Abstract
This study focuses on the development of a nanocomposite membrane using polyvinyl chloride and nanocellulose@titanium aluminate to effectively filter and adsorb dye from wastewater. Various analytical techniques were used such as FTIR, XRD, SEM, and TGA to characterize the synthesized adsorptive nanocomposite membrane. The thermal and electrical properties of the membrane were measured using a static system. The study investigated the effect of different adsorbent dosages, pH levels, and dye concentrations on the membrane’s adsorption ability. The PVC-NC@TALCM nanocomposite membrane was found to remove 98.6% of MB dye when loaded with 5% titanium aluminate at pH 10. Kinetic and isotherm studies revealed that the adsorption of MB onto the membrane followed pseudo-second-order kinetics and Freundlich isotherms, respectively. The researchers also highlighted the economic and eco-friendly nature of the PVC-NC@TALCM nanocomposite membrane as a viable option for the removal of MB from industrial wastewater.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The elimination of heavy metals and dyes from drinking water and wastewater is a significant challenge for safeguarding the environment and human health globally. The release of significant quantities of heavy metals, such as nickel, chromium, copper, silver, and zinc, into water bodies, can lead to considerable environmental and health concerns, as well as an increase in wastewater treatment expenses (Adel et al., 2022).
Researchers have commonly used several techniques for treating dye solutions, including, ion exchange, electrocoagulation, advanced oxidation, photocatalytic degradation, adsorption, phytoremediation, coagulation, and flocculation (Fitch et al., 2022). However, these methods have limitations, such as the generation of large quantities of sludge, harmful by-products, bio-resistant organisms, and complex operational procedures, which hinder their success. Membrane nanofiltration is a promising technique in the treatment of water and industrial wastewater due to its low cost (Yadav et al., 2022).
The membrane nanofiltration method suffers from concentration polarization and membrane fouling, which ultimately leads to a decrease in separation efficiency, an increase in operating costs, and a shortening of membrane lifetime. Polymeric membranes are extensively employed in water purification and wastewater treatment (Pan et al., 2022). In the future, photocatalytic methods will be the most promising methods to treat complex systems, because they consume cheap photons from the UV-near visible region, degrade the organic pollutants and recover metals in one system (Ahmadi et al., 2023).
Different significant membrane types have been developed, such as microfiltration, nanofiltration, ultrafiltration, catalytic and conducting membranes, and their applications and processes, such as wastewater treatment and bio-processing (Wang et al., 2021). Membranes that respond to stimuli have been extensively studied to reduce fouling on the membrane surface and provide steady water treatment performance. In this paper, a simple one-step impregnation of nanomaterials such as nanocellulose on membrane surfaces was created to provide self-cleaning properties when exposed to polluted wastewater (Wang et al., 2022a).
Polyvinyl chloride (PVC) membrane has unique properties, such as hardness, excellent resistance to abrasion, acid, alkali, and microbial corrosion, and low cost, which make it a popular choice in various applications. Certain studies have investigated the use of phase inversion techniques in manufacturing ultrafiltration and microfiltration flat PVC membranes. However, when processing a solution containing protein-like substances, the hydrophobic nature of PVC can lead to severe fouling on the membrane surface (Zhang et al., 2013).
Cellulose is one of the most abundant natural polymers on Earth, derived from the biosphere, and present in plants and certain living organisms. It is a glucose polymer, with about 40–50% of the polymer in crystalline form, while the remaining 25–35% is made up of amorphous hemicellulose. Hemicellulose is composed of various monomers including xylose, arabinose, galactose, and mannose. Lignin makes up the majority of the remaining material at 18–24%, with small amounts of minerals, wax, and ash present as well (Praveen Kumar, et al., 2019).
The unique properties of cellulose and its derivatives have led to their use in various applications, such as in the production of paper, textiles, and packaging materials, as well as in biomedical and pharmaceutical applications. In recent years, there has been growing interest in the use of nanocellulose (NC) in various fields, including water treatment, due to its high surface area, low toxicity, and renewable nature (Seddiqi et al., 2021).
One of the potential applications of NC in water treatment is as an adsorbent material. Adsorption refers to the process of attaching pollutants, such as heavy metal ions, dyes, and organic compounds, to the surface of the adsorbent material. NC has been shown to have excellent adsorption properties for various pollutants, making it a promising material for use in water treatment (Perumal et al., 2022).
In recent years, there has been a growing interest in using nanocellulose (NC) as an adsorbent material for water treatment. One approach that has gained attention is the composite technique of impregnating porous substrates with multiple grades of NC, which has shown promising results in enhancing the adsorption efficiency of pollutants. This renewed interest in NC for water treatment has led to the development of novel NC-based adsorbents (Qiao et al., 2021).
