Abstract
Oilseed rape (Brassica napus L.) is sensitive to heat stress during the reproductive stage, but it is not clear whether the male and female reproductive organs differ in their sensitivity to heat stress. In this study, full diallel crossing experiments were conducted among four genotypes of B. napus under control, moderate and high heat stress conditions for five days immediately before and two days after crossing. General combining ability (GCA), specific combining ability (SCA) and reciprocal effects were analyzed to evaluate the genetic basis of heat stress tolerance in male and female reproductive organs. High female temperature (Tf) and high male temperature (Tm) reduced the number of fertile pods and seeds set per floret, and the significant Tf × Tm interaction indicated that female reproductive organs were more sensitive to heat stress than male reproductive organs. There were no overall GCA, SCA or reciprocal effects across all combinations of Tf and Tm. However, a significant reciprocal × Tf effect was found, suggesting that genotypes differed in their ability to set fertile pods and seeds as Tf increased. The relative heat tolerance of G1 as a female increased as Tf increased, and the relative heat tolerance of G2 as a male decreased as Tf increased. In summary, reciprocal diallel crossing has demonstrated that female reproductive organs of B. napus are more sensitive than male to transient heat stress at the early flowering stage, and genotypes differ in relative heat tolerance in the male and female reproductive organs as Tf increases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Global grain production is at risk from predicted temperature increases in the twenty-first century, with estimated decline of 10–25% by 2100 (IPCC 2014). Temperature extremes reduce crop grain yield during the reproductive stage in most crop plants (Hatfield and Prueger 2015). The frequency of extreme temperature events is expected to increase by 2050 (Battisti and Naylor 2009).
Canola (Brassica napus L.), or oilseed rape, is the third most important oilseed produced globally. Brassica species are most sensitive to heat stress during the reproductive period (Gan et al. 2004; Annisa et al. 2013; Chen et al. 2019). Short periods of high temperature from the beginning of flowering reduce canola grain yields in the field (Angadi et al. 2000; Morrison and Stewart 2002) and the glasshouse (Young et al. 2004). Heat stress sensitivity in canola began at the green bud stage (BBCH 53) (Chen et al. 2020), which coincided with the beginning of meiosis. As few as three days of transient heat stress from the green bud stage to two weeks after first open flower reduced pod numbers and seed yield on the main stem (Chen et al. 2020). Short periods of heat stress for up to five weeks after first open flower on the main stem continued to reduce pod numbers and seed yield on the branches (Chen et al. 2020).
Heat stress affects various stages of plant reproduction, including meiosis, pollen and ovule number and viability, pollen germination and growth in the stigma, fertilisation and early embryo and seed development. Polowick and Sawhney (1988) reported that ovule development in B. napus is abnormal under high temperatures. Transient heat stress also reduced pollen viability in B. napus, but this alone could not explain the associated reduction in seed and pod development (Young et al. 2004; Morrison et al. 2016; Chen et al. 2020). The evidence to date does not clearly define differential effects of heat stress on male and female reproductive organs immediately before and after pollination, nor does it address the question of whether heat stress tolerance is under different genetic control in male and female reproductive organs at during reproduction.
Controlled crossing among genotypes in a full diallel design (Griffing 1956) is potentially useful to assess the relative impact of transient daily heat stress on male and female reproductive organs. This will permit the estimation of general combining ability (GCA), specific combining ability (SCA) and reciprocal (maternal or sex-linked) effects under different heat stress conditions. In a full diallel crossing design, heat stress would be applied independently to male and female reproductive organs prior to and immediately following hand pollination. This may reveal differences in the sensitivity of male and female reproductive organs to heat stress, and GCA, SCA and reciprocal effects for heat tolerance. Prior studies in canola (Young et al. 2004; Chen et al. 2020) indicate that the transient heat stress should be applied to male and female plants beginning at green bud stage, several days before the first flowers open on the main stem. Four days of heat stress applied to male or female plants prior to hand pollination adversely effected fertile pod and seed production of B. napus, and there was a synergistic impact when heat stress was applied to both gametophytes (Young et al. 2004). However, Young et al. (2004) only used one genotype and few replicates so it is not clear whether the heat stress affected male and female gametophytes equally, or if genotypes vary in heat stress tolerance in the male and female reproductive organs.
This study investigated the relative impact of heat stress on male and female reproductive organs of four genotypes of B. napus in a full diallel hand-crossing experiment under control, moderate and high heat stress conditions. This also permitted us to assess whether the four genotypes expressed heat stress tolerance differentially in male and female reproductive organs.
Materials and methods
Plant materials and management
Two Australian canola cultivars ATR Stingray (G1) and AV-Ruby (G3) and two Chinese canola cultivars Zhongyou821 (G2) and ZY001 (G4) were used as parents in the crossing program. These four cultivars showed a range of responses to heat stress at the early flowering stage in preliminary field experiments at The University of Western Australia (UWA) (Chen et al., unpublished).
