Abstract
In order to rapidly adapt to the evolving climate and sustainably nourish the growing global population, plant breeders are actively investigating more efficient strategies to enhance crop yields. In this study, we present the development of a bread wheat mapping population and backcross breeding program, serving as a valuable genetic resource for mapping the effects of different alleles on trait performance. We employed innovative methodologies to rapidly introgress traits into the bread wheat cultivar. Specifically, we utilized two parents, including Tosunbey x Tahirova2000, in a recombinant inbred line population, in addition, a backcross strategy was applied with line 148 (obtained by crossing Tosunbey x Tahirova2000 with high gluten quality) as the recipient parent of the Nevzatbey cv., known for its awnless feature. The two most important applications of the rapid breeding method are extending the light period and breaking dormancy in early harvested seeds. Both applications were successfully implemented in our study. Our vegetation periods ranging from approximately 50–60 days. Additionally, an early genotype in our developed population was harvested in 40 days. Considering that the genotype underwent a 15-day vernalization period, the generation cycle, including vernalization, drying, and refrigeration, was completed in a total of 64 days. Notably, we employed not only biochemical markers for selection but also incorporated the rapid generation advance technology known as ‘speed breeding’, allowing us to develop BC5F1 within a span of two years. We posit that this approach proves instrumental in swiftly transferring genes for multiple target traits into adapted wheat cultivars or in pyramiding desirable traits within elite breeding material.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the next three decades, the global human population is projected to increase by 25%, reaching a total of 10 billion (Hickey et al. 2017). It becomes imperative to attain substantial genetic gains in yield and relatied traits to align with the projected demand. This task must be accomplished notwithstanding the destabilizing impacts of climate change on crop production (Ober et al. 2021). Traditional breeding methods have successfully generated crops that are both nutritious and high-yielding, facilitating mechanized harvesting to meet the escalating food demands of the growing population. However, developing an improved cultivar through traditional plant breeding involves cycling and evaluating numerous generations, a time-consuming process necessary for introducing and testing multiple traits of interest (Hickey et al. 2019). Recently, the technology known as ‘speed breeding’ for rapid generation advancement has been successfully implemented in bread wheat (Alahmad et al. 2018). Speed breeding, with its flexible protocols and methodology that significantly reduces generation time, emerges as a pivotal approach in expediting breeding and research programs. Speed breeding in fully enclosed, controlled-environment growth chambers can accelerate plant development for research purposes, including the phenotyping of adult plant traits, mutant studies, and transformation. The use of supplemental lighting in a glasshouse environment allows rapid generation cycling through single seed descent (SSD) and holds potential for adaptation to larger-scale crop improvement programs. This technique enables the attainment of up to six generations per year for spring wheat, durum wheat, and barley (Watson et al. 2018). Moreover, several phenotyping protocols tailored to the speed breeding system have been devised, facilitating the characterization and selection of crucial traits. Examples include seminal root traits contributing to drought adaptation (Richard et al. 2015), grain dormancy for tolerance against pre-harvest sprouting (Hickey et al. 2010), and disease-related traits, including adult plant resistance to leaf rust (Riaz et al. 2016), stripe rust (Hickey et al. 2011), and yellow spot (Dinglasan et al. 2016) in bread wheat as well as above and below ground traits in durum wheat (Alahmad et al. 2018).
Cereal breeding programs typically require a substantial timeframe of 10–15 years to transfer novel genes into adapted germplasm. Recognizing the imperative for accelerated delivery times, the application of biotechnology emerges as a pivotal strategy. Various breeding methodologies leveraging molecular markers have been implemented to enhance progress in this context. These strategies include marker-assisted recurrent selection (Eathington et al. 2007), genome-wide or genomic selection (Poland et al. 2012), marker-assisted gene pyramiding (Costa et al. 2010), and marker-assisted backcrossing (Septiningsih et al. 2009). It is crucial to note that the efficacy of these techniques relies heavily on efficient phenotyping, involving the measurement or quantification of lines, hybrids, or cultivars, and the identification of genotype–phenotype relationships. Despite these advancements, continuous improvements in crop breeding efficiency remain imperative to meet the escalating demands for agricultural commodities in the future (Hickey et al. 2017). Marker-assisted selection (MAS) is constrained by its applicability only when the target gene or quantitative trait loci (QTL) responsible for the trait of interest are identified. Consequently, MAS proves to be less feasible for complex traits, particularly those with limited knowledge regarding the underlying genetic controls (Budak 2013). The application of trait introgression, developed to involve the combination of both speed breeding and marker-assisted selection (MAS), could be implemented in the chosen set after phenotypic selection within the speed breeding system (Hickey et al. 2017). Recent advancements in plant genomics, coupled with the decreasing cost of sequencing, have empowered plant researchers to refocus their attention towards crop plants. Despite these advancements, the prolonged generation times of numerous crop plants persist as a significant entry barrier. However, the integration of these tools and resources with speed breeding presents a compelling incentive for plant breeders to directly engage in research on crop plants. This amalgamation has the potential to further expedite research in crop improvement.
In the framework of this investigation, the viability of the speed breeding method was evaluated through a comprehensive series of steps: (1) establishment of a mapping population through the crossbreeding of Tosunbey cv., and Tahirova2000 cv., (2) implementation of a backcross program utilizing marker-assisted selection, involving the pairing of Nevzatbey cv. with line 148, and (3) generation of elite seeds through the application of the speed breeding method. These meticulous procedures were undertaken to systematically assess the effectiveness and potential applications of the speed breeding method within the scope of this study.