Another class of materials that have been extensively studied for water treatment applications is nano metal oxides, such as TiO2 nanoparticles. These inorganic materials possess unique properties, including high surface area, hydrophilicity, and catalytic activity under UV radiation, which enhance their ability to remove pollutants from water. Furthermore, the incorporation of TiO2 nanoparticles into modified membranes has been shown to improve the membrane’s antifouling/self-cleaning capabilities, making them more efficient for water treatment (Voisin et al., 2021).
This study aims to develop a novel filter membrane composed of a nanocomposite mixture of polyvinyl chloride (PVC) and nanocellulose, which will be further reinforced with titanium aluminate (TiAl2O4) nanoparticles. The resulting membrane will be assessed for its ability to remove methylene blue dye at different concentrations. Additionally, the research will investigate the impact of varying the concentration of TiAl2O4 nanoparticles on the physical and chemical properties of the membranes. The study will examine the effects of dye concentration, pH, membrane dose, and temperature on the adsorption of dye from aqueous solutions. A range of isothermal and kinetic models will be employed to evaluate the adsorption behavior and desorption process. The experimental results of batch dye adsorption will be analyzed using the PVC-NC @ TALCM membrane.
2 Experimental
2.1 Materials
The agricultural wastes were received from Egyptian farms as the source of cellulose. Polyvinyl chloride (PVC), tetrahydrofuran (THF), dimethyl formamide (DMF), sodium hydroxide (NaOH), isopropyl alcohol, β-diketones, carboxylic acids, Titanium tetra isopropoxide (TTIP), nitric acid, isopropanol, ammonium hydroxide NH4OH, titanium chloride (TiCl4), and methylene blue (MB) from Sigma Aldrich, throughout the investigation, distilled water from the central lab was utilized. Other chemical products were used such as distilled water, HCl, isopropyl alcohol, and NH4OH (25%) solutions. All of the chemicals were used in this paper under analytical grade.
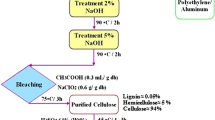
2.2 Purification of agricultural wastes as a source of cellulose
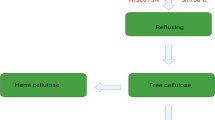
Agricultural wastes with varying cellulose concentrations, such as sawdust, sugarcane bagasse, and rice straw, were collected and dried in the sun before being chopped into little pieces (1–3 cm) (Debnath et al., 2021). The cut wastes were washed with distilled water and acetone, then dried overnight in an oven at 60 °C and chosen for further cellulose. 10 g of the cellulosic materials undergo alkali treatment with 250 ml sodium hydroxide (1% wt/v) solution for 5 h and refluxed at 120 °C to remove the hemicelluloses. The insoluble residue (cellulose) will be collected by filtering at the end of the extraction and rinsed extensively with distilled water until the filtrate was neutral. The resultant cellulosic materials were dried in the air before being mixed with dimethylsulfoxide (DMSO) and heated in a water bath at 80 °C for 3 h. The product was then filtered, washed with distilled water, and dried in the open air (Shaheen & Emam, 2018).
2.3 Chemical method for preparation of nanocellulose
NanoCellulose (NC) was synthesized in spherical form rod-like highly crystalline nanocrystals. In this respect, 5 g of nanocellulose was obtained from 15 g of lignocellulosic material by acid hydrolysis and refluxing of dignified and hemicellulose (free cellulose) with 250 ml of 5N sulfuric acid for 5 h at 50 °C under strong agitation to remove amorphous silica and form Cellulose nanocrystals. By adding fivefold the quantity of water to the reaction mixture, the hydrolysis was stopped. Before centrifugation, the resulting mixture was allowed to cool to room temperature. The fractions were cleaned with distilled water and centrifuged regularly. After at least five washing cycles, the centrifugation process come to an end, and the supernatant liquor became turbid (Vincent & Kandasubramanian, 2021).
2.4 Preparation of titanium dioxide nanoparticles by Sol–Gel Method
The schematic diagram in Fig. 1 shows that Titanium tetra isopropoxide (TTIP), nitric acid, isopropanol, and deionized water were used to synthesize the TiO2 nanoparticles via the sol–gel method through the hydrolysis of TTIP.4 ml of TTIP was dropped slowly into the vigorously stirred 5 ml isopropanol solution After 15 min of stirring, 75 ml of deionized water was added drop-by-drop to the prepared mixture, causing the production of a white precipitate. This white precipitate converted into the white solution after a little period. The solution was then thoroughly stirred while being kept at a temperature of 80 °C for a period of one hour.2.5 ml of acetic acid was added drop-by-drop to this solution and the resulting solution was stirred for 5 h.1 ml of Nitric acid was added drop-by-drop for the peptization after 5 h of stirring, and the resultant solution was left to agitate for another 2 h. The solution was dried at 60 °C in the vacuum oven after being naturally cooled to room temperature. The produced powder was pounded into a fine powder using a mortar and pestle, and it was then annealed for 3 h at 500 °C (Pawar et al., 2019).