A pilot experiment was conducted from April to November 2015 in a glasshouse and controlled environment rooms (CERs) at UWA, Crawley, Western Australia (31.986° S 115.822° E). The main experiment was conducted in the same months in 2016. In both experiments, seeds of the four cultivars were sown on six sowing dates one week apart with sufficient plants to permit multiple crosses on the main stem between cultivars according to the experimental plan. Seeds were sown in seedling trays in a growth chamber at 15 °C constant temperature. After 12 d, the seedling trays were transferred to a cold room (6 °C day, 2 °C night) for vernalisation for 4 wk. After vernalisation, all plants were transplanted into 90 mm square olive pot (Garden City Plastics, Australia) with one plant per pot and grown in a glasshouse before and after heat treatment in CERs. Each pot was filled with canola potting mix, comprising 50% fine composted pine bark, 20% coco peat and 30% brown river sand plus 1.0 g kg–1 of gypsum with final pH ~ 6.0. The average temperature in the glasshouse was 22 °C day/16 °C night, under natural daylength (10.5–12.5 h) and average light intensity at midday of 635 mmol m–2 s–1 photosynthetically active radiation (PAR). At the green bud stage, the plants were transferred to CERs for heat treatments.
Heat treatments in CERs
The diurnal temperature fluctuation in the heat treatments followed the description in previous experiments (Chen et al. 2019, 2020). The pilot study had two treatments (CERs): control (L) with transient maximum/minimum temperatures of 25 °C/15 °C, and high-temperature treatment (H) with transient maximum/minimum temperatures of 35 °C/25 °C (TC1 and TC3 in Fig. 1a, Chen et al. 2020). The main experiment had three treatments (CERs): L and H as per the pilot study, and moderate temperature treatment (M) with transient maximum/minimum temperatures of 32 °C/22 °C (TC2 in Fig. 1a, Chen et al. 2020).
Pod and seed development of hybrids after the female parent lines treated with low (Tf_L), moderate (Tf_M) and high (Tf_H) temperature were crossed with male parent lines treated with low (Tm_L), moderate (Tm_M) and high (Tm_H) temperature in the main experiment (2016). a Total pods per floret (TPF), b fertile pods per floret (FPF), c seeds per floret (SPF) and d maximum pod length (MPL)
All CERs were set to 425 μmol m–2 s–1 PAR for 16 h and 8 h dark period each day, 65% relative humidity, with water supplied automatically by drippers to pots to maintain soil moisture at approximately 90% field capacity (Chen et al. 2020).
The temperature treatment of female plants (Tf) was L or H in the pilot experiment, and L, M and H in the main experiment. Likewise, the temperature treatment of male plants (Tm) was L or H in the pilot experiment, and L, M and H in the main experiment.
Experimental design
In the pilot study, a full diallel cross including reciprocals was executed for G1, G2, G3 and G4 in each combination of Tf (L or H) and Tm (L or H). Each cross was repeated up to five times (biological replicates) per cross/temperature combination at various times according to plant availability from multiple sowings. After crossing, female plants were fully randomised on a glasshouse bench.
In the main experiment, a full diallel cross including reciprocals was executed for G1, G2, G3 and G4 in each combination of Tf (L, M or H) and Tm (L, M or H). Each cross was repeated up to five times (biological replicates) per cross/temperature combination at various times according to plant availability from multiple sowings. The full factorial of nine combinations of male and female temperatures was used in this experiment, and a full diallel crossing design among the four genotypes was established at each of the nine heat level combinations (total 16 crosses at each heat level) (Supplemental Fig. S1A). After crossing, female plants were fully randomised on a glasshouse bench (Supplemental Fig. S1B).
Crossing and growth to maturity
Pairs of parent plants, one designated as male and one as female, were moved from the glasshouse to the CER of the designated heat treatment at the green bud stage following the experimental design. In the pilot study, crossing occurred in the CER after seven days of pre-treatment at L or H. In the main experiment, crossing occurred in the CER after five days of pre-treatment at L, M or H. Three to five florets were emasculated on each female plant, and pollen was moved from newly opened flowers on the male parent plant to hand pollinate the emasculated female flowers. Hand-pollinated buds were covered with selfing bags to prevent contamination with pollen from other plants. After pollination, all female plants remained in their designated heat treatment for another two days, before they were moved back to the glasshouse until maturity. Any remaining florets on the main stem or branches were trimmed so that only the hand-pollinated florets were retained until maturity (Supplemental Fig. S1B).