Material and methods
Establishment of the plant growing room for speed breeding
The conversion of a south-facing corridor in the faculty of Bioengineering at Karamanoğlu Mehmetbey University of building into a plant growth room was undertaken in this study. A designated area of 20 m2 within the corridor was enclosed using an aluminum frame, and six tables measuring 90 × 180 cm were installed. Each table was equipped with two G2 LED lamps, each with a power output of 160 W, manufactured by Planktekno Light Company in Turkey. The spectrum values for the LED lamps are detailed in Fig. 1a, illustrating a broad-spectrum with a peak value at a wavelength of 660 nm. Positioned on the table floor at a height of 100 cm, the LED lamps were assessed for their Lux values at various heights using a Trotec BF06 Luxmeter. The PPFD (Photosynthetic Photon Flux Density) values were determined in µmol/m2/s by applying the coefficient provided by the LED manufacturer (Table 1). Notably, the plants positioned 80 cm away from the LED lamps received a photon intensity of 300 µmol/m2/s. The experimental results substantiate the efficacy of the LED lamps, indicating their adequacy for fostering the growth of healthy plants.
The lighting schedule for the plant growth room was configured with a 21-h light period followed by a 3-h dark period. Within this regimen, Plantekno LEDs remained illuminated for 9 h, from 1 to 10 am. At 10 am, the Plantekno LEDs were turned off, and the room’s curtain opened automatically. Subsequently, during this period, two AltunLEDs emitting white light with a power of 120 W each, along with sunlight from the windows, illuminated the room for 3 h. Between 1 and 10 pm, the Plantekno LEDs resumed lighting for another 9 h, and the room’s curtain closed automatically. Subsequently, between 10 pm and 1 am, the room remained in darkness for 3 h (Fig. 1b).
Temperature settings for the room were maintained at 25 ± 1 °C during the illuminated period and 18 ± 1 °C during the dark period, facilitated using an air conditioner (Mitshubishi SRK71M-S) for both heating and cooling. Ventilation for the plant growth room was achieved by opening windows between 10 am and 1 pm, especially during the summer. The relative humidity within the room fluctuated between 60 and 70% in winter and 50–60% in summer. To prevent excessive humidity, a dehumidifier (Tropec TTK 70 E) was employed. Reflective films were applied to the walls and aluminum frame of the plant growth room to enhance light efficiency (Fig. 2).
Growing the mapping population
In this study, a total of 10,000 F2 seeds resulting from the Tosunbey/Tahirova2000 cross were sown in the field. From this F2 generation, 1000 spikes were carefully selected in the field based on their tall and early heading characteristics. Subsequently, the seeds obtained from these selected spikes were utilized to establish a recombinant inbred line population using the speed breeding method. The selection criteria of tall and early heading genotypes aimed at utilizing this population for drought tolerance studies. To introduce rye translocation into the mapping population, Tahirova2000, which carries the 1BL.1RS rye translocation, was employed as a parent. Seeds from the resulting recombinant inbred line population underwent a vernalization process in a petri dish within a refrigerator at 4 °C in the dark for approximately 30 days. Following vernalization, the plants were transferred to multiple pots measuring 6.5 cm in height and 4 × 4 cm in diameter. A white peat bedding substrate (Klasmann TS1 from Germany) served as the soil source in the pots, and the seeds were manually sown, with one plant per tray. Fertilization was initiated after stem elongation using a fertilizer containing a combi nutrient, considering that the peat already contained plant nutrients in the early stages of plant development. Additionally, a 1% calcium nitrate solution was applied weekly through foliar spraying (Ghosh et al. 2018). Various developmental stages of the plants, including first leaf emergence, third leaf emergence, booting, heading date, anthesis, and harvest time, were meticulously recorded. The single seed descent method, as proposed by Brim (1966), was employed to advance the population. Harvesting occurred approximately 17–20 days after flowering, and the harvested spikes were dried in an incubator for one week at an average temperature of 35 °C. The dried seeds underwent a soaking process in Petri dishes for five days at 4 °C, followed by germination in plant growth cabins at 20 °C, marking the commencement of the vernalization process for the germinating seeds. This population showcases favorable genetic diversity and phenotypic variation, underscoring its suitability for continued development and utilization in breeding programs.
Marker-assisted backcross breeding
In the hybridization process, Nevzatbey, characterized by the awnless feature, and with 7x + 9y HMW-GS was employed as the male parent to verify the hybrid nature of F1 plants, as the awnless trait is dominant. The female parent was the coded line 148 with 17x + 18y HMW-GS, and an awned line obtained from crossing Tosunbey with Tahirova2000. F1 and BC1F1 seeds were generated within a climate-controlled greenhouse. Subsequent generations, including BC2F1, BC3F1, and BC4F1, were cultivated using the speed breeding method in the plant growing room, culminating in the production of BC5F1 generation seeds. Vernalization for 30 days was applied to both the recurrent parent and hybrid genotypes. Germinated seeds were then transferred to jiffy tablets (diameter of 4 mm), and vernalization was conducted in a lightened growth cabinet at 4 °C with a 12-h illuminated period. After completing the vernalization process, genotypes were transferred to pots at four different sowing times with one-week intervals. Pots with a volume of 2.5–3.5 L (Akyuz from Turkey) were utilized in the backcrossing program. The growth period of the plant, in which the first crossing was performed, was designated as the generation period. Peat welding, fertilization programs, and other procedures were executed similarly to those in the mapping population operations.
