Abstract
Thrips are a worldwide threat to Capsicum because they damage fruits, flowers and leaves directly by feeding, and indirectly by vectoring tospoviruses such as tomato spotted wilt virus. Therefore, growers would benefit from thrips-resistant varieties. Previously, a quantitative trait locus (QTL) that provides resistance to Frankliniella occidentalis has been identified. Here we explore the potential of this QTL for breeding thrips-resistant varieties by studying its effect on two thrips species (i.e. F. occidentalis and Thrips tabaci) in four different Capsicum annuum backgrounds. We observed differences in thrips resistance between different genetic backgrounds, both in plants that have the resistance allele for the QTL region in homozygous state as well as in plants with the susceptibility allele in homozygous state. This suggests the presence of factors in these backgrounds that either increase or reduce thrips resistance. Altogether, we confirmed the major effect of the QTL on thrips resistance in all four genetic backgrounds to both F. occidentalis and T. tabaci, thus showing its general applicability as a source for breeding thrips-resistant Capsicum varieties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thrips are a major pest in vegetable, fruit and ornamental crops worldwide (Kirk and Terry 2003). Thrips is the common name for insects of the order of Thysanoptera, that includes over 5500 described species. Thrips damage the plant directly by feeding on the flowers, fruits and leaves, leading to silvering spots, deformation, reduced growth, and altered carbon allocation, resulting in reduced marketable yield (Chisholm and Lewis 1984; Welter et al. 1990; Shipp et al. 1998). Thrips also affect their hosts indirectly, through the transmission of viruses, causing economic losses in many crops and making thrips an important threat to the agricultural and horticultural sector (Mumford et al. 1996; Riley et al. 2011).
Only a few thrips species are currently reported to transmit tospoviruses, of which Western flower thrips (Frankliniella occidentalis Pergande) is the most important due to its worldwide occurrence (Ullman et al. 2002; Kirk and Terry 2003; Riley et al. 2011). Western flower thrips has become the most important pest species in European greenhouses as well as in fields and orchards in the Mediterranean climate since its accidental introduction in 1983 (Van Lenteren and Loomans 1999).
Before introduction of F. occidentalis from western North America, Thrips tabaci was the most prevalent thrips species in Europe (Van Lenteren and Loomans 1999). Nowadays, T. tabaci is a major pest in field vegetables including onion, leek, cabbage, tobacco, garlic and cotton, but it also infests greenhouse crops such as tomato, pepper, cucumber, and ornamentals. Thrips tabaci has also been reported to transmit tomato spotted wilt virus (TSWV) in Capsicum, although studies have shown that only male–female populations can transmit some isolates of TSWV and do so at a low rate (Wijkamp et al. 1995).
Therefore, it is important to explore the use of suitable sources for breeding broad spectrum thrips-resistant varieties. Host plant resistance to thrips has been previously observed and characterized in Capsicum (Maharijaya et al. 2011, 2012; Van Haperen et al. 2019; Visschers et al. 2019a, b). Maharijaya et al. (2011) identified CGN16975 as a resistant accession, as lower damage scores for both Thrips parvispinus (Karny) and F. occidentalis were observed. A significant inhibition of larval development of F. occidentalis first instar larvae was observed on leaves of this accession (Maharijaya et al. 2012; Van Haperen et al. 2019). The resistance to F. occidentalis was mapped to a single QTL on chromosome 6 that explained about 50% of the variation (Maharijaya et al. 2015). However, it is not known how effective the QTL is in other susceptible backgrounds. If the QTL is functional in other backgrounds, the QTL can be widely exploited to breed thrips-resistant varieties.
The goal of this study is to further explore the potential of this QTL in different breeding programs for thrips-resistant varieties. We investigate the effect of the chromosome 6 QTL on F. occidentalis larval development in four Capsicum annuum backgrounds, obtained from two breeding companies. In addition, we study the effect of the QTL on T. tabaci larval development in the same genetic backgrounds to determine whether this source of resistance is effective to multiple thrips species.