Measurements on male plants
Pollen viability was measured on male plants using the acetocarmine method (Heslop-Harrison 1992). Plants in the glasshouse were moved to CERs (L and H) on the day that first open flowers appeared on plants. Three newly opened flowers were harvested from L and H CERs on the third, fifth and seventh days of heat treatment for pollen viability tests. Flowers were harvested between 11:00 and 12:00 h each day. One drop of 1% acetocarmine was placed on top of a microscope slide. Pollen grains were dispersed into the stain, and covered with a cover slip. After 2–3 min, viable and non-viable pollen were observed under a microscope (Olympus, Shinjuku, Tokyo, Japan) with 600-fold magnification. Pollen viability was recorded on 200 pollen grains per flower. Bright orange stained pollen was counted as viable, and pollen that did not absorb the stain was counted as non-viable.
Measurements on female plants
Pods and seeds on each hand-pollinated floret were recorded at maturity. Pods less than 2 cm in length were not counted unless they contained a seed, and only pods with at least one seed were counted as fertile. Seeds were counted if they were at least 50% fully developed. Average total pods per floret (TPF), fertile pods per floret (FPF) and seeds per floret (SPF) were calculated for each female plant. The length of the longest pod on each female plant was also measured and recorded as maximum pod length (MPL).
Linear Mixed Model and Data analysis
Diallel joint analyses were based on the following linear mixed model:
where \({\varvec{y}}\) is the vector of observations; \({\varvec{f}}\) is the vector of fixed effects of female temperature (Tf); \({\varvec{m}}\) is the vector of fixed effects of male temperature (Tm); \({\varvec{f}}_{{\varvec{m}}}\) is the vector of fixed effects of Tf × Tm interaction; \({\varvec{g}}\) is the vector of random effects of GCA, with \(g \sim {\text{N}}\left( {0,\user2{I}\sigma _{{GCA}}^{2} } \right)\) where \(\sigma_{GCA}^{2}\) is the variance of the GCA; \({\varvec{s}}\) is the vector of random effects of SCA, with \(s \sim {\text{N}}\left( {0,\user2{I}\sigma _{{SCA}}^{2} } \right)\) where \(\sigma_{SCA}^{2}\) is the variance of SCA; \({\varvec{r}}\) is the vector of random effects of reciprocal (REC), with \(\user2{r} \sim {\text{N}}\left( {0,\user2{I}\sigma _{{REC}}^{2} } \right)\) where \(\sigma_{REC}^{2}\) is the variance of REC; \({\varvec{g}}_{{\varvec{f}}}\) is the vector of random effects of GCA × Tf, with \(\user2{g}_{\user2{f}} {\text{ }} \sim {\text{N}}\left( {0,\user2{I}\sigma _{{GCAxTf}}^{2} } \right)\) where \(\sigma_{GCAxTf}^{2}\) is the variance of GCA × Tf; \({\varvec{s}}_{{\varvec{f}}}\) is the vector of random effects of SCA × Tf, with \(\user2{s}_{\user2{f}} {\text{ }} \sim {\text{N}}\left( {0,\user2{I}\sigma _{{SCAxTf}}^{2} } \right)\) where \(\sigma_{SCAxTf}^{2}\) is the variance of SCA × Tf; \({\varvec{r}}_{{\varvec{f}}}\) is the vector of random effects of REC × Tf, with \(\user2{r}_{\user2{f}} {\text{ }} \sim {\text{N}}\left( {0,\user2{I}\sigma _{{RECxTf}}^{2} } \right)\) where \(\sigma_{RECxTf}^{2}\) is the variance of REC × Tf; \({\varvec{g}}_{{\varvec{m}}}\) is the vector of random effects of GCA × Tm, with \(\user2{g}_{\user2{m}} {\text{ }} \sim {\text{N}}\left( {0,\user2{I}\sigma _{{GCAxTm}}^{2} } \right)\) where \(\sigma_{GCAxTm}^{2}\) is the variance of GCA × Tm; \({\varvec{s}}_{{\varvec{m}}}\) is the vector of random effects of SCA × Tm, with \(\user2{s}_{\user2{m}} {\text{ }} \sim {\text{N}}\left( {0,\user2{I}\sigma _{{SCAxTm}}^{2} } \right)\) where \(\sigma_{SCAxTm}^{2}\) is the variance of SCA × Tm; \({\varvec{r}}_{{\varvec{m}}}\) is the vector of random effects of REC × Tm, with \(\user2{r}_{\user2{m}} {\text{ }} \sim {\text{N}}\left( {0,\user2{I}\sigma _{{RECxTm}}^{2} } \right)\) where \(\sigma_{RECxTm}^{2}\) is the variance of REC × Tm; e is the vector of random residual effects, \({\mathbf{e}} \sim {\text{N}}\left( {0,\user2{I}\sigma _{\varepsilon }^{2} } \right)\) where \(\sigma_{\varepsilon }^{2}\) is the residual variance; \({\varvec{X}}_{{\varvec{f}}}\), \({\varvec{X}}_{{\varvec{m}}}\), \({\varvec{X}}_{{{\varvec{fm}}}}\), \({\varvec{Z}}_{{\varvec{g}}}\), \({\varvec{Z}}_{{\varvec{s}}}\), \({\varvec{Z}}_{{\varvec{r}}}\), \({\varvec{Z}}_{{{\varvec{gf}}}}\), \({\varvec{Z}}_{{{\varvec{sf}}}}\), \({\varvec{Z}}_{{{\varvec{rf}}}}\), \({\varvec{Z}}_{{{\varvec{gm}}}}\), \({\varvec{Z}}_{{{\varvec{sm}}}}\), and \({\varvec{Z}}_{{{\varvec{rm}}}}\) are the respective incidence matrices and \({\varvec{I}}\) is the identity matrix of appropriate dimension denoting that the random effects have identical and independent distributions.