Screening of hybrid seeds was conducted using SDS-PAGE and PCR methods. The detection of High Molecular Weight Glutenin Subunits (HMW-GS) in wheat genotypes was conducted using the Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) method, as outlined by Masci et al. (2000) and Gianibelli et al. (2001). For this purpose, one-third of the hybrid seeds was utilized for SDS-PAGE analysis, while the remaining two-thirds of the segment containing the embryo were reserved for sowing in the next generation. For the PCR, genomic DNA isolation from young leaves was performed using the Zymo ZRPlant/Seed DNA Miniprep D6020 kit following the kit’s procedure. Primer sequences utilized in the study are provided in Table 2 The classical PCR reaction was carried out with 1 U Taq DNA polymerase, 1 X PCR Buffer solution (containing 1.5 mM MgCl2), forward and reverse primers, 200 µM dNTP, and 50 ng DNA sample in a total volume of 20 µL. The PCR protocol included an initial cycle of 5 min at 95 °C, followed by 35–38 cycles of 30 s at 94 °C, 30 s at 58–60 °C, and 30 s at 72 °C. A final extension cycle of 2 min at 72 °C was also included. The annealing temperature was optimized for Bx and By primers, and samples were amplified at the optimized annealing temperature. The amplified products were analyzed by electrophoresis in a 1.5% agarose gel and visualized after staining with ethidium bromide. Gel images were captured using the BioRad (ChemiDoc MP) gel imaging system.
Seeds displaying heterozygosity for the 17x + 18y and 7x + 9y High Molecular Weight Glutenin Subunits (HMW-GS) in the GluB1 allele were selected as parents for the subsequent generation. PCR screening in the first backcross generation was implemented to validate the SDS-PAGE results.
Production of single spike for elite seed production
In the context of variety protection breeding and the exploration of the speed breeding method’s applicability for enhancing the seed production of newly developed lines, a comprehensive approach was implemented. Initially, the High and Low Molecular Weight Glutenin Subunits (HMW-GS and LMW-GS) were determined using the SDS-PAGE method in 60 seeds sourced from the elite line. The lines were found to exhibit the same HMW-GS and LMW-GS patterns. Subsequently, 60 seeds were planted in pots with a diameter of 7 × 7 cm, a height of 8 cm, and a volume of 0.30 L. The fertilization and maintenance processes mirrored those outlined in the work package for developing the mapping population. Following the growth period, the seeds from the 60 harvested plants were subjected to a five-day drying process in an incubator. From the dried seeds, 500 were stored at 4 °C for four days, and subsequently, they were allowed to germinate at 20 °C for two days. The germinated seeds were then planted in multiple pods with a height of 6.5 cm and a diameter of 4 cm. This approach aimed to not only ensure variety protection but also to assess the potential of the speed breeding method in expediting the seed production of newly developed lines, facilitating their rapid introduction into production.
Results
Cultivation of recombinant inbred line population
To establish the recombinant inbred line mapping population, F1 seeds were generated through the crossbreeding of Tosunbey and Tahirova2000. Subsequently, F1 seeds were sown to produce F2 seeds, and about 10,000 F2 seeds were meticulously selected in the field, prioritizing traits such as tall stature, early maturation, and desirable panicle characteristics. From this selection, 1000 F3 generation spike were chosen under field conditions. The seeds obtained from these selected spikes were employed in the development of the recombinant inbred line population using a speed breeding method, leading to the acquisition of seeds from the F8 generation.
In the cultivation process, plants within the recombinant inbred line population underwent vernalization in a refrigerator at 4 °C for approximately 30 days (Table 3). During the F3 generation, two distinct sets of trays were organized based on the number of buds on the tillers. A total of 868 seeds were sown in 84-cell trays with a bud height of 5 cm and a bud diameter of 3.5 × 3.5 cm, determined by the number of tiller buds. Additionally, 70-cell trays with a bud height of 6.5 cm and a bud diameter of 4 × 4 cm were utilized. Harvesting took place 56 days after the sowing date. Considering the use of vernalized wheat, an additional 30 days were included in the cultivation period, resulting in the completion of one generation in 86 days. The extension of the vernalization process to 30 days, from the initially planned 15–20 days in this study, was due to the identification of vernalization genes in Tosunbey and Tahirova2000 varieties, aiming to account for potential vernalization gene combinations in the offspring.
Moving on to the F4 generation, 784 seeds were sown in 70-cell trays with a bud height of 6.5 cm and a bud diameter of 4 × 4 cm. Harvesting occurred 57 days after sowing, and as the level of homozygosity increased, distinctions in flowering and maturation dates among genotypes became more pronounced. Late-flowering lines were harvested on the 69th day when the plants were still notably green, and seeds harvested while still green exhibited no issues with germination (Fig. 3a).Concerns about losing late-flowering lines due to non-germination were minimal, aligning with the research’s goal of creating early flowering lines, although it was acknowledged that the total number of lines set in the research’s might not be reached (Fig. 3b).
In the subsequent F5 generation, 730 seeds were sown in 70-cell trays with a bud height of 6.5 cm and a bud diameter of 4 × 4 cm. Like the previous generation, the increase in homozygosity percentage led to a heightened difference in flowering times between early and late-flowering lines. While early flowering plants were ready for harvest 53 days after sowing, some genotypes among the early-flowering lines did not flower during the harvest period, either due to new flowering or lack of vernalization. The juxtaposition of late-flowering lines resulted in a visual pattern, as shown in Fig. 3c.
In the F6 generation, 680 seeds were planted in 70 cm multiple pots. Harvesting took place after 56 days, and the subsequent drying and refrigeration procedure was carried out for these seeds, consistent with the process in every generation. The 627 germinated seeds were then planted in 70 cm multiple pots as the F7 generation. In this generation, plants were harvested 54 days after planting, following the speed breeding procedure. As this generation marked the conclusion of plant cultivation the plants were expected to reach full maturity. With the anticipation that all seeds would mature in this generation, a total of 627 lines were obtained.