2.5 Synthesis of titanium aluminate
Although metallic alkoxides have high reactivity with water, which favors the hydrolysis process and results in the immediate precipitation of the metallic hydroxide, the alkoxides were employed as precursors in the synthesis of titanium aluminate in our work. In this regard, 43.92 g of titanium sec-butoxide and 63.57 g of aluminum sec-butoxide were transferred to 1L three-necked glass flask. After that, in the glove box, these alkoxides were shaken well under a nitrogenous atmosphere in the three-ended balloon with a Teflon blade coated with Thin-Film Metallic Glass (TFMG), for 30 min, to be homogenized. The alkoxides moisture will become almost viscous, and exhibit yellow transparent color. To solve the alkoxides moisture, add 100 ml isopropyl alcohol and shake for another two hours. Finally, 30 ml of 1.7 M H2O and 0.5 M HCI and solution in isopropyl alcohol solution were added under constant shaking, to avoid the inactivity atmosphere of the balloon (Ha & N. et al., 2020).
2.6 Synthesis of membranes
The asymmetric membranes were made using the phase inversion approach as the following; The polymer solution, which included polyvinyl chloride (PVC) and nanocellulose (NC), was dissolved in dimethyl formamide (DMF), and the mixture was then mixed with titanium oxide nanoparticles and rapidly agitated for 24 h at 60 °C, as shown in Table 1, before being manually cast with a 150 mm-thick casting knife at room temperature on a clean glass plate. As illustrated in Fig. 2, the membrane surface was exposed to air at room temperature (about 26 °C) for free-convective solvent evaporation. After a 60 s delay, a distilled water bath was used to submerge the membranes at 26ºC. After 15 min, the polymeric skin layer was removed and submerged in distilled water for 24 h to ensure that all residual solvent was removed(Deng & Li, 2021). Digital caliper equipment (Electronic outside Micrometer, IP54 type OLR) was used to measure the thickness of the final membrane between 135 and 150 mm. Two processes must be completed to obtain the final membrane: First, the contact angle concentration must be optimized to get a membrane with the highest rejection and flux. The second stage is to look at the influence of different nanoparticle adsorbent concentrations on dye rejection (Sun et al., 2021).
2.7 Membrane characterization
FTIR spectral analysis of membrane was performed using FTIR, Model (Thermo Scientific Nicolet iS10 Spectrometer) produced by thermo fisher scientific (USA) company in the spectral range from 4000 cm−1 to 400 cm−1, a beam splitter is KBr/Ge mid-infrared optimized, with resolution 4 cm−1 (Park et al., 2021). X-ray diffraction was employed to obtain the phases of the samples. X-ray diffraction (Philips PW3040/60 X, pert PRO P Analytical-Netherlands) was used to obtain the patterns. Continuous measurements were taken from 5 to 90 degrees while the samples were on a glass slide. (Jain et al., 2022). All samples were observed by scanning electron microscope model (Zeiss evo 10) attached with EDX unit (Energy Dispersive X-ray Analysis), with accelerating voltage 500 K.V. magnification14x up to 100000x(Agarwalla & Mohanty, 2022). TGA analysis was performed on the Perkin-Elmer TGA7 thermo balance. The dried samples were analyzed under a nitrogen atmosphere in the temperature range of 5-600ºC, at a scanning rate of 20ºC /min.
2.7.1 Water content
The water content test determines the hydrophilic properties of the nanocomposite membrane and evaluates the difference between the membrane’s wet and dry weight. How to calculate water content values is shown in Eq. (1).
where Wdry is the weight of the dry membrane after it has dried in the oven to the desired weight. And Wwet is the membrane’s weight after 48 h of immersion in distillate water (Torre-Celeizabal et al., 2022).
2.8 Dye Adsorption Experiments
At room temperature, 0.1 g of a PVC-NC@TALCM membrane was submerged in 50 mL of MB solution and swirled at 400 rpm. The free MB was analyzed at a wavelength of 664 nm, and at different intervals of time using UV–the visible spectrophotometer Shimadzu (UV-2600).
The influence of initial dye concentration (10, 15, 20, 25, 30 ppm), dose (0.1, 0.5, 0.8 g), and effect of pH (5, 7, 9) was studied. From Eqs. 2 and 3, both dye uptake efficiency (R %), and the equilibrium adsorption capacity (qe) can be calculated.
where V is the volume (L), W is the weight of the membrane (g), and the equilibrium and starting concentrations of MB dye are Ce (mg/L) and Co, respectively (Agarwalla & Mohanty, 2022).