The analyses were performed using the ASReml-R package (Butler et al. 2009). Fixed and random effects were estimated using the Restricted Maximum Likelihood/Best Linear Unbiased Prediction (REML/BLUP) method in ASReml. The significance of fixed effects was tested using the Wald test and the significance of random effects using the likelihood ratio test in the ASRemlPlus-R package (Brien 2016).
Results
Pilot study
In the pilot study, 5216 seeds were harvested from 2412 florets which were hand-crossed in the 64 hybrid combinations of the diallel among G1, G2, G3 and G4 repeated across the four Tf × Tm combinations (L × L, L × H, H × L and H × H). On average, the control treatment (L × L) had 5.47 hybrid seeds per floret (SPF), L × H had very few seeds harvested (SPF = 0.68), and H × H and H × L did not set seed. Therefore, it was decided to add a moderate heat stress treatment (32/22 °C day/night) in the main experiment, with the duration of heat pre-treatment reduced from 7 to 5 d before crossing, to facilitate the evaluation of heat stress on male and female organs.
Main experiment
In the main experiment, there were 144 hybrid combinations of the diallel among G1, G2, G3 and G4 repeated across the nine Tf × Tm combinations (L × L, L × M, L × H, M × L, M × M, M × H, H × L, H × M and H × H). A total of 2467 florets were hand-pollinated (Supplemental Fig. S1A), with 1086 pods collected from 516 plants. Of these 727 pods were fertile and 6722 seeds were harvested (Supplemental Fig. S1B).
The Wald test of fixed effects revealed large Tf, Tm, and Tf × Tm effects for TPF, FPF, SPF and MPL (Table 1). Heat stress had an adverse effect on both female and male reproductive organs. The significant Tf × Tm interaction confirms that heat stress had a more significant impact on female reproductive organs than male reproductive organs (Fig. 1). Moderate or high heat stress on male reproductive organs resulted in higher TPF, FPF, SPF and MPL than moderate or high stress on female reproductive organs (Fig. 1).
The likelihood ratio test of random effects estimated using the REML/BLUP method revealed no significant GCA, SCA or reciprocal effects overall, but significant reciprocal × Tf effects for TPF, FPF and SPF (Table 1). The lack of a significant GCA indicates that the four varieties had the same average response to heat stress across the nine Tf/Tm combinations, and the lack of a significant SCA indicates that no specific combinations of the four parents had a consistent heterotic response to heat stress across the nine Tf/Tm combinations. Reciprocal effects were also not significant when averaged across the whole experiment. Interaction effects of Tm or Tf by GCA and SCA were not significant; that is, there was no consistent change in GCA or SCA across the nine Tf/Tm combinations (Table 1).
However, there was a significant reciprocal × Tf effect for TPF, FPF and SPF (Table 1), indicating that the reciprocal effects changed as Tf increased. This was observed as a change in ranking of G1, G2, G3 and G4 for TPF, FPF and SPF as males or females as Tf increased (Table 2). There was no significant reciprocal × Tm effect, that is, relative genotypic performance for heat stress tolerance as male or female did not change as Tm increased.
Reciprocal × Tf effect for total pods per floret (TPF)
G2 was the best-performing female for TPF across all Tf and its ranking as a male increased as Tf increased. G3 was ranked lowest as a male across all Tf and its ranking as a female decreased as Tf increased. G1 was highly ranked as a male for TPF at moderate Tf, but not as a female (Table 2).
Reciprocal × Tf effect for fertile pods per floret (FPF)
G2 was also the best-performing for FPF female across all Tf and its ranking as a male increased as Tf increased. G3 was ranked low for FPF as a male across all Tf and its ranking as a female decreased as Tf increased. G1 was also highly ranked as a male for FPF at moderate Tf, but not as a female (Table 2).
Reciprocal × Tf effect for seeds per floret (SPF)
In contrast to TPF and FPF, G1 was the highest-ranking female for SPF at moderate and high Tf, and the highest-ranking male at moderate Tf. G2 was highly ranked as a female at moderate Tf, but not as a male. The ranking of G3 as a female fell for SPF as Tf increased. G4 was ranked highly as a male for SPF across all Tf but lowest as a female across all Tf (Table 2).