In a comprehensive assessment across all generations, the vernalization period for the plants ranged from 29 to 33 days. Despite this, it was observed that in some generations, there were plants heading without undergoing vernalization, possibly due to specific genetic factors. The vernalized plants exhibited the development of first leaves. The emergence of the third leaf varied between 5 and 8 days, depending on the generations. The time for plants in the population to reach the booting period ranged between 25 and 28 days, again dependent on the generations. In all generations, plants headed in approximately one month, and flowering occurred 3–4 days after heading. Harvesting was conducted roughly 20 days after flowering. Figure 3d presents a photo of the 2-leaf stages of the F5 generation plants.
Briefly, the F1 to F8 generations of Tosunbey and Tahirova2000 were bred focusing on traits such as height and early maturity. Vernalization lasted 30 days, different flowering times were observed among generations, and 627 lines were obtained at the end of the process, indicating genetic influences on branching and flowering.
Backcross program and molecular analysis
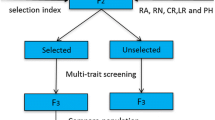
The backcross program involved using the awnless Nevzatbey cv. as the male parent in hybridizations, resulting in awnless F1 plants. The BC1F1, BC2F1, BC3F1, and BC4F1 generations were successfully cultivated using the speed breeding method, and seeds of the BC5F1 generation were obtained. Prior to initiating the hybridization program, optimization of the plant growth room and cultivation of summer varieties were carried out to ensure smooth operations. Once confirmed, the hybridization program commenced. Parental and hybrid genotypes underwent a 30-day vernalization process, followed by the transfer of germinated seeds to jiffy tablets for further vernalization in a lighted growth cabinet. After completing the vernalization process, genotypes were transferred to pots in the plant growth room at four different planting times. In the backcross program, each hybrid plant exhibited different development in each backward generation due to varying combinations of vernalization and photoperiod genes. Data on development periods were not collected for individual plants as in the population study. The generation time was recorded based on the earliest plant and the first hybrid spike. The generation period for hybridized seeds in the BC1F1 generation was 75 days, reduced to 64 days in the BC2F1 generation. The BC3F1 generation took 70 days from seed sowing to hybrid harvesting. In the BC4F1 generation, a five-week vernalization period was implemented, resulting in the harvest of the first hybrid spike in 60 days. Despite a 7-day increase in vernalization time, the generation period was shortened by 10 days, achieving a three-day reduction in the total generation time compared to other backcross generations. More than 100 hybrid seeds were obtained in each generation, and they underwent scanning using SDS-PAGE and PCR methods. Seeds carrying heterozygous genes encoding the 17x + 18y and 7x + 9y High Molecular Weight Glutenin Subunits in the GluB3 allele were selected as parents for the next generation. Figure 4a illustrates the selection of hybrid seeds carrying specific genes for the next backcrossing program, with emphasis on the heterozygous state of the GluB3 allele. This selection process was consistently applied in each backcross generation (Fig. 4b–d). The successful execution of the backcross program and molecular analyses demonstrates the potential for genetic improvement and trait selection in subsequent generations.
The R3-encoded primer was utilized to distinguish lines carrying and not carrying the Bx17 gene. The band produced by line 148 carrying the Bx17 gene is indicated with a yellow arrow. The Nevzatbey (NB) variety, lacking the relevant gene, exhibited double bands (highlighted by a red arrow). As known, the gene in the Nevzatbey variety, situated in the relevant locus, encodes the 7 + 9 High Molecular Weight Gluten subunit. Considering that lines 1, 2, 3, and 4 are heterozygous for the relevant gene, the bands they produce are represented by both yellow and red arrows. Some pictures of the seeds, plants and vernalization processes in the hybridization program are given Fig. 5a–d.
In summary, the backcross program involves the use of awnless Nevzatbey cv. was used as male parent producing awnless F1 plants. Thanks to speed breeding, it has successfully bred subsequent generations (BC1F1 to BC5F1). Before hybridization, plant growth room conditions were optimized and summer varieties were planted. Each hybrid plant developed differently due to different vernalization and photoperiod genes. Over 100 hybrid seeds were obtained in each generation screened for specific glutenin subunits. Seeds carrying the desired genes were selected as parents for the next generation, ensuring successful genetic improvement and trait selection in subsequent generations.
Production of single spike and molecular analyses for elite seed production
In our investigation, single spike from an elite line, developed for seed production, were efficiently produced using the speed breeding method. The primary objective of this endeavor is to assess the applicability of the speed breeding method in preserving variety purity for variety conservation breeding. Additionally, we aim to expedite the seed production of newly developed lines, facilitating their swift integration into production.
Initially, the High and Low Molecular Weight Glutenin Subunits were identified in 60 seeds of the line through the SDS-PAGE method (Fig. 6). Consistent band patterns were observed among the lines, leading to the planting of 60 seeds in pots with a diameter of 7 × 7 cm, a height of 8 cm, and a volume of 0.30 L. Genotypes were harvested 60 days after planting and subsequently dried in an incubator for a period of 5 days (Table 4). Among the dried seeds, 500 were stored at 4 °C for 4 days, followed by a germination period of two days at 20 °C. The germinated seeds were then transplanted into multiple pots with a height of 6.5 cm and a mesh diameter of 4 × 4 cm. Harvesting was conducted 59 days after planting, adhering to the rapid breeding method (Table 4).
