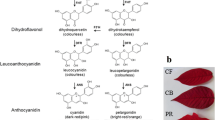
Abstract
In general, carnations (Dianthus caryophyllus) have each of four kinds of anthocyanins acylated by malic acid. A few carnation cultivars are known to display a peculiar dusky color supposedly caused by anthocyanic vacuolar inclusions (AVIs). The hereditary pattern suggests that the peculiar color is controlled by a single recessive factor tightly linked with existence of AVIs containing non-acylated anthocyanins. To diversify the peculiar color carnation, we produced a bluish purple line displaying a highly novel metallic appearance by crossbreeding. By subjecting the line to ion-beam irradiation, we generated metallic reddish purple, metallic crimson and metallic red lines. The major anthocyanin of the metallic bluish purple and reddish purple lines was pelargonidin 3,5-diglucoside, whereas that of the metallic crimson and red lines was pelargonidin 3-glucoside. All four metallic lines did not have transcripts for anthocyanin malyltransferase. Metallic crimson and red lines did not express the acyl-glucose-dependent anthocyanin 5-O-glucosyltransferase gene. In contrast to the dusky color types, metallic lines have highly condensed AVIs and water-clear vacuolar sap in the petal adaxial epidermal cells. Differences in the number of AVIs on the abaxial side were observed within mutants containing the same anthocyanin, thereby affecting their shade and hue. We demonstrated that (1) a factor generating the AVIs is inactivated anthocyanin malyltransferase gene, (2) AVIs in water-clear vacuolar sap in the adaxial epidermal cells generate the novel metallic appearance, and (3) ion beam breeding is a useful tool for increasing metallic colors by changing anthocyanin structure and the level of AVIs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carnation (Dianthus caryophyllus) is one of the most important horticultural crops around the world. Development of novel flower colors has great impact both economically and culturally; therefore, it is essential and challenging to create novel colors. Carnations have basic colors consisting of cyanic and non-cyanic molecules; anthocyanins are responsible for the cyanic colors, flavonoids are responsible for yellow color and chlorophyll is responsible for green color. There are also orange or brown colors achieved by combination of the pigments mentioned above.
Four major anthocyanins are known in carnation: pelargonidin 3-malylglucoside (Pg3MG), cyanidin 3-malylglucoside (Cy3MG), pelargonidin 3,5-cyclicmalyldiglucoside (Pg3,5cMdG), and cyanidin 3,5-cyclicmalyldiglucoside (Cy3,5cMdG) (Bloor 1998; Nakayama et al. 2000). All of these anthocyanins are glycosylated and are normally acylated by malic acid. One carnation cultivar has a specific anthocyanin as its major anthocyanin; carnation cultivars having anthocyanins Pg3MG, Cy3MG, Pg3,5cMdG or Cy3,5cMdG are generally red, dark red, pink or purple, respectively. Although violet carnations accumulating delphinidin have been produced by genetic engineering (Tanaka and Ohmiya 2008), we have chosen another path to achieve novel carnation colors with non-genetically modified organisms. The structural simplicity of anthocyanin constituents makes it laborious to produce novel flower colors in carnation. The fact that wild Dianthus species and their relatives have the same anthocyanins as those found in carnation cultivars increases the difficulty in enriching flower color variation by crossing carnation cultivars with wild Dianthus species and relatives.
Some carnation cultivars are known to display dusky and metal flower colors (defined herein as peculiar colors) when their petal epidermal cells contain anthocyanin inclusions in vacuoles. Pigment inclusions were investigated in several flower species and referred to as anthocyanic vacuolar inclusions or AVIs (Markham et al. 2000). However, information on the relationship between AVIs and peculiar colors is limited (Morita et al. 2005, Zhang et al. 2006), and there are no reports on the hereditary pattern of peculiar coloration and AVIs other than our preliminary report (Okamura et al. 2012). Furthermore, there are very few kinds of peculiar color phenotypes in carnation. We focused on diversifying peculiar colors in an attempt to create a variety of novel colors in carnation. Mutation induction using gamma and X-rays have been used successfully to create new flower colors in horticultural crops including carnations (the FAO/IAEA Mutant Variety Database, http://mvgs.iaea.org/). We have already succeeded in breeding a variety of new colors in carnation by ion-beam irradiation (Okamura et al. 2003). Since ion beams deposit high energy locally and densely on genomic DNA resulting in a few changed traits with reduced undesired alterations as compared to X- or gamma- ray treatment (Tanaka et al. 2010), we recognized that mutagenesis by ion beams would be quite an effective technique to diversify colors while retaining peculiar color expression.