Correlations between traits across nine combinations of female and male temperatures
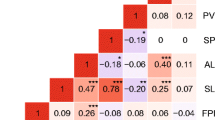
TPF had a weak positive correlation with the other three traits (FPF, SPF and MPL) in the low Tm and Tf treatments, but these correlations increased as Tf increased (Fig. 2). In contrast, FPF always had a strong positive correlation with SPF, especially at high Tf (Fig. 2). MPL had a moderate to strong positive correlation with FPF and SPF in most treatments. TPF, FPF and MPL may be useful surrogates for, or complement SPF in large-scale experiments to assess heat tolerance.
Correlations between total pods per floret (TPF), fertile pods per floret (FPF), seeds per floret (SPF) and maximum pod length (MPL) across the nine combinations of three temperature treatments (low, moderate, high) of male and female reproductive organs in the main experiment. × indicates “Not significant”
Pollen viability
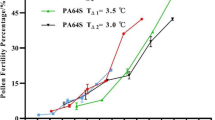
Flowers in most of the genotypes started showing heat stress symptoms, such as small half-opened flowers with protruding styles and small stamens, after 7 d in the H treatment; therefore, pollen viability tests were not continued beyond the seventh day. The average pollen viability of the four genotypes was > 95% on day 0, and gradually fell to 75% after 7 d of the H treatment (Fig. 3).
Discussion
We conducted controlled environment experiments that separated heat stress treatments on male and female reproductive organs, in order to identify differences in the sensitivity of these organs to heat stress. The pilot study suggested that the female reproductive organs of B. napus were more sensitive to heat stress than the male reproductive organs in terms of SPF, but we were unable to detect any genotypic variation for stress tolerance in the high (H) stress treatment as very few seeds were formed. In the main experiment, we added a moderate (M) heat stress treatment with maximum transient daily heat stress of 32 °C and discovered that heat treatments on both the female and male reproductive organs prior to hand-pollination reduced subsequent pod and seed formation. However, the significant Tf × Tm interaction (Table 1) and the relatively higher impact of Tf compared to Tm on TPF, FPF, SPF and MPL (Fig. 1) confirms that the impact of heat stress is greater on the female than the male reproductive organs. Heat stress on the male reproductive organ caused a reduction in pollen viability (Fig. 3), but by itself this reduction in pollen viability could not explain the full impact of Tm on TPF, FPF, SPF and MPL.
In our study, the heat treatment occurred during reproduction from 5 d before crossing to 2 d after crossing. As a result, the temperature treatment on males (Tm) occurred during meiosis and pollen development in the male reproductive organs, pollen germination on the stigma, pollen tube growth in the style, and fertilisation of the ovary. Temperature treatment on females (Tf) occurred during meiosis and ovary development in the female reproductive organs, and fertilization of the ovary. There were major differences in the impact of Tm and Tf on seed and pod set in the main experiment. The number of hybrid seeds per floret (SPF) was relatively high in the moderate temperature treatment on males (Tm_M) and control temperature on females (Tf_L) (Fig. 1c), but there were very few SPF in the moderate temperature treatment on females (Tf_M) and control temperature on males (Tm_L) (Fig. 1c). In contrast, the high temperature treatment seriously reduced SPF in both female (Tf_H) and male (Tm_H) (Fig. 1c). Similar relative results were observed for TPF, FPF, and MPL (Fig. 1). We conclude that female reproductive organs are more sensitive than male to transient daily heat stress, but that the high temperature treatment (Tm_H and Tf_H) has a large negative impact on both male and female reproductive organs and pollen tube growth and fertilisation.
It was reported previously that heat stress on the male reproductive organ did not disrupt the synergid-derived, pollen tube guidance system to the micropyle in B. napus (Young et al. 2004). While more research is required on the impact of heat stress on pollen tube growth and fertilisation, our results here and previously (Chen et al. 2020) support the conclusion that heat stress has its greatest impact on reducing pod and seed set in B. napus during the period from one week prior to two weeks after flowers open, and this impact is mostly observed in the female reproduction organs.
The main experiment also aimed to identify any differences in heat tolerance among genotypes, if they exist. Our unique approach analysed results from a full diallel crossing experiment with selfs and reciprocals (Griffing 1956), including a complete diallel for each of the nine heat-stress treatments. No significant GCA occurred across the experiment; that is, none of the genotypes consistently contributed superior or inferior genetic values for TPF, FPF, SPF or MPL (Table 1). Also, no significant SCA or reciprocal effects occurred across the experiment.