3 Result and discussion
3.1 FTIR analysis of nanocomposite membranes
The FTIR spectrum of PVC-NC and PVC-NC@TALCM membranes at different titanium aluminate concentrations is given in Fig. 3. Figure 3 shows a band at 2239 and 1331 cm−1 that might be created by CH2’s asymmetric stretching and deformation vibrations, and absorption peaks at 1031 and 829 cm−1 caused by CH’s in-plane and out-plane bending modes, which are PVC’s distinctive peaks. The nanocellulose will have a peek at 3409 cm−1 about the stretching vibration of the hydroxyl group (OH), and the two absorption peaks at around 1688,1401 may be ascribed to the absorption of the bending vibrations of (C-O), as can be observed from the spectrum of PVC /NC) (Rana et al., 2021). Upon the combination between the PVC/NC and titanium aluminate, the intensity of the −OH stretching band increases in the brooding and moving to the higher wavelength. This may be due to the formation of hydrogen bonds, between the hydroxyl groups of titanium aluminate with the aldehyde group of nanocellulose. It’s shown that the strength of peaks at 485, 486.4, and 479 cm−1 are increased, and move to the higher wavelength with an increasing the % of TiAl2O4 loading on PVC/NC membrane (Ahmad & Guria, 2022).
3.2 XRD analysis of nanocomposite membranes
XRD pattern of PVC/NC in Fig. 4 shows a diffraction peak at 2θ of 17.3°, while the role of X-ray diffraction, of the inorganic nanocomposite, e.g., TiAl2O4 can be investigated through the full measurement range. In this regard, the XRD diffraction patterns of PVC-NC@TALCM at 1%, 3%, and 5%, of titanium aluminate exhibits the varied type of crystalline phase’s peak; this reveals the presence of some crystalline peak indicating the complete homogenous distribution of TiAl2O4 in the PVC/NC membrane matrix. Furthermore, the increase in TiAl2O4% leads to the appearance of a sharp intense peak at about 2θ of 24.13, 25.93, and 33.5 for 1%, 3%, and 5% of TiAl2O4 respectively (Fahoul et al., 2021). Multiple peaks with lower intensities appear at 2θ of 27.8, 34.6, 43.59, and 50.35, 38.17, and 48.55 attributed to (220) and (311) of TiAl2O4% respectively. The previous interpretation points to the formation of multiple phases in the new material composed of combined semicrystalline and amorphous phases. Further, an increase in TiAl2O4 concentrations leads to an increase in peak sharpness and hence sample crystallinity that forms a lamellar composite structure. The diffraction peaks that arise can be accurately indexed to TiAl2O4 with a face-centered cubic spinal structure. This suggested that in the experimental conditions used in this study, the TiAl2O4 spinal phase formed completely. In addition, there were no impurities found in the synthesized sample (Kunde & Sehgal, 2020). The Scherer equation estimated the average crystallite size to be around 100 nm. The average crystallite size was also determined by atomic force microscopy, as shown in Fig. 4.
3.3 SEM analysis of nanocomposite membranes
SEM image of PVC-NC@TALCM in Fig. 5 indicates that stable membranes of PVC-NC@TALCM have been obtained through the preparation process. When NC was added to PVC, a stable matrix was formed due to physicochemical interactions. The matrix becomes strengthened after TiAl2O4 was added, due to the physical reinforcement. The subsequent addition of TiAl2O4 to the matrix provides sufficient crosslinking which enhances the overall functional performance and roughness.
SEM images in Fig. 5 reveal the growth of nano/micro clusters of TiAl2O4 nanotubes through the membrane surface. The clusters are non-regular in shape and non-uniform in size. The tubes are very smooth, with an outer diameter of 550 nm, and a tube length of 825 ± 51.7 nm. Figure 5 shows irregular clusters and is almost similar to the morphology of titanium aluminate nanoparticles in Fig. 5b. By increasing the TiAl2O4 ratio in the membrane, the number of overgrowing microclusters was decreased, but their sizes were increased. The TiAl2O4 appeared in uniform circular holes and homogeneous distribution, as shown in the insets with increasing TiAl2O4 as shown in Fig. 5c.
3.4 TGA analysis of nanocomposite membranes
By analyzing the thermal stability of the prepared composite, it can be said that the addition of titanium aluminate (TiAl2O4) helped in the thermal stability of the basic polymer as shown in Fig. 6. We note that the degradation of PVC occurs in three stages. The first stage is the exit of the solvent, which is a very small stage because there is a small percentage of the solvent in the membrane that ends at 150 °C, followed by a second stage that intersects around the exit of functional groups from the base polymer, and ends at 334 °C, and the final stage is carbonation ended at 485 °C and expresses the gaseous vacuum of carbon gas, that affects the result of the analysis at the end of the slit.