Firstly, we found breeding lines that display peculiar color from among our gene pool and investigated the heredity of peculiar color. Then, we made several crosses to breed a line displaying a novel metallic appearance and a more bluish purple color than any other existing cultivars. Subsequently, we tried to diversify metallic color by ion-beam irradiation. This study discusses the relationship between peculiar colors, constituents of anthocyanins and related compounds, the genes and characteristics of AVIs involved in the color expression.
Materials and methods
Cultivation
Carnation cultivars and breeding lines were cultivated in a greenhouse at Central Laboratories for Key Technologies, Kirin Co., Ltd. (Sakura, Tochigi, Japan) under natural light conditions. The temperature was maintained between 16 and 30 °C.
Crossing experiment
Carnation cultivars ‘Beam Cherry’ (pink, normal color) and ‘Nazareno’ (dusky purple, peculiar color with AVIs) (Fig. 1a), breeding lines ‘07MC4’ (pink, normal color), ‘04MC1’ (pink, normal color), ‘06MP1’ (pink, normal color), and ‘01MA1’ (dusky purple, peculiar color with AVIs) were used. Female parental plants were emasculated just before anthesis and pollinated with the pollen of male parental plants. Pollinated flowers were covered with paper bags to avoid accidental pollination until mature seeds were harvested. Seeds were planted in 128-cell trays, and seedlings with six leaves were transplanted and grown in the greenhouse.
Flower phenotypes (a), microscopic photographs of transverse cross-sections of petal epidermal cells (b), and the structures of anthocyanins (c) of carnation cultivars; (left to right), ‘Beam Cherry’ (pink, no AVIs), ‘Red Vital’ (red, no AVIs), and ‘Nazareno’ (dusky purple, AVIs rich). Scale bars in microscopic photographs indicate 30 μm
Mutagenesis
The surfaces of carnation petals were sterilized with 70 % ethanol for 30 s and then with 0.5 % sodium hypochlorite for 3 min, rinsed three times with sterilized water and placed in petri dishes containing MS medium (Murashige and Skoog 1962) supplemented with 2 mg/l zeatin, 30 g/l sucrose and 7 g/l agar (MSZ medium). The samples were irradiated with 320 MeV carbon ions (LET 76 keV/μm) from an AVF cyclotron at Japan Atomic Energy Agency (Takasaki, Gunma, Japan). After irradiation, tissues were transferred onto fresh MSZ medium and cultured at 25 °C in a growth chamber under a 16-hour photoperiod supplied by fluorescent lights (50 μE m−2 s−1). Regenerated shoots were transferred onto MS medium supplemented with 30 g/l sucrose and 7 g/l agar for rooting. The rooted shoots were acclimatized in the greenhouse. Cuttings were collected and rooted in 128-cell trays, and then grown in the greenhouse as described above.
Isolation, identification and quantitative determination of anthocyanins
The major anthocyanins and related compounds of ‘Lines A–D’ were analyzed using high-performance liquid chromatography (HPLC). Anthocyanins were extracted from carnation petals with 10 % acetic acid. Aliquots (10 μl) of the extracts were analyzed using the HP1100 system equipped with a photodiode array detector (Agilent Technologies-Yokokawa Analytical Systems, Tokyo, Japan) and an Inertsil ODS-2 column (4.6 × 250 mm, GL science, Tokyo, Japan) at 40 °C with a flow rate of 0.8 ml/min. Absorption spectra were monitored at 200-600 nm. A linear gradient of 10–50 % of solvent B (1.5 % H3PO4, 40 % acetonitrile, 50 % acetic acid) in solvent A (1.5 % H3PO4) ran over a 40 min period. Anthocyanins were detected and quantified based on their absorption at 505 nm, and the concentrations were calculated as pelargonidin 3-rutinoside equivalents. Flavonoids were detected and quantified based on their absorption at 360 nm, and the concentrations were calculated as quercetin 3-rutinoside equivalents.
Observation of petal epidermal cells
Transverse sections (100 μm thick) of mature petals were prepared using a Plant Microtome (model MTH-1, Nippon Medical & Chemical Instruments Co., Ltd., Osaka, Japan). The sections were put on glass slides and observed with a microscope (Olympus Biological Microscope model BX53).
