Abstract
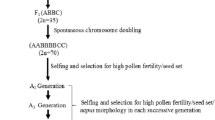
This study was conducted to assess the cytoplasm effects of Brassica napus and B. juncea on the some characteristics of B. carinata, as well as the phylogenetic distances separating the three species. Alloplasmic lines of B. carinata were developed from B. napus × B. carinata and B. juncea × B. carinata hybrids by recurrent backcrossing to the BC7 generation. Sixteen populations from three generations were compared for a number of characteristics. Plants with the cytoplasm of B. napus flowered later, had shorter filaments and longer pistils, lower pollen amount, lower seed set, lower petal length and width and different petal color; plants with the cytoplasm of B. juncea had shorter pistils and filaments, and lower petal length and width than their corresponding euplasmic sibs, respectively. The results suggest that the cytoplasm is involved in the development of flower organs. The natural species, B. carinata showed a balance between the nucleus and cytoplasm. The cytoplasm from B. napus showed a stronger disturbing effect than that of B. juncea, suggesting that B. carinata might be genetically closer to B. juncea than to B. napus. The significant difference in the alloplasmic effect of the cytoplasms of B. napus and B. juncea also suggests that in B. carinata the B genome may play a greater role than the C genome.








Similar content being viewed by others
References
Banga SS, Labana KS, Medhi BN (1984) Alternaria incidence in some alloplasmic lines of Indian mustard (Brassica juncea (L.) Coss.). Theor Appl Genet 67:195–196
Banga SS, Deol JS, Banga SK (2003) Alloplasmic male-sterile Brassica juncea with Enarthrocarpus lyratus cytoplasm and the introgression of gene(s) for fertility restoration from cytoplasm donor species. Theor Appl Genet 106:1390–1395
Bannerot H, Boulidard L, Chupeau Y (1977) Unexpected difficulties met with the radish cytoplasm in Brassica oleracea. Eucarpia Cruciferae Newsl 2:16
Beversdorf WD, Weiss-Lerman J, Erickson LR, Souza-Machada V (1980) Transfer of cytoplasmically-inherited triazine resistance from bird’s rape to cultivated oilseed rape (Brassica campestris and B. napus). Can J Genet Cytol 22:167–172
Correns C (1908) Die Rolle Der männlichen Keimzellen bei der Geschlechts-bestimmung der gynodioecischen pflanzen. Ber Deut Bot Ges 36:686–707
CSAJ (The Color Science Association of Japan) (1991) Handbook of color science. Asakura Publ. House, Tokyo
Deol JS, Shivanna KR, Prakash S, Banga SS (2003) Enarthrocarpus lyratus-based cytoplasmic male sterility and fertility restorer system in Brassica rapa. Plant Breed 122:438–440
Egashira H, Ogawa R, Kanno H, Tanisaka T, Imanishi S (1999) Pistillate-parental differences and the ability to produce interspecific hybrids between Lycopersicon esculentum and ‘peruvianum-complex’ species (L. peruvianum and L. chilense). Plant Breed 118:253–258
Getinet A, Rakow G, Raney JP, Downey RK (1997) Glucosinolate content in interspecific crosses of Brassica carinata with B. juncea and B. napus. Plant Breed 116:39–46
Heyn FW (1976) Transfer of restorer genes from Raphanus to cytoplasm male sterile Brassica napus. Eucarpia Cruciferae Newsl 1:15–16
Hinata K, Konno N (1979) Studies on a male-sterile strain having the Brassica campestris nucleus and the Diplotaxis muralis cytoplasm. I. On the breeding procedure and some characteristics of the male sterile strain. Jpn J Breed 29:305–311
Iwasa S (1963a) Studies on the alloplasmatic effect in tribe Brassiceae I. On the carinata-cytoplasmic Brassica Pekinensis induced by the successive backcrosses. J Fac Agric Kyushu Univ 12:201–212
Iwasa S (1963b) Studies on the alloplasmatic effect in tribe Brassiceae. II. Several conspicuous characteristics appeared in the carinata-cytoplasmic pekinensis plant. J Fac Agric Kyushu Univ 12:213–228
Kanada I, Kato M (1997) Effect of Brassica oxyrrhina cytoplasm on Raphanus sativus. Breed Sci 47:57–65