As for other composites that contain titanium aluminate in different proportions, we note an increase in the extent of the crushing of the materials and an expansion in the extent of the crushing stages, where we note that the first stage is within 150 °C, while the second stage is much at 323, 345 and 246 for the ratios from 1 to 3% for titanium aluminate Which confirms the high thermal stability of the composite for the original membrane. We also note that the amount of burnt sample decreased with an increase in the percentage of titanium aluminate, which confirms the thermal stability of the prepared materials. It also appears from the increase in the proportion of titanium aluminate in the final crushing stage that the intermediate stages appear for the basic components of the nanomaterials used, Al, TiO2.
3.5 Adsorption performance evaluation
3.5.1 Initial dye concentrations on the uptake efficiency
The influence of initial dye concentrations on the absorption efficiency of the PVC-NC@TALCM membrane is shown in Fig. 7. With increasing MB concentration, the removal efficiency of PVC-NC@TALCM membrane increased from 65.3 to 83.4 mg/g corresponding to MB concentration 10 ppm and 30 ppm in case of 5% PVC-NC@TAlCM, whereas in case of 3% and 1% membrane ratio, the removal efficiency was 73 and 65.3% and for PVC/NC 41%. The adsorption of MB dye onto PVC-NC@TALCM membrane is enhanced with increasing MB dye initial concentration; this may be due to the saturation of PVC-NC@TAlCM membrane adsorption sites. This is probably due to adsorption site saturation since the driving force generated by the increasing dye concentration becomes constant, sufficient to overcome mass transfer between the solid and liquid phases (Gharbani & Mehrizad, 2022).
3.5.2 Adsorbent dose effect
At all indicated mixing ratios (1, 3, 5%), the maximum removal efficiency of MB dye was (88.2, 97.6, and 98.6%) respectively as shown in Fig. 8. The presence of extra adsorption sites enhanced the available surface area for the adsorption process to be completed, leading to a high removal efficiency in the sequence of 5% PVC-NC@TALCM > 3% PVC-NC@TALCM >%1 PVC-NC@TALCM > PVC-NC (Sabarish & Unnikrishnan, 2018a).
3.5.3 Effect of pH
The influence of pH on MB adsorption by PVC-NC@TAlCM at different ratios (5%, 3%, and 1%) membranes was investigated, with the findings shown in Fig. 9. It was observed that the uptake efficiency initially increased with increasing pH for all membrane composite ratios and reached its maximum at pH = 10, these results are in good agreement with many researchers (Gohr, et al., 2022). Increasing the pH of methylene blue dye solution causes electrostatic reactions between the MB dye and the PVC-NC@TAlCM membrane, and it is observed especially in cases of 5% and 3% with a maximum removal efficiency of 98.6%, leading to an increase in the percentage of negative hydroxyl ion, and this leads to an increase the dye uptake process on the surface of the membrane (Lu et al., 2018). However, the positive charges on the surface of the PVC-NC@TAlCM adsorbent membrane and the positive charge of the methylene blue dye solution compete at lower pH levels.
3.6 Adsorption Kinetics
The behavior of uptakes MB by PVC-NC@TALCM nanocomposite adsorption kinetics was investigated using pseudo-first and pseudo-second-order kinetic models applied to batch adsorption findings.
The pseudo-first-order model’s linear form is as follows:
where qe and qt (mg g−1) are the adsorption capacities at equilibrium and at time t (min), respectively, and k1 (min−1) is the pseudo-first-order rate constant. The slope and intercept of plots of log (qe-qt) vs t, as illustrated in Fig. 10, were used to determine the rate constants k1, qe, and correlation coefficients r2(Vedula & Yadav, 2022).
The linear expression for the pseudo-second-order rate expression is:
qe and qt are the adsorption capacities at equilibrium and at time t (mg g−1), respectively, while k2 (g mg−1 min−1) is the pseudo-second-order rate constant. We can derive the rate constants k2, qe, and correlation coefficients r2 using linear plots of t/qt vs t. (Fig. 11).
Two kinetic models’ parameters are shown in Table 2, with the pseudo-first-order kinetic model’s correlation coefficient (r2) being 0.95. When we applied the pseudo-second-order kinetic model at 25 °C, we discovered that the correlation coefficient r2 ranged from 0.99 to 1 (or nearly equal to 1) in all cases, implying that correlation coefficients (r2) derived from the pseudo-second-order kinetic model are much higher than correlation coefficients derived from the pseudo-first-order kinetic model. We may infer that both variables (r2) and qe indicated that the adsorption of MB onto PVC-NC@TALCM nanocomposite membrane followed the pseudo-second-order kinetic model, indicating that the chemical adsorption process is the rate-limiting step between MB and PVC-NC@TALCM nanocomposite(Hisada et al., 2019).