However, there were highly significant Reciprocal × Tf effects (Table 1), indicating that genotypes change in ranking as males and females for TPF, FPF and SPF as Tf increases (Table 2). This was observed as (i) a difference in the ability to set pods and seed when acting as a male or female, as witnessed by the high rank of G4 as a male but its low rank as a female, and (ii) a change in ranking as Tf increased, for example, G1 increased in rank as a female as Tf increased, and G2 decreased in rank as a male as Tf increased. G1 formed relatively more fertile pods and seeds as a female under heat stress than other genotypes, but relatively fewer as a male.
It is important to emphasise that these experiments are specific to hand-pollination, and the results from self-pollination may differ. Canola breeders who seek improvements in heat stress tolerance will ask: do genotypes differ in heat tolerance during selfing, and are there differences in the genetics of heat tolerance in female or male reproductive organs? Our results demonstrate that differences exist in the genetics of heat tolerance in male and female reproductive organs: G1 was more tolerant in female reproductive organs and less tolerant in male reproductive organs than other genotypes, and G4 was relatively more tolerant in male reproductive organs as Tf increased. This source of heat tolerance in the female reproductive organs of G1 and male reproductive organs in G4 will not be revealed in a simple screening test based on self-pollination, where both male and female reproductive organs are exposed to heat stress.
This has implications for breeding for heat tolerance. If screening is based on reproductive success during self-pollination, and genetic differences exist in heat tolerance in female and male reproductive organs, then a potential source of heat tolerance genes may be missed, as we discovered when we found no GCA for heat tolerance among the four test genotypes. A crossing program must aim to recombine heat tolerance in female and male reproductive organs from different genotypes. Cowling et al. (2019) proposed a breeding program for heat tolerance in a self-pollinating crop with rapid two-year cycles of recurrent selection, where heat tolerance was gradually recombined in the population with grain yield and other low heritability traits, based on optimal contributions selection on an index composed of all traits and with priority selection for heat tolerance index.
Our experiments confirm that heat stress affects the interaction of male and female reproductive organs during the process of fertilisation, embryo formation and seed and pod development. Pollen viability was reduced by 20–25% after 7 days of exposure to the high temperature of 35 °C, but this does not account for all of the loss in subsequent TPF, FPF and SPF caused by high Tm, suggesting that pollen viability is not a good indicator of heat stress tolerance in B. napus. This is consistent with previous reports in B. napus (Young et al. 2004; Morrison et al. 2016; Chen et al. 2020). However, this is in contrast to cereal crops, in which the maintenance of pollen fertility could serve as basis for the selection of reproductive tolerance to heat stress (Dolferus et al. 2011; Thistlethwaite et al. 2020).
In these experiments, the significant reciprocal × Tf interaction suggests that the expression of genes for tolerance differs between male and female reproductive organs, and this varies across genotypes. If heat stress tolerance has low to moderate heritability and differs across reproductive organs in different genotypes, as appears to be the case in B. napus based on this and other experiments (Chen et al. 2019, 2020), then selection for heat stress tolerance must be included along with grain yield and other economically important traits as a long-term breeding strategy. Frequent recombination will be necessary to combine unique heat stress tolerance genes expressed in the male or female reproductive organs of different genotypes in order to achieve the long-term goal of increased heat stress tolerance and high grain yield (Cowling et al. 2019).
Canola F1 hybrids are better suited to regions experiencing heat stress than open-pollinated cultivars (Pokharel et al. 2020), suggesting a shift to F1 hybrid canola cultivation under predicted hotter climates in the future. Parental selection is critical for canola hybrid production. Using two rapeseed lines with contrasting oil content, Hua et al. (2012) found that the maternal genotype greatly affected seed oil content in the subsequent F1 hybrid. In canola hybrids, the maternal genotype should be selected for heat tolerance to improve both F1 seed production and subsequent heat stress tolerance of the F1 hybrid.
Previous research on heat stress tolerance in crop plants focused on heat stress tolerance in pollen and pollen pistil interactions (Snider and Oosterhuis 2011), because the male part was considered as more sensitive to heat stress than the pistil (Mascarenhas and Crone 1996). However, evidence for pistil sensitivity to heat stress is accumulating. In tomato, a high-temperature treatment after pollination prevented fertilisation and resulted in ovule abortion (Iwahori 1966). In wheat, three days of high temperature (30 °C) during meiosis did not alter pollen germination, but prevented pollen tube guidance to the ovules due to an increase in ovule abnormalities and a decrease in the proportion of functional ovules (Saini et al. 1983). In Brassica, pollen germination under heat stress does not seem to be a major issue. A heat shock treatment at 60 °C for 24 h to mature pollen grains of B. juncea did not affect pollen viability and the ability to set pods (Rao et al. 1992). Three B. napus cultivars subjected to 36 °C had good pollen germination (Morrison et al. 2016). Reproductive organs of B. napus plants are negatively affected by heat stress, resulting in reduced male and female reproductive organ viability (Polowick and Sawhney 1988); most flower buds remaining closed but having protruding stigmas, as also observed in B. rapa (Annisa et al. 2013). Stamen size decreased and the anthers had abnormal microsporogenesis. The female part of the flower, although normal in appearance, did not set seed and had aberrant ovule development (ovular abnormalities) under high temperatures (Polowick and Sawhney 1988). Heat stress disrupts ovule development, fertilisation, early embryo growth and seed development in B. napus (Young et al. 2004; Chen et al. 2020). In this paper, we show that heat stress affects both male and female reproductive organs, but with a greater impact on the female than male, and that the genetic expression of heat tolerance differs in female and male reproductive organs.