Expression analysis and genomic PCR analysis
Carnation petals were frozen in liquid nitrogen and ground with a mortar and pestle; total RNAs were then extracted using an RNeasy mini kit (QIAGEN, Hilden, Germany). First-strand cDNAs were synthesized from 500 ng of each total RNA using an oligo dT adapter primer (Takara Bio, Ohtsu, Japan) and M-MLV Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA). PCR was performed with SapphireAmp Fast PCR Master Mix (Takara Bio) using the following two primer sets: DcAMalTFwd (5′-ATGACACTTTCGCCGCTTGATAATTTG-3′) and DcAMalTRev (5′-CTAGAAAGAGGATAATCCAAGCCACC-3′); DcAA5GTFwd (5′-ATGAACATGTCATGCAAGTTTG-3′) and DcAA5GTRev (5′-CCTCGAGTACCATTTTTGCG-3′). The PCR conditions were as follows: 1 min denaturation at 94 °C; then 30 cycles of 5 s denaturation at 98 °C, 5 s annealing at 58 °C, and 20 s extension at 72 °C. For genomic PCR, DNAs were extracted from carnation petals or leaves using DNeasy Plant Mini Kit (QIAGEN) according to the manufacture’s manual. Primer sets used to amplify the AA5GT regions were designed as follows: 5GT5′F, 5′-GATCGATGAATTGTAAGTTGTAACC-3′, and 5GT3′R, 5′-AATTCGGTGGCAAAGCCGCCGGAAC-3′ (for detection of AA5GT region including full length of coding region); 5GT5′F and 5GTR09, 5′-CTGTTTCCACCATGAGTTCGACATCTTC-3′ (for detection of AA5GT 5′ region); 5GTF01, 5′-GCCAGGCAACGGAGACATAACGTCC-3′, and 5GT3′R (for detection of AA5GT 3′ region) (Fig. 3b). To detect the AA5GT/Ty1dic1 genome region, the primer set (5′UTF and Ty1Rev) designed in previous report were used (Nishizaki et al. 2011). Genomic PCR were performed using Prime Star GXL DNA polymerase (Takara Bio) with the following conditions: 3 min for denaturation at 96 °C, then after 30 cycles of 10 s denaturation at 96 °C, 15 s annealing at 56 °C, and 90 s extension at 68 °C.
Results
Heredity of the peculiar color phenotype and linkage with the presence of AVI
Among our carnation collections, we found several cultivars and breeding lines that have dusky purple flowers (similar to ‘Nazareno’ in Fig. 1a). Microscopic observation revealed that all of the peculiar color lines had AVIs in their petal epidermal cells (similar to ‘Nazareno’ in Fig. 1b). In order to examine the hereditary pattern of the peculiar color phenotype, we conducted reciprocal crosses between the breeding lines ‘07MC4’ and ‘04MC1’, both of which have pink petals (similar to ‘Beam Cherry’ in Fig. 1a) and no AVIs in their petal epidermal cells (similar to ‘Beam Cherry’ in Fig. 1b). These crosses were conducted because we previously found that both of these normal color lines produced some peculiar color descendants by self-pollination. Plants obtained from the cross ‘07MC4’ × ‘04MC1’ consisted of 69 normal and 25 peculiar color plants (Table 1). This result was consistent with a 3:1 segregation ratio (χ2 = 0.128, P = 0.72). Microscopic observation showed that all of the 25 peculiar color plants had AVIs in a large part of their petal epidermal cells. Plants obtained from the cross ‘04MC1’ × ‘07MC4’ also showed a 3:1 segregation ratio (139 normal color: 47 peculiar color, χ2 = 0.007, P = 0.93). When ‘07MC4’ was crossed with the peculiar color line ‘01MA1’, 55 normal and 49 peculiar color plants were obtained (=1:1, χ2 = 0.346, P = 0.56). Almost all of the plants obtained by the cross between peculiar color lines (‘01MA1’ × ‘Nazareno’) had peculiar flower color (59 out of 60 plants). Petal epidermal cells of the ten randomly chosen peculiar color plants from each of the latter three crossings were microscopically examined, revealing that all of them had AVIs in their petal epidermal cells. These facts are consistent with the hypothesis that the peculiar color phenotype is conferred by AVIs formation, which is controlled by a single recessive allele. These results also suggest that both ‘07MC4’ and ‘04MC1’ lines are heterozygous for this locus.