Materials and methods
Overview of experiments and plant material
In this study, larval development of Frankliniella occidentalis and Thrips tabaci were studied in four different genetic backgrounds (background 1 and 2 in experiment 1A–B, background 3 and 4 in experiment 2A–B). Plants with the resistance allele in homozygous state (“R”) and plants with the susceptibility allele in homozygous state (“S”) for the QTL region on chromosome 6 were selected for phenotyping with F. occidentalis (experiment 1A and 2A) and T. tabaci (experiment 1B and 2B).
An overview of plant material per experiment can be found in Table 1. BASF Vegetable Seeds Netherlands produced the two BC3S1 introgression lines that resulted from crosses between the resistance donor Capsicum annuum CGN16975 and two susceptible C. annuum breeding lines. A single F1 plant was used to make the backcrosses for each population (BP1 with background 1, and BP 2 with background 2, Table 2). Backcrosses and marker assisted selection were used to produce BC3 families. Seeds from CGN16975, Capsicum annuum accession AC1979, were obtained from the Centre for Genetic Resources, The Netherlands. Bejo Zaden BV produced two BC2S1 lines that resulted from a cross between a resistant F3 plant obtained from the mapping population of Maharijaya et al. (2015) with the QTL region on chromosome 6 in homozygous state and two susceptible C. annuum backgrounds (BP3 with background 3, and BP4 with background 4, Table 2). For each background, the best BC2 with the QTL in heterozygous state and the highest % of recurrent background was selected to produce the BC2S1, using foreground and background selection. Markers flanking the QTL on chromosome 6 were used to select for the presence of the resistance allele in the next generations (Table 3). Background selection was applied for backgrounds 3 and 4 using SNP markers evenly distributed over all chromosomes. BC3 plants (background 1 and 2) and BC2 plants (background 3 and 4) that were heterozygous for the QTL region on chromosome 6 were selfed to obtain plants with either the resistance or the susceptibility allele in homozygous state for the QTL region on chromosome 6 in the different backgrounds.
For the BC3S1 background 1 and 2, seeds were sown end of June 2018 together with CGN16975A, a first generation inbred line of a CGN16975 plant from the CGN16975 seed batch (obtained from the Centre for Genetic Resources, The Netherlands) as resistant reference and backcross parents 1 and 2 (BP1 and BP2) as susceptible references. For the BC2S1 background 3 and 4, seeds were sown end of March 2019 together with CGN16975A and seeds from the same seed batch (resistant F3 line) that was used as a resistance donor for the BC2S1 as resistant references. A second generation inbred line from susceptible accession CGN17219 (CGN17219A) was used as a susceptible reference. All seeds were sown in potting compost in a greenhouse of Unifarm, Wageningen University and Research, Wageningen, The Netherlands. The plants were grown at 25 °C, with a photoperiod of L16:D8, and 70% relative humidity. Orius laevigatus (Fieber) was obtained from Entocare C.V. (Wageningen, The Netherlands) and used to control thrips during culturing. Plants were watered three times a week. Two times per week, nutrients were added.
DNA extraction and genotyping
Leaves from the BC3S1 plantlets with background 1 and 2 were sampled at a plant age of 2,5 weeks and sent to BASF Vegetable Seeds for DNA extraction and genotyping. Leaves from the BC2S1 plantlets with background 3 and 4 were sampled at a plant age of 3 weeks and sent to Bejo Zaden BV for DNA extraction and genotyping. KASP-markers were designed based on SNPs flanking the 2-lod interval of the QTL region as previously defined by Maharijaya et al. (2015) (Table 3) and used for plant selection. Plants with the resistance (R) or susceptibility (S) allele in the QTL-region on chromosome 6 in homozygous state were selected for phenotyping at a plant age of 10 weeks (background 3 and 4) or 12 weeks (background 1 and 2).
Thrips rearing and synchronization
A population of Frankliniella occidentalis Pergande and a population of Thrips tabaci Lindeman were used to phenotype the plants for thrips resistance. The population of F. occidentalis originated from Greenhouse Horticulture of Wageningen University and Research, Bleiswijk, The Netherlands and was reared on Phaseolus vulgaris beans. As F. occidentalis is a polyphagous species and feeds on multiple crops, it is expected that thrips rearing history does not affect the level of resistance observed, as has been shown previously in tomato by Vosman et al. (2018). The population of T. tabaci originated from Allium ampeloprasum var. porrum plants grown in a tunnel near Unifarm, Wageningen University and Research, Wageningen, The Netherlands and reared on leaf pieces of the same species. Each population was maintained in glass jars covered with a thrips proof gauze at 25 °C, L16:D8, and 70% relative humidity.