3.7 Adsorption Isotherm
The adsorption isotherms were studied to understand the adsorption performance of MB onto PVC-NC@TALCM membranes. Both Langmuir and Freundlich models were applied to discover the appropriate model to explain MB adsorption (Zhou et al., 2021).
Ce is the equilibrium concentration of adsorbate (mg/l) in the Langmuir and Freundlich model, the adsorbent’s equilibrium adsorption capacity (mg/g) is qe, while the maximum adsorption capacity (mg/g) is qm). Table 3 illustrates the linear form of this model.
Langmuir and Freundlich constants (L/mg) are denoted by KL and Kf, respectively. Kf denotes the maximal adsorption capacity (mg/g), while n denotes the adsorption intensity. The slope and intercept of the plot Ce/qe vs Ce were used to get the Langmuir maximum uptake qm and KL, The Freundlich constants Kf and n were determined by plotting ln qe versus ln Ce.
Adsorption favorability is typically predicted using the value of 1/n. If 1/n = 0, adsorption is irreversible, beneficial if (0 1/n 1), and unfavorable if 1/n > 1(Hebbar et al., 2018).
Table 3 displays the isotherm constants that were determined by linearly fitting the experimental data. (Figs. 12, 13). The current experimental findings fit the Freundlich model of adsorption. This result shows that incorporating titanium aluminate into PVC-NC@TALCM heterogeneously alters the membrane surface and enhances an Adsorption protocol described by Freundlich.
The Langmuir and Freundlich isotherms were used to characterize the connection between the number of MB adsorbed and its equilibrium concentration in solution at room temperature in this study, with the findings shown in Table 3.
3.8 Adsorption thermodynamic
Thermodynamics studies also elucidate adsorption mechanisms. Kc, ΔH, ΔS, and ΔG for adsorption were calculated by Vant Hoffer equation as shown below:
T is the absolute temperature (K), R is the universal gas constant (8.314 J mol−1 K−1), Kc is the thermodynamic equilibrium constant at different temperatures, ΔS is the entropy change in J mol−1 K−1, ΔG is the Gibbs’ free energy in kJ mol−1, and ΔH is the enthalpy change in kJ mol−1 (Gharbani & Mehrizad, 2022).
At MB C0 = 200 mg L−1, the values for all parameters indicated in Table 4 were calculated. For the adsorption system, ΔG was negative. As a result, physical adsorption occurred spontaneously. The negative ΔG value of MB adsorption by membrane surface increased as the temperature rose, indicating that the MB dye’s adsorption strength increased as well. Because of the positive ΔH (29,391.36 kJ mol−1), MB adsorption is endothermic. The positive ΔS (67.9986 J mol−1 K−1) indicated that the membrane surface had high MB affinity (Gohr, et al., 2022).
3.9 Adsorption Process Mechanism
The adsorption mechanisms of dye by membrane surface are complex, Electrostatic, van der Waals, π-π stacking, and hydrogen bonding interactions are all possible. The zeta potential was examined to have a better understanding of the contact forces between the membrane surface and the MB. Due to the protonation of the membrane surface, the zeta potential of the modified membrane surface was 4.8:-1.2 mV at pH 3 and 10, respectively (Figs. 14 and 15). These findings suggested that the most important contact between the surface and the MB was electrostatic interaction. We may deduce that the interaction of -SO3 in the MB molecule with the OH group on the composite membrane surface explains the adsorption mechanism of MB (Wang et al., 2022b). During MB adsorption, the π-π stacking interaction may be minimal. We can also say that the adsorption is due to the hydrogen bonding interaction. New sustainable nanofiltration membranes (PVC/CNC) with excellent initial separation performance, for desalination or treatment of industrial wastewater. The prepared membrane will be manufactured integrated with absorbent particles using nano-sized nanocrystals of cellulosic CNC (cellulose nanocrystals) combined with polyvinyl chloride, to be more flexible, durable, high potential flux, high biological and good chemical resistance due to its hydrophilicity, bio-compatibility, and it is inexpensive. Another advantage of a prepared membrane is it’s self-cleaning which helps in sustainability, it’s also economic and environmentally friendly, which in turn will enhance the chemical properties and capabilities of the nanofiltration membranes, reducing the number of chemicals used for the desalination or treatment processing. For this purpose, polyvinyl chloride-cellulose nanocrystal (PVC/CNC) membranes will be prepared by the phase inversion method by dissolving and blending PVC and CNC solutions. In addition, varying amounts of nano-sized metal oxide will be employed as an adsorbent to generate nanocomposite nanofiltration membranes with high rejection and flux. The effect of the polymer binder blend ratio (PVC/CNC) and nanometal oxide concentration on the physicochemical characteristics of membranes will also be investigated (Peng et al., 2022). Producing of nanofiltration membranes for semi-industrial purposes, to increase the water treatment operating efficiency process and increase energy savings.