Notably, when cultivated in 7 cm diameter pots, the plants exhibited tillering, with their spike reaching approximately 7–8 cm. Conversely, when grown in 4 cm pots, the plants did not display tillering, and their spiks remained around 3–4 cm in size. Figure 7a–d show photographs of plants grown in the rapid breeding room and where elite seed production was attempted.
Briefly, we efficiently produced single spikes from an elite seed line using speed breeding. Our aim was to evaluate its effectiveness for cultivar maintenance and rapid seed production. First, 60 seeds were analyzed for glutenin subunits and planted in 7 × 7 cm pots. After harvesting in 60 days, the seeds were subjected to drying, germination and transplanting in smaller pots.
Discussion
The most constraining factor in modern plant breeding is the duration needed for the production of segregation populations or the dependence on seasonal fluctuations in the development of genetic material. In breeding initiatives oriented towards line development, following the selection and intermating of parents, it typically necessitates 4–6 plant generations to yield genetically stable homozygous lines suitable for field evaluation (Bhatta et al. 2021). To expedite this process, plant breeders have employed various strategies, including doubled haploid technology (Forster et al. 2007) and shuttle breeding (Ortiz et al. 2007). In contrast to doubled haploid technology, speed breeding is suitable for diverse germplasm and eliminates the need for specialized labs for in vitro culturing (Hickey et al. 2019). This approach entails cultivating plant populations in environments conducive to early flowering, thereby hastening the generation time (González‐Barrios et al. 2021). In this technique, plant breeders can develop genetic material in the segregation population regardless of the season (Watson et al. 2018). The benefits of speed breeding are substantial. From early experiments with artificial lighting to the utilization of cutting-edge technologies such as LED lights and solar power, speed breeding demonstrates its potential to revolutionize crop breeding. The speed breeding technique comprises three main innovation points. Firstly, the lighting duration is extended to 22 h per day, mimicking the 16-h daylight period typical of summer greenhouse programs. Secondly, seed harvesting occurs approximately 15–20 days after anthesis, while the grains are still green. Unlike previous practices that harvested seeds at physiological maturity, this approach aims to shorten the generation time. Lastly, harvested seeds are dried in an incubator and stored at 4 °C for 3–5 days in a refrigerator to break seed dormancy and ensure germination. Researchers have developed and detailed the protocols of the speed breeding method, testing it on various plants in greenhouse and plant growing cabin conditions. Bread wheat, durum wheat, barley, oats, chickpeas, peas, forage peas, quinoa, and Brachypodium distachyon were among the species investigated. The research revealed that many plants can be cultivated in small areas using this method, which can be implemented in small plant growth cabins at low costs (Ghosh et al. 2018).
The speed breeding method has been employed for two crucial applications: extending the light period and breaking dormancy in early-harvested seeds. Our study successfully executed both applications. The vegetation periods in our research ranging approximately between 50 and 60 days. Notably, within the population we developed, an early genotype was harvested in just 40 days. Taking into account a 15-day vernalization period for this genotype, the entire generation cycle, including vernalization, drying, and refrigeration procedures, was accomplished in a total of 64 days. Speed breeding technology is gaining prominence in contemporary plant breeding programs. This technique utilizes optimal light quality, light intensity, day length, and temperature control to expedite photosynthesis and flowering. Additionally, early seed harvest is incorporated to reduce the generation time. Speed breeding protocols have proven effective in the development of commercial peanut varieties (O’Connor et al. 2013), chickpea varieties (Gaur et al. 2007) and oat (Kigoni and Arbelaez 2023). Furthermore, protocols have been established for grass pea, lentil, and quinoa to accelerate breeding and research (Chiurugwi et al. 2019). These advancements hold the potential to yield new varieties in the near future (Ghosh et al. 2018; Lulsdorf and Banniza 2018). Watson et al. (2018) reported that depending on the cultivar or accession, plants reached anthesis in 35–39 days (wheat, with the exception of Chinese Spring) and 37–38 days (barley), while it took 26 days to reach heading in B. distachyon. Alahmad et al. (2018) used the speed breeding method to select different quantitative traits in durum wheat. A thousand genotypes in the F2 generation were selected for root rot, leaf rust, plant height, number, and angle of seminal roots. Astonishingly, they managed to complete an entire generation of durum wheat in a mere 77 days. Potts et al. (2023) reported that under specific LED-supplemented glasshouse setups, up to six generations of wheat and barley per year were achieved at a density of 1000 plants/m2. Hickey et al. (2017) applied a similar trait introgression technique in wheat to rapidly introgress grain dormancy and triple rust resistance into two Australian spring wheat cultivars within a two-and-a-half year period.
Speed breeding has emerged as a transformative technique in the development of mapping populations. Traditionally, generating diverse segregating populations for genetic mapping was a time-consuming process. However, the ability of speed breeding to rapidly cycle through generations has revolutionized this field. In this study, we utilized speed breeding applications and selection methods for production and cultivation of mapping population. In this study we combined single seed descent (SSD) selection method with speed breeding technique. Speed breeding incorporation of supplemental lighting in a glasshouse environment facilitates rapid generation cycling through the application of single seed descent (SSD), presenting the potential for adaptation to larger-scale crop improvement programs. Jähne et al. (2020) stated that specific breeding methods, such as single plant selection (SPS) and SSD, align exceptionally well with the principles of speed breeding. In contrast, conventional methods such as traditional selection techniques, encompassing pure line, recurrent, bulk, mass, and pedigree selection do not align with the accelerated breeding demands and precision required by speed breeding in modern agricultural contexts (Jagadish et al. 2016). SSD is frequently employed in breeding and research programs to streamline the development of homozygous lines after a cross of traits. In addition, speed breeding can be utilized to quickly introgress genes or haplotypes into elite lines through marker-assisted selection, offering an accelerated approach to genetic improvement (Hickey et al. 2017). Speed breeding using SSD programs is highly efficient, especially for cereal crops like wheat and barley (Potts et al. 2023). In another study, similar approaches have been used in peanut breeding research, where controlled conditions, continuous light, optimal temperature, and SSD were combined in a greenhouse setting. These efforts consistently reduced the generation time for full-season maturity peanut cultivars from 145 to 89 days (O'Connor et al. 2013).