Synchronized first instar larvae (L1) of both thrips species were obtained by allowing female adults to lay eggs for 24 h on snack cucumbers, after which the adults were removed and the cucumbers were transferred to a new glass jar and kept in a growth cabinet at 25 °C. After 4 days for F. occidentalis and 5 days for T. tabaci, the new synchronized first instar larvae emerged.
Detached leaf assay
For background 1 and 2, 40 BC3S1 plants per background were selected for phenotyping, of which 20 had the resistance (“R”) allele and 20 plants had the susceptibility (“S”) allele in homozygous state. For background 3 and 4, 10 BC2S1 plants, 5 with the “R” allele and 5 with the “S” allele in homozygous state per background were selected for phenotyping. Larval development from first into second instar larvae was used as a parameter for thrips resistance (Van Haperen et al. 2019). The first four fully opened leaves of each plant were collected. Each leaf was placed with the petiole in 1.5% water agar in a Petri Dish (BD Falcon, tight-fit, 50 × 9 mm). Five first instar larvae were added to each leaf. At 3, 5 and 7 days after infestation, the developmental stage of the larvae was determined. The fraction of first instar larvae that did not develop into the next developmental stage was used as a resistance parameter. This development occurs in 1–2 days (F. occidentalis) and 1–3 days (T. tabaci) under optimal conditions (Lublinkhof and Foster 1977).
Statistical analysis
The fraction L1 was transformed as y = arcsine(√x). Fractions L1 on plants with background 1 and 2 were analysed separately from fractions L1 on plants with background 3 and 4, as these experiments were conducted at different time points. The transformed fractions L1 on plants with background 1 and 2 (experiment 1) and plants with background 3 and 4 (experiment 2) were used in two Three-Way analysis of variance (ANOVAs) to see whether there was an effect of the QTL genotype carrying either the resistance “R” or susceptibility “S” allele for the QTL region on chromosome 6, an effect of background (background 1 and 2, and background 3 and 4 respectively), and an effect of thrips species on larval development. When no significant three-way interaction was observed, the Three-way ANOVAs were repeated excluding the three-way interactions to confirm the significant two-way interactions. Subsequently, two-way interactions that were not significant (P > 0.05) were excluded. A post hoc Fisher’s Protected LSD was used to determine which groups showed significant differences in larval development. The PVE% for each background was determined by dividing the sum of squares for QTL genotype by the total corrected sum of squares.
Results
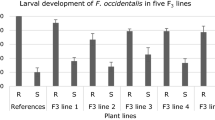
Two Three-Way ANOVAs with QTL genotype (“R” or “S” allele), thrips species (F. occidentalis or T. tabaci) and genetic background (background 1 and 2, and background 3 and 4 respectively) as factors followed by a Fisher’s Protected LSD were used to determine the effects of each factor on larval development, possible interactions between these factors, and whether larval development was significantly different between groups. In all four backgrounds investigated, the level of thrips resistance was higher in leaves of plants that have the resistance (“R”) allele in homozygous state for the QTL region on chromosome 6, than on leaves of plants that have the susceptibility (“S”) allele for the QTL region, both in the lines with background 1 and 2 (Table 4; experiment 1; Supplementary data Table S1) and in the lines with background 3 and 4 (Table 5; experiment 2; Supplementary data Table S2). This is evident based on the high fraction of L1 which indicates resistance as only few L1s developed into the next larval stage. A significant difference between group “R” and group “S” was observed for both thrips species (P < 0.001 in all cases). The explained variance of the QTL genotype was 52,4% and 58,6% for background 1 and 2, and 62,4% and 74,1% for background 3 and 4 respectively. In addition, we observed a significant difference between the two thrips species on the different groups of plants. Frankliniella occidentalis showed a significantly lower fraction of L1 compared to T. tabaci. In background 1 and 2 (experiment 1) this difference was observed in all QTL genotypes, but in background 3 and 4 (experiment 2) only in the plants with the “S” allele for the QTL region on chromosome 6 and the susceptible reference. We also observed a significant difference in F. occidentalis larval development when comparing group “R” and group “S” with genetic background 1 to group “R” and group “S” with genetic background 2 respectively, whereas these groups with different backgrounds do not show a significant difference in T. tabaci larval development. This observation resulted in a significant interaction between background and thrips species for background 1 and 2 (P = 0.004). This interaction was not observed in background 3 and 4 (P = 0.294). In genetic background 3 and 4, we observed a significantly higher fraction L1 for each thrips species in group “R” with background 4, compared to group “R” with background 3, whereas the fractions L1 in group “S” did not significantly differ between backgrounds for each thrips species respectively. This resulted in a significant interaction between QTL genotype (group “R” and group “S”) and background (P < 0.001). This significant interaction was not observed in background 1 and 2 (P = 0.611).