Kato M, Jodo S, Tanaka T (1990) Effect of Abyssinian mustard cytoplasm on CC genome of Brassica. Jpn J Breed 40:475–484
Kihara H (1951) Substitution of nucleus and its effects on genome manifestations. Cytologia 16:177–193
Liu Ai-hua, Wang Jian-bo (2006) Genomic evolution of Brassica allopolyploids revealed by ISSR marker. Genet Resour Crop Evol 53:603–611
Lu CM, Kato M (2001) Fertilization fitness and its relation to chromosome number in interspecific progeny between B. napus and B. rapa: a comparative study using natural and resynthesized B. napus. Breed Sci 51:73–81
Matsuzawa Y, Mekiyanon S, Kaneko Y, Bang SW, Wakui K, Takahata Y (1999) Male sterility in alloplasmic Brassica rapa L. carrying Eruca sativa cytoplasm. Plant Breed 118:82–84
Mizushima U (1961) Utilization of alloplasm to plant breeding. Recent Adv Breed 2:44–52
Mizushima U, Katsuo K (1953) On the fertility of artificial amphidiploid between Brassica nigra Koch and B. oleracea L. Tohoku J Agric Res 4:1–14
Mizushima U, Katsuo K (1958) Elimination of self incompatibility in the common cabbage, Brassica oleracea. L., by means of substitution of nucleus. Proc 10th Int Congr Geneti vol 2. p 191
Momotaz A, Kato M, Kakihara F (2000) Variation in seed fertility and fatty acid composition in the allohexaploids between Brassica carinata and Sinapis species with the advance of generations. Breed Sci 50:91–99
Pearson OH (1972) Cytoplasmically inherited male sterility characters and flavor components from the species cross Brassica nigra (L.) Koch. × B. oleracea L. J Am Soc Hort Sci 97:397–402
Prakash S, Chopra VL (1990) Male sterility caused by cytoplasm of Brassica oxyrrhina in B. campestris and B. juncea. Theor Appl Genet 79:285–287
Prakash S, Ahuja I, Upreti HC, Dinesh Kumar V, Bhat SR, Kirti PB, Chopra VL (2001) Expression of male sterility in alloplasmic Brassica juncea with Erucastrum canariense cytoplasm and the development of a fertility restorer system. Plant Breed 120:479–482
Rashid A, Rakow G, Downey RK (1994) Development of yellow seeded Brassica napus through interspecific crosses. Plant Breed 112:127–134
Sigareva MA, Earle ED (1997) Direct transfer of a cold-tolerant Ogura male-sterile cytoplasm into cabbage (Brassica oleracea ssp. capitata) via protoplast fusion. Theor Appl Genet 94:213–220
Sodhi YS, Pradhan AK, Verma JK, Arumugam N, Mukhopadhyay A, Pental D (1994) Identification and inheritance of fertility restorer genes for ‘tour’ CMS in rapeseed (Brassica napus L.). Plant Breed 112:223–227
Starzycki M, Starzycka E, Matuszczak M, Krzymanski J (1999). New alloplasmatic forms of winter oilseed rape created by interspecific crossing. Proc 10th Int Rapeseed Congr, Canberra, CD-ROM
Thormann CE, Ferreira ME, Camargo LEA, Tivang JG, Osborn TC (1994) Comparison of RFLP and RAPD markers to estimating genetic relationships within and among cruciferous species. Theor Appl Genet 88:973–980
Tokumasu S, Kato M (1988) Chromosomal and genetic structure of Brassicoraphanus related to seed fertility and the presentation of an instance of improvement of its fertility. Euphytica 39:145–151
Zhang B, Lu CM, Kakihara F, Kato M (2002) Effect of genome composition and cytoplasm on petal colour in resynthesized amphidiploids and sesquidiploids derived from crosses between Brassica rapa and Brassica oleracea. Plant Breed 121:297–300
Acknowledgment
The first author is grateful to the Japanese government for awarding her a MONBUSHO scholarship.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s10681-007-9476-5
Rights and permissions
About this article
Cite this article
Chang, C.T., Uesugi, R., Hondo, K. et al. The effect of the cytoplasms of Brassica napus and B. juncea on some characteristics of B. carinata, including flower morphology. Euphytica 158, 261–270 (2007). https://doi.org/10.1007/s10681-007-9424-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-007-9424-4