Figure 16 Zeta potential curve as a function of solution pH obtained for (PVC-NC@TAlCM).
3.9.1 The novelty of the prepared Nanocomposite (PVC-NC@TiAl2O4)
New sustainable nanofiltration membranes (PVC/NC) with excellent initial separation performance, for desalination or treatment of industrial wastewater. The prepared membrane will be manufactured integrated with absorbent particles using nano-sized nano cellulosic NC combined with polyvinyl chloride, to be more flexible, durable, high potential flux, high biological and good chemical resistance due to its hydrophilicity, and bio-compatibility, and is inexpensive. Another advantage of prepared membranes is self-cleaning which helps in sustainability, it’s also economic and environmentally friendly, which in turn will enhance the chemical properties and capabilities of the nanofiltration membranes, reducing the number of chemicals used for the desalination or treatment processing. For this purpose, polyvinyl chloride- nanocellulose (PVC/NC) membranes will be prepared by the phase inversion method by dissolving and blending PVC and NC solution. In addition, varying amounts of nano-sized titanium aluminate will be employed as an adsorbent to generate nanocomposite nanofiltration membranes with high rejection and flux. The impact of the polymer binder blend ratio (PVC to NC) and titanium aluminate concentration on the physicochemical properties of membranes will also be investigated. Producing of nanofiltration membranes for semi-industrial purposes, to increase the water treatment operating efficiency process and increase energy savings. As shown in Table 5, we note the high percentage of removal of the membrane compared to other materials that contain other nanomaterials. This is likely due to the increased surface area and porosity of the nanocomposite membrane, which allows for more efficient adsorption of the dye molecules. Additionally, the presence of nanocellulose in the composite membrane can provide additional binding sites for dye molecules, further increasing its adsorption capacity. Furthermore, the presence of TiAl2O4 in the composite membrane can also contribute to its adsorption capacity by providing additional binding sites and increasing its hydrophilicity. Finally, these studies have also demonstrated improved thermal and electrical properties compared to other membranes, which can further enhance their performance in dye removal applications.
4 Conclusion
In this study, a novel PVC-NC@TALCM nanocomposite filtration membrane by adding titanium aluminate to the PVC-NC@TALCM matrix.The resulting nanocomposite membrane was characterized using XRD, FTIR, SEM, TGA, and optical microscopy analyses, which confirmed its efficacy for the removal of methyl blue (MB) from industrial wastewater.
The results of the experiments showed that the 5% PVC-NC@TALCM nanocomposite filtration membrane had an efficiency of 98.6% at an initial dye concentration of 30 ppm and pH 10. The adsorption isotherm of MB on the PVC-NC@TALCM nanocomposite filtration membrane was described by the Freundlich model, which is a multilayer adsorption model. The adsorption kinetics followed the pseudo-second-order model, and the maximum adsorption capacity (qe) was found to be 141.83 mg g-1.
Furthermore, the adsorption process was found to be spontaneous and endothermic, accompanied by an increase in entropy. This indicates that the process was thermodynamically favorable and could proceed without the input of energy. Moreover, the PVC-NC@TALCM nanocomposite membrane was found to be self-cleaning, which is an important feature for sustainability as it reduces the number of chemicals required to clean the membrane.
The results of this study demonstrate that the PVC-NC@TALCM nanocomposite membrane is an economic, environmentally friendly, and effective adsorbent for the removal of MB from industrial wastewater.
Data availability
Not available.
Abbreviations
- PVC-NC@TALCM:
-
Polyvinyl chloride–nanocellulose@titanium aluminate
- PVC:
-
Polyvinyl chloride
- NC:
-
Nanocellulose
- Ti2ALO4 :
-
Titanium aluminate
- THF:
-
Tetrahydrofuran
- DMF:
-
Dimethylformamide
- TTIP:
-
Titanium tetra isopropoxide
References
Adel, M., et al. (2022). Removal of heavy metals and dyes from wastewater using graphene oxide-based nanomaterials: A critical review. Environmental Nanotechnology, Monitoring & Management, 18, 100719.
Agarwalla, A., & Mohanty, K. (2022). Comprehensive characterization, development, and application of natural/Assam Kaolin-based ceramic microfiltration membrane. Materials Today Chemistry, 23, 100649.
