evol Biol 16:551–557
Lowry DB, Modliszewski JL, Wright KM, Wu CA, Willis JH (2008) The strength and genetic basis of reproductive isolating barriers in flowering plants. Phil Trans R Soc Lond B 363:3009–3021
Mallet J (2007) Hybrid speciation. Nature 446:279–283
Marques I, Rosselló-Graell A, Draper D, Iriondo JM (2007) Pollination patterns limit hybridization between two sympatric species of Narcissus (Amaryllidaceae). Am J Bot 94:1352–1359
Marques I, Nieto Feliner N, Draper Munt D, Martins-Loução MA, Fuertes Aguilar J (2010) Unraveling cryptic reticulate relationships and the origin of orphan hybrid disjunct populations in Narcissus. Evolution 64:2353–2368
Marques I, Nieto Feliner N, Martins-Loução MA, Fuertes Aguilar J (2011) Fitness in Narcissus hybrids: low fertility is overcome by early hybrid vigour, absence of exogenous selection and high bulb propagation. J Ecol 99:1508–1519
Martin NH, Willis JH (2007) Ecological divergence associated with mating system causes nearly complete reproductive isolation between sympatric Mimulus species. Evolution 61:68–82
Mayr E (1942) Systematics and the origin of species. Columbia University Press, New York
Meléndez-Ackerman EJ, Campbell DR, Waser NN (1997) Hummingbird behavior and mechanisms of selection on flower color in Ipomopsis. Ecology 78:2532–2541
Nosil P, Crespi BJ (2006) Experimental evidence that predation promotes divergence during adaptive radiation. Proc Natl Acad Sci USA 103:9090–9095
Ramsey J, Bradshaw HD, Schemske DW (2003) Components of reproductive isolation between the monkeyflowers Mimulus lewisii and M. cardinalis (Phrymaceae). Evolution 57:1520–1534
Rieseberg LH (1997) Hybrid origins of plant species. Annu Rev Ecol Syst 28:359–389
Rieseberg LH, Blackman BK (2010) Speciation genes in plants. Ann Bot 106:439–455
Schwarz D, McPheron BA (2007) When ecological isolation breaks down: sexual isolation is an incomplete barrier to hybridization between Rhagoletis species. Evol Ecol Res 9:829–841
Seehausen O, Terai Y, Magalhaes IS, Carleton KL, Mrosso HDJ, Miyagi R et al (2008) Speciation through sensory drive in cichlid fish. Nature 455:620–626
Shmida A, Dafni A (1989) Blooming strategies, flower size and advertisement in the “Lily Group” geophytes of Israel. Herbertia 45:111–123
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. Freeman, New York
Taylor EB, Boughman JW, Groenenboom M, Sniatynski M, Schluter D, Gow JL (2006) Speciation in reverse: morphological and genetic evidence of the collapse of a three-spined stickleback (Gasterosteus aculeatus) species pair. Mol Ecol 15:343–355
Thompson JD (2001) How do visitation patterns vary among pollinators in relation to floral display and floral design in a generalist pollination system? Oecologia 126:386–394
Tiffin P, Olson MO, Moyle LC (2001) Asymmetrical crossing barriers in angiosperms. Proc R Soc Lond B 268:861–867
Turelli M, Moyle LC (2007) Asymmetric postmating isolation: Darwin’s corollary to Haldane’s rule. Genetics 176:1059–1088
Utelli A-B, Roy BA (2000) Pollinator abundance and behavior on Aconitum lycoctonum (Ranunculaceae): an analysis of the quantity and quality components of pollination. Oikos 89:461–470
Vallejo-Marín M, Dorken ME, Barrett SCH (2010) The ecological and evolutionary consequences of clonality for plant mating. Ann Rev Ecol Syst 41:193–213
Whittemore A, Schaal BA (1991) Interspecific gene flow in sympatric oaks. Proc Natl Acad Sci USA 88:2540–2544
Wolf DE, Takebayashi N, Rieseberg LR (2001) Predicting the risk of extinction through hybridization. Conserv Biol 15:1039–1053
Acknowledgments
The authors thank D. Draper, E. Salvado, S. Albano, H. Silva, J. Soler, E. Laguna, P. Pérez, J. Pérez, M.J. Albert for field support, R.G. Albaladejo, M. Alarcón, J. Aldasoro, A. González, J. M. Iriondo, two anonymous referees and M. Vallejo-Marín, Evol. Ecol. Associate Editor, for their useful comments on a previous version of the manuscript, D. Gilson for linguistic assistance. This work has been supported by a PhD-fellowship to I.M. from FCT, Ministério da Ciência e do Ensino Superior, Portugal (SFRH/BD/19053/2004) and a FPVI European-funded Integrated Infrastructure Initiative “SYNTHESYS“(ES-TAF 023-2004).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Marques, I., Aguilar, J.F., Martins-Loução, M.A. et al. Spatial–temporal patterns of flowering asynchrony and pollinator fidelity in hybridizing species of Narcissus . Evol Ecol 26, 1433–1450 (2012). https://doi.org/10.1007/s10682-012-9554-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-012-9554-6