We further scrutinized our carnation collections for the segregation of normal and the peculiar color phenotype. We found that the carnation cultivars ‘Beam Cherry’ (pink, normal color, no AVIs) and ‘Red Vital’ (red, normal color, no AVIs) produced no peculiar color plants when they were crossed with ‘Nazareno’ (dusky purple, peculiar color, AVIs rich) (Table 1). In contrast, the breeding line ‘06MP1’ (pink, normal color, no AVIs) produced 24 peculiar plants out of 45 seedlings after crossing with ‘Nazareno’ (Table 1). These results are consistent with the idea that the ‘Beam Cherry’ and ‘Red Vital’ are homozygous for the wild-type allele, and ‘06MP1’ is heterozygous for a recessive allele that is required to express the peculiar color.
Anthocyanin composition in the peculiar color phenotype
To gain more insight into the expression of the peculiar color phenotype, we analyzed the anthocyanins of the cultivars and breeding lines. Normal color carnations, ‘Beam Cherry’, ‘07MC4’ and ‘04MC1’ had Pg3,5cMdG, and ‘Red Vital’ had Pg3MG as its major anthocyanin (Table 2). Peculiar color carnations, ‘01MA1’ and ‘Nazareno’ had Pg3,5dG as their major anthocyanin. This result suggested that the non-acylated type anthocyanins may play an essential role in AVIs formation. Furthermore, we investigated the anthocyanins of five randomly selected individuals from both peculiar and normal color populations obtained from the cross ‘07MC4’ × ‘04MC1’. Whereas all five of the peculiar color individuals predominantly contained Pg3,5dG, a non-acylated anthocyanin, all five of the normal colored flowers predominantly contained Pg3,5cMdG, an acylated anthocyanin. These results suggest the correlation between the presence of non-acylated anthocyanins and AVIs formation, at least in the carnation materials used here. Since the addition of a malate moiety to anthocyanins is catalyzed by anthocyanin malyltransferase (AMalT) and carnation has a single copy of the AMalT gene (Abe et al. 2008; Umemoto et al. 2009), our examination of the heredity of peculiar color and anthocyanin acylation strongly suggest that peculiar color is conferred by the inactivation of the AMalT gene.
Creating novel peculiar color lines
From the peculiar color plants obtained by the cross ‘07MC4’ × ‘04MC1’, we selected an individual with the most metallic, bluish purple flower. Stability of the unique flower color was confirmed after vegetative propagation and then we designated the individual as ‘Line A’ (Fig. 2a). We further tried to diversify the metallic flower color by the ion-beam breeding technique (Okamura et al. 2012). Petal segments of ‘Line A’ were irradiated with 320 MeV carbon ions and cultured in vitro to obtain regenerated plants. Flowers of the ion-beamed plants were examined in greenhouses. The frequency of occurrence of flower color mutants increased as the irradiation dose increased (Table 3). Among a variety of mutants obtained, we finally selected three mutants displaying the most metallic colors and named them ‘Line B’ (metallic reddish purple), ‘Line C’ (metallic crimson) and ‘Line D’ (metallic red), respectively (Fig. 2a). Others in Table 3 are consisting of one Pink and one Slightly Metallic Pink from a 20 Gy irradiation, and five Dusky Violet mutants.
Flower phenotypes (a), microscopic photographs of transverse cross-sections of petal epidermal cells (b), and the structures of anthocyanins (c) of carnation breeding lines; (left to right), ‘Line A’ (metallic bluish purple, AVIs rich), ‘Line B’ (metallic reddish purple, AVIs average), ‘Line C’ (metallic crimson, AVIs rich) and ‘Line D’ (metallic red, AVIs average). Scale bars in microscopic photographs indicate 30 μm
Characteristics of AVIs in the metallic color lines
Petal epidermal cells of the four metallic color lines, ‘Lines A-D’, were microscopically observed to investigate the characteristics of AVIs (Fig. 2b). All four of the metallic lines have AVIs throughout the epidermal cells on the petal adaxial side. The color of vacuolar sap on the adaxial side of petal epidermal cells is much lighter in the four metallic lines than that of typical dusky purple types such as ‘Nazareno’, and the AVIs of the four metallic lines are highly condensed. In addition, differences in the number of anthocyanin inclusions on the abaxial side were observed for mutants containing the same anthocyanin (Fig. 2), a phenomenon that affects shade and hue as well. That is, in the petal epidermal cells on the abaxial side, the level of pigment inclusion is much lower and the color of the vacuolar sap is much deeper in ‘Lines B and D’ compared with those of ‘Lines A and C’. Unique, blue inclusions were observed in most of the abaxial epidermal cells of ‘Line C’ (Fig. 2b).