Conclusion
Both the male and female reproductive organs of B. napus are sensitive to heat stress, but the impact of heat stress is disproportionately greater on the female than the male. Heat stress tolerance is under different genetic control in the female and male reproductive organs. Pollen viability is not a good predictor of heat stress tolerance in canola. The lack of significant GCA and SCA and significant reciprocal × Tf interaction in these experiments indicates that crossing will be necessary to recombine genetic tolerance in male and female reproductive organs.
Availability of data and material
All critical data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Change history
21 July 2021
A Correction to this paper has been published: https://doi.org/10.1007/s10681-021-02892-y
Abbreviations
- CER:
-
Controlled environment room
- FPF:
-
Fertile pods per floret
- GCA:
-
General combining ability
- MPL:
-
Maximum pod length
- SCA:
-
Specific combining ability
- SPF:
-
Seeds per floret
- L:
-
Low temperature (control) treatment
- M:
-
Moderate temperature treatment
- H:
-
High temperature treatment
- Tm:
-
Temperature on male plants
- Tf:
-
Temperature on female plants
- TPF:
-
Total pods per floret
References
Angadi SV, Cutforth HW, Miller PR, McConkey BG, Entz MH, Brandt SA, Volkmar KM (2000) Response of three Brassica species to high temperature stress during reproductive growth. Can J Plant Sci 80(4):693–701
Annisa A, Chen S, Turner NC, Cowling WA (2013) Genetic variation for heat tolerance during the reproductive phase in Brassica rapa. J Agron Crop Sci 199:424–435. https://doi.org/10.1111/jac.12034
Battisti DS, Naylor RL (2009) Historical warnings of future food insecurity with unprecedented seasonal heat. Science 323(5911):240–244. https://doi.org/10.1126/science.1164363
Brien C (2016) asremlPlus: augments the use of ‘asreml’ in fitting mixid models. R package version 2.0–9. https://CRAN.R-project.org/package=asremlPlus.
Butler D, Cullis B, Gilmour A, Gogel B (2009) Analysis of Mixed Models for S–language Environments: ASReml-R Reference Manual. https://vsn.klever.co.uk/downloads/asreml/release3/asreml-R.pdf.
Chen S, Guo Y, Sirault X, Stefanova K, Saradadevi R, Turner NC, Nelson MN, Furbank RT, Siddique KHM, Cowling WA (2019) Nondestructive phenomic tools for the prediction of heat and drought tolerance at anthesis in Brassica species. Plant Phenomics 2019:3264872. https://doi.org/10.34133/2019/3264872
Chen S, Stefanova K, Siddique KHM, Cowling WA (2020) Transient daily heat stress during the early reproductive phase disrupts pod and seed development in Brassica napus L. Food Energy Security 9(4):e00262. https://doi.org/10.1002/fes3.262
Cowling WA, Li L, Siddique KHM, Banks RG, Kinghorn BP (2019) Modeling crop breeding for global food security during climate change. Food Energy Security 8(2):e00157. https://doi.org/10.1002/fes3.157
Dolferus R, Ji X, Richards RA (2011) Abiotic stress and control of grain number in cereals. Plant Sci 181(4):331–341. https://doi.org/10.1016/j.plantsci.2011.05.015
Gan Y, Angadi SV, Cutforth H, Potts D, Angadi VV, McDonald CL (2004) Canola and mustard response to short periods of temperature and water stress at different developmental stages. Can J Plant Sci 84(3):697–704. https://doi.org/10.4141/P03-109
Griffing B (1956) Concept of general and specific combining ability in relation to diallel crossing systems. Aust J Biol Sci 9(4):463–493. https://doi.org/10.1071/BI9560463
Hatfield J, Prueger J (2015) Temperature extremes: effect on plant growth and development. Weather Clim Extremes 10:4–10
Heslop-Harrison JS (1992) Cytological techniques to assess pollen quality. In: Cresti M, Tiezzi A (eds) Sexual plant reproduction. Springer, Berlin, pp 41–48. doi:https://doi.org/10.1007/978-3-642-77677-9_4
Hua W, Li R-J, Zhan G-M, Liu J, Li J, Wang X-F, Liu G-H, Wang H-Z (2012) Maternal control of seed oil content in Brassica napus: the role of silique wall photosynthesis. Plant J 69(3):432–444. https://doi.org/10.1111/j.1365-313X.2011.04802.x
IPCC (2014) Climate change 2014: synthesis report. In: Pachauri RK, Meyer LA (eds) Contribution of working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. IPCC, Geneva, Switzerland
Iwahori S (1966) High temperature injuries in tomato V Fertilization and development of embryos with special reference to the abnormalities caused by high temperature. J Jpn Soc Hortic Sci 35(4):55–62. https://doi.org/10.2503/jjshs.34.33