Discussion
In this study we showed the effect of the QTL on chromosome 6 in different Capsicum annuum backgrounds, using two thrips species. Pepper plants carrying the CGN16975 resistance allele in homozygous state for the QTL region on chromosome 6 showed a significantly higher level of thrips resistance to both F. occidentalis and T. tabaci in all four backgrounds tested, than plants that carry the susceptibility allele in homozygous state for this QTL region. This indicates that one of the genes in the selected interval affects larval development in both thrips species, or that two closely linked genes each affect one of the thrips species. We also observed significant differences in susceptibility to thrips between the different backgrounds: susceptibility to F. occidentalis in background 1 was significantly higher compared to background 2, and background 4 had significantly higher levels of resistance to both thrips species compared to background 3 when the plants carry the resistance allele in homozygous state. This indicates that additional factors in the genetic background outside of the QTL region also contribute to thrips resistance, affecting F. occidentalis, T. tabaci, or both thrips species. Maharijaya et al. (2015) calculated a broad sense heritability of 0.93–0.96 for the F. occidentalis resistance parameters, which suggests that the phenotypic variation is hardly affected by environmental factors (within the setup used in these experiments). Therefore there must be genetic factors other than the QTL on chromosome 6 that also affect resistance. This is as expected, as the QTL on chromosome 6 explains only 45–50 percent of the variance in the F2 population studied by Maharijaya et al. (2015). The genetic factors determining the unexplained part of the variance were presumably too small to be detected in the original mapping population, as was also suggested by Maharijaya et al. (2015). Also in Arabidopsis thaliana (Méndez-Vigo et al. 2013) and rice (Liao et al. 2001; Cheng et al. 2011) a significant effect of genetic background on QTLs has been shown. Therefore it is important to validate the effect of QTLs of interest in plants that have different alleles for the QTL region, but also show high similarities in the background outside of the QTL region. In addition, it is important to validate the effect of the QTL in different backgrounds, as additional factors that affect thrips resistance might be present or lacking.
We show that the resistance mechanism underlying the QTL, inhibition of larval development, affects both F. occidentalis and T. tabaci in all backgrounds tested. A significantly higher level of inhibition of larval development was observed for both thrips species in plants that carry the resistance allele for the QTL region, compared to plants that carry the susceptibility allele. In addition, the resistance source CGN16975, from which the QTL is derived, showed a significant effect on thrips damage caused by Thrips parvispinus (Karny) (Maharijaya et al. 2011). However, as the mapping of the resistance QTL derived from CGN16975 was done by screening larval development and damage of F. occidentalis, we do not know whether our QTL also affects larval development of T. parvispinus. Nevertheless, our study of the QTL effect on two thrips species shows that this QTL is a valuable source for breeding varieties with a broad range of resistance to thrips. Similar findings of QTLs conferring resistance to natural thrips populations consisting of two thrips species, i.e. T. tabaci and Frankliniella schultzei (Trybom), was found in cowpea, although it was not determined whether each QTL contributed to resistance to both thrips species, or to only one of the tested thrips species (Muchero et al. 2010). Contrary to our observations, no correlation was found between resistance to F. occidentalis and T. tabaci in another pepper study (Visschers et al. 2019a). However, in that study overall thrips resistance was compared in different Capsicum accessions, while our study focused on the effect of a QTL on thrips larval development within and between different backgrounds. In a follow up study Visschers et al. (2019b) also evaluated other thrips species, in which they found a correlation between resistance to F. occidentalis and Scirtothrips dorsalis (Hood), but not to Thrips palmi (Karny) in C. annuum. It seems that different genetic factors play a role in different thrips-resistant Capsicum accessions, and that these factors may contribute to resistance to one or multiple thrips species.