Comprehensive analysis of anthocyanins and related compounds in the peculiar color lines
The major anthocyanins and related compounds of ‘Lines A–D’ were analyzed using HPLC (Table 4). The dominant anthocyanin of ‘Line A’ and ‘Line B’ was Pg3,5dG, whereas that of ‘Line C’ and ‘Line D’ was Pg3G. Anthocyanin concentration was compared between the lines containing the same anthocyanin. The concentration of ‘Line B’ was about 68 % of ‘Line A’, and that of ‘Line D’ was about 86 % of ‘Line C’. Major flavonoids of ‘Lines A-D’ were the same, namely kaempferol 3-(6′′-rhamnosyl-sophoroside) and kaempferol 3-sophorosid.
Expression analysis and genomic PCR analysis of the AMalT and AA5GT genes in the peculiar color lines
AMalT mediates the transfer of a malyl-group to Pg3,5dG from malyl-glucose (Abe et al. 2008). Anthocyanin 5-O-glucosyltransferase (AA5GT) catalyzes the glycosylation of Pg3G into Pg3,5dG (Matsuba et al. 2010). We analyzed the expression of the AMalT and AA5GT genes in the four metallic color lines using RT-PCR. Transcripts for AMalT were detected in ‘Beam Cherry’, whereas AMalT transcripts were not detected in any of the four metallic lines. Although AA5GT transcripts were detected in both ‘Line A’ and ‘Line B’, no AA5GT transcripts were detected in ‘Line C’ or ‘Line D’ (Fig. 3a). The expression analysis results correspond well with the anthocyanin present in each line. The results also verify the correlation between the lack of AMalT activity and AVIs.
a RT-PCR analysis of AA5GT and AMalT in a carnation cultivar and breeding lines: (left to right) cv. ‘Beam Cherry’ (pink, no AVIs), ‘Line A’ (metallic bluish purple, AVIs rich), ‘Line B’ (metallic reddish purple, AVIs average), ‘Line C’ (metallic crimson, AVIs rich) and ‘Line D’ (metallic red, AVIs average), b primer positions used for the genomic PCR for amplification of AA5GT genomic region. Primer sequences were described in Materials and methods. c Genomic PCR analysis using the genomic DNA extracted from ‘Line C’, ‘Line D’, and ‘Beam Cherry (BC)’ with the primer sets indicated above
Since there are instances that AA5GT is inactivated by transposon insertion (Nishizaki et al. 2011) and transposon insertion could be triggered by tissue culture effects (Neelakandan and Wang 2012), we carried out genomic PCR analysis to verify if the AA5GT gene itself has a mutation and to clarify what type of mutation has been generated in ‘Lines C and D’. Firstly, to confirm the AA5GT genotype of ‘Lines C and D before ion beam irradiations’, that is ‘Line A’, genomic PCR analysis was performed using the primer sets described in our previous study (Nishizaki et al. 2011). When the primer set 5′UTF and Ty1Rev, which was designed to detect specifically the AA5GT disrupted by the insertion of the transposable element (designated as AA5GT/Ty1dic1 in previous report, Nishizaki et al. 2011), was used, amplicons with the expected length were detected in both ‘Line C’ and ‘Line D’, as well as in a control ‘Beam Cherry’ (Fig. 3c). This means that ‘Line A’ harbored AA5GT-Ty1/copia and healthy AA5GT allele. Then we designed several primer sets to detect healthy AA5GT genomic region (Fig. 3b): 5GT5′F and 5GT3′R for detection of AA5GT region including full length of coding region; 5GT5′F and 5GTR09 for detection of AA5GT 5′ region; and 5GTF01 and 5GT3′R for detection of AA5GT 3′ region. When the primer sets designed to detect the full length of open reading frame, 5′ genomic region, or 3′ genomic region of healthy AA5GT were used, the expected length amplicons were detected in ‘Beam Cherry’, but not in ‘Lines C and D’ (Fig. 3c). These results suggest that a large disruption including the whole healthy AA5GT region is responsible for lacking a glucose moiety at the 5 position which was caused presumably by ion beam irradiation.