Strategic planning can be employed to establish a sequential process involving DNA marker testing, accelerated breeding, and field evaluation. Breeding strategies involving the application of molecular markers that are being applied to enhance progress include marker assisted recurrent selection (Eathington et al. 2007), genome-wide or genomic selection (Poland et al. 2012), marker-assisted gene pyramiding (Costa et al. 2010) and marker assisted backcrossing (Septiningsih et al. 2009). Notably, these techniques are reliant on efficient phenotyping of lines, hybrids, or cultivars and identification of genotype–phenotype relationships. Nevertheless, further improvements in crop breeding efficiency are required to meet future demands for agricultural commodities. The initial spring wheat variety was developed utilizing speed breeding. In this instance, speed breeding facilitated the expedited introgression of genes responsible for grain dormancy, inhibiting germination at crop maturity. This resulted in the production of a high protein milling wheat with enhanced tolerance to preharvest sprouting (Schwager 2017). Speed breeding could also expedite the identification and utilization of allelic diversity within wheat collections for resistance to leaf rust. Integration of speed breeding with DNA markers linked to established genes facilitated the discovery of novel sources of resistance (Riaz et al. 2017). Marker-assisted selection (MAS) has proven to be a valuable tool in crop improvement programs. This approach is most effective when concentrating on a small number of genes with significant effects, as seen with leaf rust resistance genes (e.g., Lr23) in bread and durum wheat (Chhetri et al. 2017), as well as Yr15 in durum wheat (Yaniv et al. 2015). Moreover, MAS can only be implemented if the target gene or quantitative trait loci (QTL) responsible for the trait of interest are identified. Consequently, MAS becomes less feasible for complex traits with limited understanding of the underlying genetic controls (Budak 2013). Alahmad et al. (2018) documented selection in early generations to enrich the resulting population with favorable allelic combinations for multiple traits. They suggested that greenhouse screening methods are adaptable and deployable systems on their own, or can be integrated with MAS if markers are accessible. In this study, novel lines were developed utilizing both the marker-assisted backcross method and a rapid breeding approach. Extensive genotypic research has been conducted on wheat quality, leading to the effective development of markers. Wheat gluten proteins, comprising high molecular weight (HMW) and low molecular weight (LMW) fractions, are a focus of investigation (Anderson et al. 2001). The HMW glutenin subunits 1A, 1B, and 1D, encoded in the Glu-1 loci on the long arm of homologous chromosomes, are denoted as Glu-A1, Glu-B1, and Glu-D1, respectively (Shewry et al. 1992). Among the genotypic factors influencing wheat quality, both high and low molecular weight glutenin subunits, as well as rye translocation, hold significant importance. HMW-GS have been extensively studied and are closely linked to wheat quality. Researchers have reported that these polymeric structures alone contribute to 47–60% of the variation in the bread quality of wheat (Rakszegi et al. 2005). Payne et al. (1987) introduced a quality score for each subunit based on the SDS sedimentation volume. Notably, the 1x, 17x + 18y, and 5x + 10y HMW-GS combinations, located in different loci, attain the highest score for protein quality. While the 2x + 12y allele pair at the Glu-D1 locus is generally associated with poorer quality traits, the 5x + 10y allele is linked to superior quality traits. The utilization of such technology for trait enhancement via backcrossing to widely grown cultivars may result in marginal advancements. However, for crops where grain quality is paramount, this strategy may prove more successful. The recovery of adaptation and grain quality characteristics through backcrossing is likely to foster the development of improved lines. This enhances the likelihood of identifying individuals possessing all desired traits using MAS in subsequent generations, rendering it more cost-effective. The approach outlined in this research has been modified for integration into speed breeding, allowing for multi-trait selection simultaneously with accelerated line development. Speed breeding has minimized the duration needed to produce RILs with a significant level of homozygosity, requiring only 12 months to reach the F6 generation.
Conclusion
We believe this to be the first report of deployment of speed breeding technique was successfully deployed in bread wheat (Triticum aestivum L.) to achieve six generations per year while imposing MAS selection for gluten proteins. In our study, the plants were subjected to a photoperiod of 21 h of light and 3 h of darkness, with a temperature of 25 °C during the light period and 18 °C during the dark period. LED lamps with a broad spectrum, emphasizing intense red light, served as the light sources. In the hybridization program study, one generation was completed in approximately 70 days. The F1 to F8 generations of Tosunbey and Tahirova2000 produced 627 lines. Also, the backcross program involves the use of awnless Nevzatbey cv. it has successfully bred subsequent generations. Over 100 hybrid seeds were obtained in each generation screened for specific glutenin subunits. Seeds carrying the desired genes were selected as parents for the next generation, ensuring successful genetic improvement and trait selection in subsequent generations. We efficiently produced single spikes from an elite seed line using speed breeding. Our aim was to evaluate its effectiveness for cultivar maintenance and rapid seed production. The results obtained indicated that 3–4 generations of winter wheat can be obtained using the rapid breeding method. The rapid breeding method has become an important and widely used approach worldwide. With this method, we implemented selection in the early generations to enrich the resulting population with favorable allelic combinations for multiple traits and it is useful to rapidly transfer genes for multiple target traits into adapted wheat cultivars or pyramiding desirable traits in elite breeding material. However, potential advantages of speed breeding technology, there are certain limitations to its widespread application in crop breeding. These limitations may include the partial loss of advancing populations, which could result in the erosion of valuable genetic variability essential for single seed descent breeding. Speed breeding must therefore be integrated with other breeding techniques as well as cost-efficient high throughput genotyping and phenotyping to speed up the generation, testing and commercial release of crop varieties.
