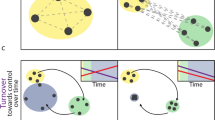
Abstract
Marine ecosystems are diverse and complex, providing significant challenges to the development of generalizable metrics of ecosystem health. Of particular concern is the varied form of change caused by multiple human activities, which limits the capacity to generate a single measure to encapsulate the overall condition of the ecosystem. Here we consider how successional theory can help to simplify our understanding of marine community structure, especially when viewed in context of human disturbance. During succession, the emergent properties of communities change in predictable ways. As communities mature, there is an increase in total production and biomass, the mean size of organisms, the level of internal recycling of food and nutrients, and the mean trophic level. Using a set of multi-species trophic models, we explore the changes in community structure that are likely to occur during succession. These changes include increases in biomass within trophic levels due to decreased rates of energy and food loss through trophic and production inefficiencies, and potential shifts from top-down control early in succession to bottom-up later. Because human activities disproportionately favor early-successional species, we can gain insights by considering community degradation in the context of succession being played in reverse. Indicators of health based on ecological succession thus provide a mechanistic view to measure the impact of human activities (both positive and negative) on marine ecosystems.
Similar content being viewed by others
References
Abrams PA (1993) Why predation rate should not be proportional to predator density. Ecology 74:726–733
Anderson CNK et al (2008) Why fishing magnified fluctuations in fish abundance. Nature 452:835–839
Arkema KK, Abramson SC, Dewsbury BM (2006) Marine ecosystem-based management: from characterization to implementation. Front Ecol Environ 4:525–532
Aronson RB, Precht WF (1997) Stasis, biological disturbance, and community structure of a Holocene coral reef. Paleobiology 23:326–346
Bascompte J, Melian CJ, Sala E (2005) Interaction strength combinations and the overfishing of a marine food web. Proc Natl Acad Sci USA 102:5443–5447
Brown JH, Gillooly JF, Allen AP, Savage VM, West GB (2004) Toward a metabolic theory of ecology. Ecology 85:1771–1789
Calder WA (1984) Size, function, and life history. Harvard University Press, Cambridge
Christensen V (1995) Ecosystem maturity—towards quantification. Ecol Model 77:3–32
Connolly SR, Roughgarden J (1999) Theory of marine communities: competition, predation, and recruitment-dependent interaction strength. Ecol Monogr 69:277–296
Crossman DJ, Choat JH, Clements KD, Hardy T, McConochie J (2001) Detritus as food for grazing fishes on coral reefs. Limnol Oceanogr 46:1596–1605
Dayton PK (1985) Ecology of kelp communities. Ann Rev Ecol Syst 16:215–245
Dayton PK, Tegner MJ, Edwards PB, Riser KL (1998) Sliding baselines, ghosts, and reduced expectations in kelp forest communities. Ecol Appl 8:309–322
DeMartini EE, Friedlander AM, Sandin SA, Sala E (2008) Differences in fish-assemblage structure between fished and unfished atolls in the northern Line Islands, central Pacific. Mar Ecol Prog Ser 365:190–215
Diaz RJ, Rosenberg R (2008) Spreading dead zones and consequences for marine ecosystems. Science 321:926–929
Doney SC, Fabry VJ, Feely RA, Kleypas JA (2009) Ocean Acidification: the other CO2 problem. Ann Rev Marine Sci 1:169–192
Duffy JE (2002) Biodiversity and ecosystem function: the consumer connection. Oikos 99:201–219
Estes JA et al (2011) Trophic downgrading of Planet Earth. Science 333:301–306
Fänge R, Grove D (1979) Digestion. In: Hoar WS, Randall DJ, Brett JR (eds) Fish Physiology, vol VII. Energetics and Growth. Academic Press, New York, pp 161–260
Frankenberg D, Smith KL Jr (1967) Coprophagy in marine animals. Limnol Oceanogr 12:443–450
Friedlander AM, DeMartini EE (2002) Contrasts in density, size, and biomass of reef fishes between the northwestern and the main Hawaiian islands: the effects of fishing down apex predators. Marine Ecol-Progr Ser 230:253–264
Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL (2001) Effects of size and temperature on metabolic rate. Science 293:2248–2251
Grigg RW (1983) Community structure, succession and development of coral reefs in Hawaii. Mar Ecol Prog Ser 11:1–14