Discussion
Aiming to rationally diversify peculiar colors in carnation, we attempted to elucidate the hereditary pattern of the peculiar color phenotype and to identify genes involved in color expression. The segregation ratio of normal color to peculiar color individuals in the cross between two lines that previously segregated peculiar color by self-pollination, referred to herein as peculiar color segregators, turned out to be close to 3:1 (Table 1). In addition, the segregation ratio of normal to peculiar in the cross between the peculiar color segregator and peculiar color line was close to 1:1. These results indicate that the expression of the peculiar color phenotype is controlled by a single recessive allele. Major anthocyanins of all the normal color lines were anthocyanins acylated by malate, whereas the anthocyanins of all the peculiar color lines were non-acylated anthocyanins (Table 2). The enzyme catalyzing the addition of malate to the anthocyanin glycoside moiety in carnation is AMalT (Abe et al. 2008). Carnation is known to have single copy of the AMalT gene (Umemoto et al. 2009). Along with the fact that all the metallic lines with AVIs had no expression of the AMalT gene (Fig. 3a), we identified AMalT as the gene that controls peculiar color expression. This finding suggests that peculiar color lines have a amalt/amalt genotype and peculiar color segregators have a AMalT/amalt genotype.
Only when homologous genes are homozygous for inactivated AMalT (amalt) will non-acylated anthocyanin be synthesized, resulting in peculiar color expression. Therefore, all the descendants from the cross between peculiar color lines should display peculiar coloration; however, among 60 descendants from the cross, only one (about 1.7 %) displayed a normal color (Table 1). An allelic gene that is inactivated by the insertion of a transposon is reported to be reactivated by the excision of the inserted transposon (Itoh et al. 2002). Such a transposition event might have occurred in the descendants from the cross between the peculiar color lines.
Microscopic observation of petal epidermal cells revealed the presence of AVIs on the adaxial side of all lines displaying a peculiar color (Note in Table 1 and Figs. 1 and 2). AVIs formation in carnation is attributed, in part, to the accumulation of anthocyanins that are insoluble and condensed due to a lack of acylation. Thus far, the peculiar colors of carnations having AVIs in petal epidermis were dusky in appearance, which was described as blue-gray by Markham et al. (2000). Expression of the dusky color was reported also in Japanese morning glory containing AVIs in their epidermal cells (Morita et al. 2005). Since pigments give a darker tone when condensed, the dusky appearance of these plants is due to the presence of AVIs in their petals.
‘Line A’ obtained from crossbreeding normal color lines, and ‘Lines B–D’ selected from mutants of ion-beamed ‘Line A’, have a novel metallic appearance strikingly different from the dusky color reported so far. ‘Lines A–D’ have highly condensed AVIs in their adaxial petal epidermal cells (Fig. 2b). ‘Lines A and B’ predominantly contain Pg3,5dG, whereas ‘Lines C and D’ accumulate Pg3G as their major anthocyanin (Table 4). Glycosylation of anthocyanin at the 5 position is mediated by AA5GT (Matsuba et al. 2010). Although ‘Lines A and B’ showed expression of AA5GT mRNA, ‘Lines C and D’ did not express this gene (Fig. 3a). Molecular analysis of the AA5GT gene in the mutant ‘Lines C and D’ revealed that in both lines the gene itself has a kind of mutation lacking the whole AA5GT region. Both lines were regenerated from tissue cultures irradiated with ion beams. Tissue culture process can cause genetic changes such as DNA sequence changes, gene amplification and transposition (Neelakandan and Wang, 2012). Different from the tissue culture mutation, a large deletion of DNA and null mutations are among the typical characteristics of ion-beamed mutants (Tanaka et al. 2010). Therefore the cause of the mutation in ‘Lines C and D’ is, we trust, the irradiation by ion beams rather than tissue culture-induced mutation. For a comparison of flower color, Fig. 1a shows normal color cultivars containing malate-acylated anthocyanin, Pg3,5cMdG or Pg3MG that is a counterpart of Pg3,5dG or Pg3G, and a dusky color variety accumulating Pg3,5dG, the same anthocyanin as ‘Lines A and B’. Variation in color hue is partly attributed to an alteration of anthocyanin structure.
Metallic lines include AVIs throughout their adaxial epidermal cells just as dusky lines do. On the other hand, the vacuolar sap of the metallic color lines is water-clear (Fig. 2b) in contrast to the transparent but colored sap of the dusky type (Fig. 1b). This observation means that metallic lines have a higher level of pigment condensation on the adaxial side than the dusky lines. The water-clear area of the epidermal cells serves like a diamond so as to transmit a lot of light that is diffused by the AVIs, resulting in the glittering appearance of the metallic lines. Differences in the number of anthocyanin inclusions on the abaxial side were observed within metallic lines containing the same anthocyanin. Anthocyanin condensation of ‘Lines B or D’ was lower than that of ‘Lines A or C’, respectively (Fig. 2b). Condensed pigments and AVIs contribute to the dark tone in petal color. Decreases of AVIs in colored vacuolar sap in the abaxial epidermal cells should create a lighter color to ‘Lines B or D’ than ‘Lines A or C’, respectively.