In the experiments with lines containing background 1 and 2, as well as with lines containing background 3 and 4, interactions between different main factors (i.e. genetic background and thrips species, and genetic background and QTL genotype respectively) were observed. These interactions can be interpreted by further analysing the main observations in the different experiments. When comparing lines with background 1 to lines with background 2, we observed significant differences in plants with the same QTL genotype (group “R” background 1 to group “R” with background 2, and group “S” with background 1 and group “S” with background 2 respectively) when screened with F. occidentalis, and not when screened with T. tabaci. This might indicate that additional genetic factors that affect F. occidentalis, but not T. tabaci performance are present, either factors in background 1 that increase F. occidentalis performance, or factors in background 2 that decrease F. occidentalis performance, and thus explain the significant interaction between these factors. In the experiment in which lines with background 3 and 4 were tested, we observed a significant interaction between QTL genotype (group “R” and group “S”) and background (background 3 and background 4). This interaction can be explained, because we observed a significantly higher resistance to both thrips species in background 4 compared to background 3 in plants that have the “R” allele. We did not observe a significant difference in larval development for both thrips species when plants have the “S” allele. These results suggest the presence of factors in background 4 that only enhance thrips resistance when the “R” allele for the QTL region on chromosome 6 is present. We did not observe a significant interaction between QTL genotype and background in the experiment with lines with background 1 and 2. These differences between the group “R” and group “S” with different backgrounds, screened with two thrips species, show that other genetic factors outside of the QTL region contribute to thrips resistance, and that these factors and their interactions should be taken into account in future research.
Altogether, this study shows the importance of studying the effect of a QTL in different genetic backgrounds, as our study indicates that different factors in the tested genetic backgrounds affect thrips resistance. Further elucidation of the resistance mechanism underlying the QTL might help in pinpointing these additional factors and their role in enhancing or reducing thrips resistance. Most importantly, we show that our QTL has a major effect on resistance to two thrips species in all four tested genetic backgrounds; thus we expect that this QTL will also be effective in other genetic backgrounds. Our findings show that this QTL can be used for breeding thrips-resistant Capsicum varieties.
References
Cheng L, Wang Y, Meng L, Hu X, Cui Y, Sun Y, Zhu L, Ali J, Xu J, Li Z (2011) Identification of salt-tolerant QTLs with strong genetic background effect using two sets of reciprocal introgression lines in rice. Genome 55:45–55
Chisholm IF, Lewis T (1984) A new look at thrips (Thysanoptera) mouthparts, their action and effects of feeding on plant tissue. Bull Entomol Res 74:663–675
Kirk WDJ, Terry LI (2003) The spread of the western flower thrips Frankliniella occidentalis (Pergande). Agric For Entomol 5:301–310
Liao CY, Wu P, Hu B, Yi KK (2001) Effects of genetic background and environment on QTLs and epistasis for rice (Oryza sativa L.) panicle number. Theor Appl Genet 103:104–111
Lublinkhof J, Foster DE (1977) Development and reproductive capacity of Frankliniella occidentalis (Thysanoptera: Thripidae) reared at three temperatures. J Kansas Entomol Soc 50:313–316
Maharijaya A, Vosman B, Steenhuis-Broers G, Harpenas A, Purwito A, Visser RGF, Voorrips RE (2011) Screening of pepper accessions for resistance against two thrips species (Frankliniella occidentalis and Thrips parvispinus). Euphytica 177:401–410