Grigg RW, Maragos JE (1974) Recolonization of hermatypic corals on submerged lava flows in Hawaii. Ecology 55:387–395
Hamilton SL, Caselle JE, Malone DP, Carr MH (2010) Incorporating biogeography into evaluations of the Channel Island marine reserve network. Proc Nat Acad Sci 107:18272–18277
Hay ME (1997) The ecology and evolution of seaweed-herbivore interactions on coral reefs. Coral Reefs 16:S67–S76
Hay ME, Fenical W (1988) Marine plant-herbivore interactions: the ecology of chemical defense. Ann Rev Ecol Syst 19:111–145
Helfman GS, Collette BB, Facey DE (1997) The diversity of fishes. Blackwell, Malden
Holling CS (1959) The components of predation as revealed by a study of small-mammal predation of the European pine sawfly. Can Entomol 91:293–320
Jackson JBC (1997) Reefs since Columbus. Coral Reefs 16:S23–S32
Jackson JBC (2001) What was natural in the coastal oceans? Proc Nat Acad Sci 98:5411–5418
Jackson JBC, Sala E (2001) Unnatural oceans. Sci Marina 65:273–281
Jackson JBC et al (2001) Historical overfishing and the recent collapse of coastal ecosystems. Science 293:629–638
Kaufman L, Sandin S, Sala E, Obura D, Rohwer F, Tschirky J (2011) Coral health index (CHI): measuring coral community health. Science and Knowledge Division, Conservation International, Arlington, VA, USA
Knowlton N, Jackson JBC (2008) Shifting baselines, local impacts, and global change on coral reefs. PLoS Biol 6:e54
Larkum AWD, Orth RJ, Duarte CM (2006) Seagrasses: biology, ecology and conservation. Springer, Dordrecht
Leopold A (1949) A Sand County almanac. Oxford University Press, Oxford
Link JS (2002) Ecological considerations in fisheries management: when does it matter? Fisheries 27:10–17
MacArthur RH, Wilson EO (1967) The theory of island biogeography. Princeton University Press, Princeton
Margalef R (1968) Perspectives in ecological theory. University of Chicago Press, Chicago
Margalef R (1997) Our Biosphere. Inter-Research, Oldendorf/Luhe
May RM (1974) Biological populations with nonoverlapping generations: stable points, stable cycles, and chaos. Science 186:645–647
Menge BA (1995) Indirect effects in marine rocky intertidal interaction webs: patterns and importance. Ecol Monogr 65:21–74
Monaco ME, Ulanowicz RE (1997) Comparative ecosystem trophic structure of three U.S. mid-Atlantic estuaries. Mar Ecol Prog Ser 161:239–254
National Research Council (2006) Dynamic changes in marine ecosystems: fishing, food webs, and future options. In: Fishing CoEEo (ed) Phase II—Assessments of the extent of change and the implications for policy, Washington, p 160
Newman MJH, Paredes GA, Sala E, Jackson JBC (2006) Structure of Caribbean coral reef communities across a large gradient of fish biomass. Ecol Lett 9:1216–1227
Odum EP (1969) Strategy of ecosystem development. Science 164:262–270
Oksanen L, Fretwell SD, Arruda J, Niemela P (1981) Exploitation ecosystems in gradients of primary production. Am Nat 118:240–261
Paine RT (1980) Food web linkage, interactions strength and community infrastructure. J Anim Ecol 49:667–685
Pandolfi JM (1996) Limited membership in Pleistocene reef coral assemblages from the Huon Peninsula, Papua New Guinea: constancy during global change. Paleobiology 22:152–176
Pandolfi JM, Jackson JBC (2001) Community structure of Pleistocene coral reefs of Curaçao, Netherlands Antilles. Ecol Monogr 71:49–67
Pandolfi JM et al (2003) Global trajectories of the long-term decline of coral reef ecosystems. Science 301:955–958
Pauly D, Christensen V (1995) Primary production required to sustain global fisheries. Nature 374:255–257
Pauly D, Christensen V, Dalsgaard J, Froese R, Torres F Jr (1998) Fishing down marine food webs. Science 279:860–863
Pérez-España H, Arreguín-Sánchez F (1999) A measure of ecosystem maturity. Ecol Model 119:79–85
Peters RH (1983) The ecological implications of body size. Cambridge University Press, Cambridge
Pikitch EK et al (2004) Ecosystem-based fishery management. Science 305:346–347
Polis GA, Sears ALW, Huxel GR, Strong DR, Maron J (2000) When is a trophic cascade a trophic cascade? Trends Ecol Evol 15:473–475
Randall JE (1967) Food habits of reef fishes of the West Indies. Stud Trop Oceanogr 5:665–847
Rassweiler A, Rassweiler T (2011) Does rapid scavenging hide non-predation mortality in coral-reef communities? Mar Freshwater Res 62:510–515