Data availability
The data supporting the fndings of this study are available from the corresponding author upon reasonable request.
References
Alahmad S, Dinglasan E, Leung KM, Riaz A, Derbal N, Voss-Fels KP, Able JA, Bassi FM, Christopher J, Hickey LT (2018) Speed breeding for multiple quantitative traits in durum wheat. Plant Methods 14:1–15
Anderson O, Hsia C, Adalsteins A, Lew E-L, Kasarda D (2001) Identification of several new classes of low-molecular-weight wheat gliadin-related proteins and genes. Theor Appl Genet 103:307–315
Bhatta M, Sandro P, Smith MR, Delaney O, Voss-Fels KP, Gutierrez L, Hickey LT (2021) Need for speed: manipulating plant growth to accelerate breeding cycles. Curr Opin Plant Biol 60:101986
Brim CA (1966) A modified pedigree method of selection in soyabeans
Budak H, Kantar M, Yucebilgili Kurtoglu K (2013) Drought tolerance in modern and wild wheat. Sci World J 2013:1–16
Chhetri M, Bariana H, Wong D, Sohail Y, Hayden M, Bansal U (2017) Development of robust molecular markers for marker-assisted selection of leaf rust resistance gene Lr23 in common and durum wheat breeding programs. Mol Breed 37:1–8
Chiurugwi T, Kemp S, Powell W, Hickey LT (2019) Speed breeding orphan crops. Theor Appl Genet 132:607–616
Costa M, Tanure J, Arruda K, Carneiro J, Moreira M, Barros E (2010) Development and characterization of common black bean lines resistant to anthracnose, rust and angular leaf spot in Brazil. Euphytica 176:149–156
Dinglasan E, Godwin ID, Mortlock MY, Hickey LT (2016) Resistance to yellow spot in wheat grown under accelerated growth conditions. Euphytica 209:693–707
Eathington SR, Crosbie TM, Edwards MD, Reiter RS, Bull JK (2007) Molecular markers in a commercial breeding program. Crop Sci 47:S-154-S−163
Forster BP, Heberle-Bors E, Kasha KJ, Touraev A (2007) The resurgence of haploids in higher plants. Trends Plant Sci 12(8):368–375
Gaur PM, Samineni S, Gowda C, Rao B (2007) Rapid generation advancement in chickpea. J SAT Agric Res 3(1)
Ghosh S, Watson A, Gonzalez-Navarro OE, Ramirez-Gonzalez RH, Yanes L, Mendoza-Suárez M, Simmonds J, Wells R, Rayner T, Green P (2018) Speed breeding in growth chambers and glasshouses for crop breeding and model plant research. Nat Protoc 13(12):2944–2963
Gianibelli M, Gupta R, Lafiandra D, Margiotta B, MacRitchie F (2001) Polymorphism of high Mr Glutenin subunits in Triticum tauschii: characterisation by chromatography and electrophoretic methods. J Cereal Sci 33(1):39–52
González-Barrios P, Bhatta M, Halley M, Sandro P, Gutiérrez L (2021) Speed breeding and early panicle harvest accelerates oat (Avena sativa L.) breeding cycles. Crop Sci 61(1):320–330
Hickey L, Lawson W, Platz G, Dieters M, Arief V, German S, Fletcher S, Park R, Singh D, Pereyra S (2011) Mapping Rph20: a gene conferring adult plant resistance to Puccinia hordei in barley. Theor Appl Genet 123:55–68
Hickey LT, Dieters MJ, DeLacy IH, Christopher MJ, Kravchuk OY, Banks PM (2010) Screening for grain dormancy in segregating generations of dormant×non-dormant crosses in white-grained wheat (Triticum aestivum L.). Euphytica 172:183–195
Hickey LT, German SE, Pereyra SA, Diaz JE, Ziems LA, Fowler RA, Platz GJ, Franckowiak JD, Dieters MJ (2017) Speed breeding for multiple disease resistance in barley. Euphytica 213:1–14
Hickey LT, Hafeez AN, Robinson H, Jackson SA, Leal-Bertioli SC, Tester M, Gao C, Godwin ID, Hayes BJ, Wulff BB (2019) Breeding crops to feed 10 billion. Nat Biotechnol 37(7):744–754
Jagadish S, Bahuguna RN, Djanaguiraman M, Gamuyao R, Prasad P (2016) Implications of high temperature and elevated CO2 on flowering time in plants. Front Plant Sci 7:166037
Jähne F, Hahn V, Würschum T, Leiser WL (2020) Speed breeding short-day crops by LED-controlled light schemes. Theor Appl Genet 133(8):2335–2342
Kigoni M, Arbelaez JD (2023) Developing intercropping breeding strategies for crop diversification and improvement of oat forage yield and quality in oat-legume mixtures. In: ASA, CSSA, SSSA international annual meeting, 2023. ASA-CSSA-SSSA
Lei, Z.S., Gale, K.R., He, Z.H., Gianibelli, C., Larroque, O., Xia, X.C, Butow, B.J., Ma W (2006) Y -type gene specific markers for enhanced discrimination of high-molecular weight glutenin alleles at the Glu-B1 locus in hexaploid wheat 43(1):94–101.