Maharijaya A, Vosman B, Verstappen F, Steenhuis-Broers G, Mumm R, Purwito A, Visser RGF, Voorrips RE (2012) Resistance factors in pepper inhibit larval development of thrips (Frankliniella occidentalis). Entomol Exp Appl 145:62–71
Maharijaya A, Vosman B, Steenhuis-Broers G, Pelgrom K, Purwito A, Visser RGF, Voorrips RE (2015) QTL mapping of thrips resistance in pepper. Theor Appl Genet 128:1945–1956
Méndez-Vigo B, Martínez-Zapater JM, Alonso-Blanco C (2013) The flowering repressor SVP underlies a novel Arabidopsis thaliana QTL interacting with the genetic background. PLoS Genet 9:e1003289
Muchero W, Ehlers JD, Roberts PA (2010) QTL analysis for resistance to foliar damage caused by Thrips tabaci and Frankliniella schultzei (Thysanoptera: Thripidae) feeding in cowpea [Vigna unguiculata (L.) Walp.]. Mol Breed 25:47–56
Mumford RA, Barker I, Wood KR (1996) The biology of the tospoviruses. Ann Appl Biol 128:159–183
Riley DG, Joseph SV, Srinivasan R, Diffie S (2011) Thrips vectors of tospoviruses. J Integr Pest Manag 2:I1–I10
Shipp JL, Hao X, Papadopoulos AP, Binns MR (1998) Impact of western flower thrips (Thysanoptera: Thripidae) on growth, photosynthesis and productivity of greenhouse sweet pepper. Sci Hortic 72:87–102
Ullman DE, Meideros R, Campbell LR, Whitfield AE, Sherwood JL, German TL (2002) Thrips as vectors of tospoviruses. Adv Bot Res 36:113–140
Van Haperen P, Voorrips RE, van Loon JJA, Vosman B (2019) The effect of plant development on thrips resistance in Capsicum. Arthropod-Plant Interact 13:11–18
Van Lenteren JC, Loomans AJ (1999) Biological control of thrips: how far are we. Bull IOBC 22:141–144
Visschers IGS, Peters JL, van de Vondervoort JAH, Hoogveld RHM, van Dam NM (2019a) Thrips resistance screening is coming of age: leaf position and ontogeny are important determinants of leaf-based resistance in pepper. Front Plant Sci 10:510
Visschers IGS, Peters JL, Timmermans LLH, Edwards E, Ferrater JB, Balatero CH, Stratongjun M, Bleeker PM, van Herwijnen Z, Glawe GA, Bruin J, van Dam NM, Macel M (2019b) Resistance to three thrips species in Capsicum spp. depends on site conditions and geographic regions. J Appl Entomol 143:929–941
Vosman B, van’t Westende WPC, Henken B, van Eekelen HDLM, de Vos RCH, Voorrips RE (2018) Broad spectrum insect resistance and metabolites in close relatives of the cultivated tomato. Euphytica 214:46
Welter SC, Rosenheim JA, Johnson MW, Mau RFL, Gusukuma-Minuto LR (1990) Effects of Thrips palmi and western flower thrips (Thysanoptera: Thripidae) on the yield, growth, and carbon allocation pattern in cucumbers. J Econ Entomol 83:2092–2101
Wijkamp I, Almarza N, Goldbach R, Peters D (1995) Distinct levels of specificity in thrips transmission of tospoviruses. Phytopathology 85:1069–1074
Acknowledgements
We thank Betty Henken for her assistance with carrying out the detached leaf assay with the BC3S1 material. We thank BASF Vegetable Seeds and Bejo Zaden B.V. for providing the BC3S1 and BC2S1 seeds and for their help in genotyping the plants. This research was supported by a Grant (TKI 1409-045) from the Ministry of Economic Affairs of The Netherlands and by breeding companies Bejo Zaden B.V. and BASF Vegetable Seeds.
Author information
Authors and Affiliations
Contributions
PvH, BV, REV and JvL conceived and designed the experiments. AFL and WS produced the BC3S1 and BC2S1 respectively. PvH carried out the detached leaf assays, data analysis and produced the first draft of the manuscript. BV, REV and JvL were involved in revising the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van Haperen, P., Voorrips, R.E., Lucatti, A.F. et al. The effect of a thrips resistance QTL in different Capsicum backgrounds. Euphytica 216, 187 (2020). https://doi.org/10.1007/s10681-020-02725-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-020-02725-4