Mascarenhas JP, Crone DE (1996) Pollen and the heat shock response. Sex Plant Reprod 9(6):370–374. https://doi.org/10.1007/BF02441959
Morrison MJ, Gutknecht A, Chan J, Miller SS (2016) Characterising canola pollen germination across a temperature gradient. Crop Pasture Sci 67(4):317–322. https://doi.org/10.1071/CP15230
Morrison MJ, Stewart DW (2002) Heat stress during flowering in summer Brassica. Crop Sci 42(3):797–803
Pokharel M, Chiluwal A, Stamm M, Min D, Rhodes D, Jagadish SVK (2020) High night-time temperature during flowering and pod filling affects flower opening, yield and seed fatty acid composition in canola. J Agron Crop Sci. https://doi.org/10.1111/jac.12408
Polowick PL, Sawhney VK (1988) High temperature induced male and female sterility in canola (Brassica napus L.). Ann Bot 62:83–86
Rao GU, Jain A, Shivanna KR (1992) Effects of high temperature stress on Brassica pollen viability, germination and ability to set fruits and seeds. Ann Bot 69(3):193–198. https://doi.org/10.1093/oxfordjournals.aob.a088329
Saini H, Sedgley M, Aspinall D (1983) Effect of heat stress during floral development on pollen tube growth and ovary anatomy in wheat (Triticum aestivum L). Funct Plant Biol 10(2):137–144. https://doi.org/10.1071/PP9830137
Snider JL, Oosterhuis DM (2011) How does timing, duration, and severity of heat stress influence pollen-pistil interactions in angiosperms? Plant Signal Behav 6(7):930–933. https://doi.org/10.4161/psb.6.7.15315
Thistlethwaite R, Trethowan R, He S, Joukhadar R, Tan D, Daetwyler H (2020) Maintaining wheat yield under high temperatures - how do current cultivars compare with what's coming? Paper presented at the GRDC Grains Research Update (Wagga Wagga), Wagga Wagga, New South Wales, Australia, February 18–19, 2020
Young LW, Wilen RW, Bonham-Smith PC (2004) High temperature stress of Brassica napus during flowering reduces micro- and megagametophyte fertility, induces fruit abortion, and disrupts seed production. J Exp Bot 55(396):485–495. https://doi.org/10.1093/jxb/erh038
Acknowledgements
This research was supported by the National Brassica Germplasm Improvement Program of The Grains Research and Development Corporation (Project DAN00208) and The University of Western Australia.
Funding
This research was supported by the National Brassica Germplasm Improvement Program of The Grains Research and Development Corporation (project DAN00208) and The University of Western Australia.
Author information
Authors and Affiliations
Contributions
SC, KHMS and WAC designed the experiments. SC and RS undertook the experiment and collected the data. SC, RS, MV, RFN, JC and WAC analyzed the data. SC, KHMS and WAC completed the data interpretation and drafted the manuscript. All authors contributed to the revision of the manuscript and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to a retrospective Open Access order.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplemental Table S1
Predicted values (PV) and standard errors (SE) for total pods per floret (TPF), fertile pods per floret (FPF), seeds per floret (SPF) and maximum pod length (MPL) after low (L), moderate (M) and high (H) heat stress treatments of male (Tm) and female (Tf) reproductive organs on parent plants in the main experiment. Supplemental Fig. S1 (A) In the main experiment, 144 hybrid hand-crossings were made in a full diallel mating design among four genotypes either as male (gi) or female (gj) where female and male parents were independently treated at low (L), moderate (M) or high (H) temperatures (Tm_L, Tm_M or Tm_H) to generate a 3×3 factorial experiment of full diallel crossings. The table indicates the number of female florets hand-pollinated and assessed in each combination on at least three plants per combination. A total of 2467 florets were assessed in 144 hybrid combinations. (B) View of the glasshouse bench near harvest in the main experiment in 2016, with 1086 pods collected from 516 plants. At harvest, 6722 seeds were harvested from 727 fertile pods. (DOCX 16 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, S., Saradadevi, R., Vidotti, M.S. et al. Female reproductive organs of Brassica napus are more sensitive than male to transient heat stress. Euphytica 217, 117 (2021). https://doi.org/10.1007/s10681-021-02859-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-021-02859-z