Lulsdorf MM, Banniza S (2018) Rapid generation cycling of an F2 population derived from a cross between Lens culinaris Medik. and Lens ervoides (Brign.) grande after aphanomyces root rot selection. Plant Breed 137(4):486–491
Ma, W., Zhang, W., Gale, K.R., (2003) Multiplex-PCR typing of high molecular weight glutenin alleles in wheat. Euphytica 134:51–60.
Masci S, D’ovidio R, Lafiandra D, Kasarda D (2000) A 1B-coded low-molecular-weight glutenin subunit associated with quality in durum wheats shows strong similarity to a subunit present in some bread wheat cultivars. Theor Appl Genet 100(3–4):396–400
O’Connor D, Wright G, Dieters M, George D, Hunter M, Tatnell J, Fleischfresser D (2013) Development and application of speed breeding technologies in a commercial peanut breeding program. Peanut Sci 40(2):107–114
Ober ES, Alahmad S, Cockram J, Forestan C, Hickey LT, Kant J, Maccaferri M, Marr E, Milner M, Pinto F (2021) Wheat root systems as a breeding target for climate resilience. Theor Appl Genet 134(6):1645–1662
Ortiz R, Trethowan R, Ferrara GO, Iwanaga M, Dodds JH, Crouch JH, Crossa J, Braun H-J (2007) High yield potential, shuttle breeding, genetic diversity, and a new international wheat improvement strategy. Euphytica 157:365–384
Payne PI, Nightingale MA, Krattiger AF, Holt LM (1987) The relationship between HMW glutenin subunit composition and the bread-making quality of British-grown wheat varieties. J Sci Food Agric 40(1):51–65
Poland J, Endelman J, Dawson J, Rutkoski J, Wu S, Manes Y, Dreisigacker S, Crossa J, Sánchez‐Villeda H, Sorrells M (2012) Genomic selection in wheat breeding using genotyping‐by‐sequencing. Plant Genome 5(3)
Potts J, Jangra S, Michael VN, Wu X (2023) Speed breeding for crop improvement and food security. Crops 3(4):276–291
Rakszegi M, Bekes F, Lang L, Tamas L, Shewry P, Bedő Z (2005) Technological quality of transgenic wheat expressing an increased amount of a HMW glutenin subunit. J Cereal Sci 42(1):15–23
Riaz A, Athiyannan N, Periyannan S, Afanasenko O, Mitrofanova O, Aitken EA, Lagudah E, Hickey LT (2017) Mining Vavilov’s treasure chest of wheat diversity for adult plant resistance to Puccinia triticina. Plant Dis 101(2):317–323
Riaz A, Periyannan S, Aitken E, Hickey L (2016) A rapid phenotyping method for adult plant resistance to leaf rust in wheat. Plant Methods 12:1–10
Richard CA, Hickey LT, Fletcher S, Jennings R, Chenu K, Christopher JT (2015) High-throughput phenotyping of seminal root traits in wheat. Plant Methods 11:1–11
Schwager R (2017) in The Land (Fairfax Media, 2017)
Septiningsih EM, Pamplona AM, Sanchez DL, Neeraja CN, Vergara GV, Heuer S, Ismail AM, Mackill DJ (2009) Development of submergence-tolerant rice cultivars: the Sub1 locus and beyond. Ann Bot 103(2):151–160
Shewry P, Halford N, Tatham A (1992) High molecular weight subunits of wheat glutenin. J Cereal Sci 15(2):105–120
Watson A, Ghosh S, Williams MJ, Cuddy WS, Simmonds J, Rey M-D, Asyraf Md Hatta M, Hinchliffe A, Steed A, Reynolds D (2018) Speed breeding is a powerful tool to accelerate crop research and breeding. Nat Plants 4(1):23–29
Yaniv E, Raats D, Ronin Y, Korol AB, Grama A, Bariana H, Dubcovsky J, Schulman AH, Fahima T (2015) Evaluation of marker-assisted selection for the stripe rust resistance gene Yr15, introgressed from wild emmer wheat. Mol Breed 35:1–12
Acknowledgements
We acknowledged the support of the “The Scientific and Technological Research Council of Türkiye” (Project Number: TÜBİTAK 116O298)
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, N.A.; methodology, N.A., B.D., H.A., S.G., A.S., C.B., M.E.S. and A.T.; software, N.A., B.D., H.A., S.G., A.S., C.B. and M.E.S; validation, N.A. and A.T., formal analysis, N.A., B.D., H.A., S.G., A.S., C.B., M.E.S. and A.T., investigation, N.A., B.D., H.A., S.G., A.S., C.B., M.E.S. and A.T.; resources, N.A.; data curation, N.A. and A.T.; writing—original draft preparation, A.T. and N.A; writing—review and editing, N.A., B.D., H.A., S.G., A.S., C.B., M.E.S. and A.T; visualization, N.A. and A.T., supervision, N.A.; project administration, N.A.; funding acquisition, N.A. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aydin, N., Demir, B., Akdag, H. et al. Accelerated breeding strategies for biochemical marker-assisted backcross breeding and mapping population development in bread wheat (Triticum aestivum L.). Euphytica 220, 116 (2024). https://doi.org/10.1007/s10681-024-03370-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-024-03370-x