Factors affecting differences in the condensation of non-acylated anthocyanins in carnation remain to be investigated. Some flavonoids, anthocyanin-biosynthesis-related compounds, have strong molecular interactions with anthocyanin molecules (Yoshida et al. 2009). We did not find a significant difference in flavonoid composition between ‘Lines A–D’ (Table 4). Anthocyanin concentration in the petals of ‘Lines B or D’ that had lower levels of anthocyanin condensation in the abaxial epidermal cells, decreased by 32 or 14 % as compared with ‘Lines A or C’, respectively, whereas the flavonoid concentration of ‘Lines B or D’ increased by 22 or 41 % as compared with ‘Lines A or C’, respectively (Table 4). We expect that analysis and comparison of tissue-specific anthocyanins and flavonoids in both the adaxial and abaxial epidermal cells could elucidate the relationship between AVIs and anthocyanin and/or flavonoid concentrations.
The mechanism causing the metallic lines is rationally explained as follows: (1) by making an intentional cross to produce homozygous AMalT-deficient alleles, a peculiar color population was obtained that contained AVIs due to the accumulation of non-acylated anthocyanins; from the peculiar color population, a line was selected that had a novel metallic appearance caused by high levels of anthocyanin condensation on the adaxial surface of petal epidermal cells; (2) a new hue, a reddish metallic color, due to structural alteration of anthocyanin glycosylation resulted from the inactivation of the AA5GT gene by ion-beam irradiation; and (3) a new tone, a light metallic color, related to the reduction in condensation level of anthocyanins on the abaxial surface of epidermal cells resulted from mutagenesis by ion-beam breeding.
Our result supports the concept that inactivation of the single-copy AMalT gene causes the accumulation of non-acylated anthocyanins, thereby resulting in the peculiar coloration of carnation. Since we have developed a variety of metallic colors using this concept, the gene AMalT is to be called the ‘metallic gene’ in carnation.
Carnation has a long history of breeding and a vast diversity of cultivated varieties have been produced. Every possible variation in color is said to have been bred, and materials to create novel colors have been exhausted. In the present study, we succeeded in creating a peculiar colored carnation with a highly metallic appearance. Diversifying the metallic color variation by a combination of crossbreeding and ion-beam breeding provided a breakthrough to address this problem. The efficiency and effectiveness of ion-beam breeding was verified in that metallic mutants with superior characteristics were induced at higher rates than those previously reported (Okamura et al. 2003 and 2012). Since AMalT serves as the acylation enzyme for cyanidin anthocyanins, Cy3MG and Cy3,5cMdG, as well, the method developed here should be equally effective in diversifying peculiar, dusky or metallic, flower colors in cyanidin-type flowers. We believe the newly created colors, dusky and metallic, are emotionally engaging by leading to the ‘wabi’ and ‘sabi’ sense of beauty in Japan, and will contribute to horticultural industry and culture in the world.