Roberts CM (1995) Effects of fishing on the ecosystem structure of coral reefs. Conserv Biol 9:988–995
Robertson DR (1982) Fish feces as fish food on a Pacific coral-reef. Mar Ecol Prog Ser 7:253–265
Rochet M-J, Trenkel VM (2003) Which community indicators can measure the impact of fishing? A review and proposals. Can J Fish Aquat Sci 60:86–99
Rogers SI, Greenwaway B (2005) A UK perspective on the development of marine ecosystem indicators. Mar Pollut Bull 50:9–19
Sala E (2006) Top predators provide insurance against climate change. Trends Ecol Evol 21:479–480
Sala E, Sugihara G (2005) Food web theory provides guidelines for marine conservation. In: Belgrano A, Scharler UM, Dunne J, Ulanowicz RE (eds) Aquatic food webs: an ecosystem approach. Oxford University Press, Oxford, pp 170–183
Sale PF (ed) (1991) The ecology of fishes on coral reefs. Academic Press, San Diego
Sandin SA, Pacala SW (2000) Regulation in populations of coral reef fish: an exploration of models and data. In: Ninth International Coral Reef Symposium, vol 1. Bali, Indonesia, pp 455–462
Sandin SA, Pacala SW (2005) Demographic theory of coral reef fish populations with stochastic recruitment: comparing sources of population regulation. Am Nat 165:107–119
Sandin SA et al (2008) Baselines and degradation of coral reefs in the northern Line Islands. PLoS ONE 3:e1548
Sandin SA, Walsh SM, Jackson JBC (2010) Prey release, trophic cascades, and phase shifts in tropical nearshore marine ecosystems. In: Terborgh J, Estes JA (eds) Trophic cascades: predators, prey, and the changing dynamics of nature. Island Press, Washington, pp 71–90
Sazima I, Sazima C, Martins Silva-Jr J (2003) The cetacean offal connections: feces and vomits of spinner dolphins as a food source for reef fishes. Bull Mar Sci 72:151–160
Scharf FS, Juanes F, Rountree RA (2000) Predator size-prey size relationships of marine fish predators: interspecific variation and effects of ontogeny and body size on trophic-niche breadth. Mar Ecol Prog Ser 208:229–248
Schmidt-Nielsen K (1984) Why is animal size so important. Cambridge University Press, Cambridge
Simberloff D (1998) Flagships, umbrellas, and keystones: is single-species management passé in the landscape era? Biol Conserv 83:247–257
Terborgh J, Estes JA (eds) (2010) Trophic cascades: predators, prey, and the changing dynamics of nature. Island Press, Washington
Trenkel VM, Rochet M-J (2003) Performance of indicators derived from abundance estimates for detecting the impact of fishing on a fish community. Can J Fish Aquat Sci 60:67–85
U.S.D.A. Forest Service (1995) The Forest Service program for forest and rangeland resources: a long-term strategic plan. Draft 1995 RPA program. In, Washington
van Rooij JM, Bruggemann JH, Videler JJ, Breeman AM (1995) Plastic growth of the herbivorous reef fish Sparisoma viride: field evidence for a trade-off between growth and reproduction. Mar Ecol Prog Ser 122:93–105
West GB, Brown JH, Enquist BJ (1997) A general model for the origin of allometric scaling laws in biology. Science 276:122–126
Williams SL, Smith JE (2007) A global review of the distribution, taxonomy, and impacts of introduced seaweeds. Ann Rev Ecol Syst 38:327–359
Williams ID et al (2011) Differences in reef fish assemblages between populated and remote reefs spanning multiple archipelagos across the central and western Pacific. J Marine Biol 2011:1–14
Wilson S, Bellwood DR (1997) Cryptic dietary components of territorial damselfishes (Pomacentridae, Labroidei). Mar Ecol Prog Ser 153:299–310
Yodzis P, Innes S (1992) Body size and consumer-resource dynamics. Am Nat 139:1151–1175
Zacharias MA, Roff JC (2001) Use of focal species in marine conservation and management: a review and critique. Aqua Conserv: Marine Freshw Ecosyst 11:59–76
Acknowledgments
The insights and creativity of J.B.C. Jackson provided the inspiration for this work. We thank F. Ballantyne IV, J.E. Smith, G.J. Williams, S.R. Connolly, and one anonymous reviewer for significant input and comments on earlier versions of this manuscript. Support for SAS was provided by an endowment from Ed Scripps.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sandin, S.A., Sala, E. Using successional theory to measure marine ecosystem health. Evol Ecol 26, 435–448 (2012). https://doi.org/10.1007/s10682-011-9533-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-011-9533-3