References
Abe Y, Tera M, Sasaki N, Okamura M, Umemoto N, Momose M, Kawahara N, Kamakura H, Goda Y, Nagasawa K, Ozeki Y (2008) Detection of 1-O-malylglucose: pelargonidin 3-O-glucose-6′′-O-malyltransferase activity in carnation (Dianthus aryophyllus). Biochem Biophys Res Comm 373:473–477. doi:10.1016/j.bbrc.2008.04.153
Bloor SJ (1998) A macrocyclic anthocyanin from red/mauve carnation flowers. Phytochemistry 49:225–228. doi:org/10.1016/S0031-9422(97)01051-0
Itoh Y, Higeta D, Suzuki A, Yoshida H, Ozeki Y (2002) Excision of transposable elements from the chalcone isomerase and dihydroflavonol 4-reductase genes may contribute to the variegation of the yellow-flowered carnation (Dianthus caryophyllus). Plant Cell Physiol 43:578–585. doi:10.1093/pcp/pcf065
Markham KR, Gould KS, Winefield CS, Mitchell KA, Bloor SJ, Boase MR (2000) Anthocyanin vacuolar inclusions—their nature and significance in flower colouration. Phytochemistry 55:327–336. doi:org/10.1016/S0031-9422(00)00246-6
Matsuba Y, Sasaki N, Tera M, Okamura M, Abe Y, Okamoto E, Nakamura H, Funabashi H, Takatsu M, Saito M, Matsuoka H, Nagasawa K, Ozeki Y (2010) A novel glucosylation reaction on anthocyanis catalyzed by acyl-glucose dependent glucosyltransferase in the petals of carnation and delphinium. Plant Cell 22:3374–3389. doi:10.1105/tpc.110.077487
Morita Y, Hoshino A, Kikuchi Y, Okuhara H, Ono E, Tanaka Y, Fukui Y, Saito N, Nitasaka E, Noguchi H, Iida S (2005) Japanese morning glory dusky mutants displaying reddish-brown or purplish-gray flowers are deficient in a novel glycosylation enzyme for anthocyanin biosynthesis, UDP-glucose:anthocyanidin 3-O-glucoside-2′′-O-glucosyltransferase, due to 4-bp insertions in the gene. Plant J 42:353–363. doi:10.1111/j.1365-313X.2005.02383.x
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nakayama M, Koshioka M, Yoshida H, Kan Y, Fukui Y, Koike A, Yamaguchi M (2000) Cyclic malyl anthocyanins in Dianthus caryophyllus. Phytochemistry 55:937–939. doi:10.1016/S0031-9422(00)00263-6
Neelakandan AK, Wang K (2012) Recent progress in the understanding of tissue culture-induced genome level changes in plants and potential applications. Plant Cell Rep 31:597–620. doi:10.1007/s00299-011-1202-z
Nishizaki Y, Matsuba Y, Okamoto E, Okamura M, Ozeki Y, Sasaki N (2011) Structure of the acyl-glucose-dependent anthocyanin 5-O-glucosyltransferase gene in carnations and its disruption by transposable elements in some varieties. Mol Genet Genomics 286:383–394. doi:10.1007/s00438-011-0655-7
Okamura M, Yasuno N, Ohtsuka M, Tanaka A, Shikazono N, Hase Y (2003) Wide variety of flower-color and -shape mutants regenerated from leaf cultures irradiated with ion beams. Nucl Instrum Methods Phys Res B 206:574–578. doi:org/10.1016/S0168-583X(03)00835-8
Okamura M, Umemoto N, Onishi N (2012) Breeding glittering carnations by an efficient mutagenesis system. Plant Biotechnol 29:209–214. doi:10.5511/plantbiotechnology.12.0104a
Tanaka Y, Ohmiya A (2008) Seeing is believing: engineering anthocyanin and carotenoid biosynthetic pathways. Curr Opin Biotechnol 19:190–197. doi:10.1016/j.copbio.2008.02.015
Tanaka A, Shikazono N, Hase Y (2010) Studies on biological effects of ion beams on lethality, molecular nature of mutation, mutation rate, and spectrum of mutation phenotype for mutation breeding in higher plants. J Radiat Res 51:223–233. doi:10.1269/jrr.09143
Umemoto N, Abe Y, Cano EA, Okamura M, Sasaki N, Yoshida S, Ozeki Y (2009) Carnation serine carboxypeptidase-like acyltransferase is important for anthocyanin malyltransferase activity and formation of anthocyanic vacuolar inclusions. 5th International Workshop on Anthocyanins 2009 in Japan, Nagoya, 15–18 September 2009, p 115
Yoshida K, Mori M, Kondo T (2009) Blue flower color development by anthocyanins: from chemical structure to cell physiology. Nat Prod Rep 26:884–915. doi:10.1039/B800165K
Zhang H, Wang L, Deroles S, Bennett R, Davies K (2006) New insight into the structure and formation of anthocyanic vacuolar inclusions in flower petals. BMC Plant Biol 6:29. doi:10.1186/1471-2229-6-29
Acknowledgments
We thank Haruka Yamamoto for excellent technical assistance. Part of this research was supported by the Research and Development Program for New Bio-industry Initiatives from the Bio-oriented Technology Research Advancement Institution of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Okamura, M., Nakayama, M., Umemoto, N. et al. Crossbreeding of a metallic color carnation and diversification of the peculiar coloration by ion-beam irradiation. Euphytica 191, 45–56 (2013). https://doi.org/10.1007/s10681-012-0859-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-012-0859-x