Ahmad, T., & Guria, C. (2022). Progress in the modification of polyvinyl chloride (PVC) membranes: A performance review for wastewater treatment. Journal of Water Process Engineering, 45, 102466.
Ahmadi, A., et al. (2023). A comparative review on adsorption and photocatalytic degradation of classified dyes with metal/non-metal-based modification of graphitic carbon nitride nanocomposites: Synthesis, mechanism, and affecting parameters. Journal of Cleaner Production, 382, 134967.
Al-Bastaki, N. and F. Banat Combining ultrafiltration and adsorption on bentonite in a one-step process for the treatment of colored waters. Resources, Conservation and Recycling, 2004. 41(2): p. 103–113.
Debnath, B., Haldar, D., & Purkait, M. K. (2021). A critical review on the techniques used for the synthesis and applications of crystalline cellulose derived from agricultural wastes and forest residues. Carbohydrate Polymers, 273, 118537.
Deng, W., & Li, Y. (2021). Novel super hydrophilic antifouling PVDF-BiOCl nanocomposite membranes fabricated via a modified blending-phase inversion method. Separation and Purification Technology, 254, 117656.
Fahoul, Y., et al. (2021). Synthesis of a novel ZnAl2O4/CuS nanocomposite and its characterization for photocatalytic degradation of acid red 1 under UV illumination. Journal of Alloys and Compounds, 889, 161708.
Fitch, A., Balderas-Hernandez, P., & Ibanez, J. G. (2022). Electrochemical technologies combined with physical, biological, and chemical processes for the treatment of pollutants and wastes: A review. Journal of Environmental Chemical Engineering, 10(3), 107810.
Gharbani, P., & Mehrizad, A. (2022). Preparation and characterization of graphitic carbon nitrides/polyvinylidene fluoride adsorptive membrane modified with chitosan for Rhodamine B dye removal from water: Adsorption isotherms, kinetics, and thermodynamics. Carbohydrate Polymers, 277, 118860.
Gohr, Sh. M., et al. (2022). Adsorption of cationic dyes onto chemically modified activated carbon: Kinetics and thermodynamic study. Journal of Molecular Liquids., 346, 118227.
Ha, T. T. N., van Giap, T., & Thanh, N. T. (2020). Synthesis of lithium aluminate for application in radiation dosimetry. Materials Letters, 267, 127506.
Hebbar, R. S., et al. (2018). Fabrication of polyetherimide nanocomposite membrane with amine-functionalized halloysite nanotubes for effective removal of cationic dye effluents. Journal of the Taiwan Institute of Chemical Engineers, 93, 42–53.
Hisada, M., Tomizawa, Y., & Kawase, Y. (2019). Removal kinetics of cationic azo-dye from aqueous solution by poly-γ-glutamic acid biosorbent: Contributions of adsorption and complexation/precipitation to Basic Orange 2 removal. Journal of Environmental Chemical Engineering, 7(3), 103157.
Jain, H., et al. (2022). Fabrication and characterization of high-performance forward-osmosis membrane by introducing manganese oxide incited graphene quantum dots. Journal of Environmental Management, 305, 114335.
Kunde, G. B., & Sehgal, B. (2020). Eco-designed iron aluminate (FeAl2O4) free-standing mesoporous films and supported ultrafiltration membranes for wastewater treatment. Journal of Environmental Chemical Engineering, 8(5), 104201.
Li, M., Effects of Natural Organic Matter on Contaminant Removal by Superfine Powdered Activated Carbon Coupled with Microfiltration Membranes. 2014.
Lu, Y., et al. (2018). Petal-like molybdenum disulfide loaded nanofibers membrane with super hydrophilic property for dye adsorption. Colloids and Surfaces a: Physicochemical and Engineering Aspects, 553, 210–217.
Pan, Z., et al. (2022). Preparation and performance of polyaniline modified coal-based carbon membrane for electrochemical filtration treatment of organic wastewater. Separation and Purification Technology, 287, 120600.
Park, K., et al. (2021). Membrane transport behavior characterization method with constant water flux in pressure-assisted forward osmosis. Desalination, 498, 114738.
Pawar, V., et al. (2019). Influence of synthesis route on structural, optical, and electrical properties of TiO2. Applied Physics A, 125(9), 657.
Peng, G., et al. (2022). Preparation and properties of PVC-based ultrafiltration membrane reinforced by in-situ synthesized p-aramid nanoparticles. Journal of Membrane Science, 642, 119993.
Perumal, A. B., et al. (2022). Extraction of cellulose nanocrystals from areca waste and its application in eco-friendly biocomposite film. Chemosphere, 287, 132084.
Praveen Kumar, G., et al., An Update on Overview of Cellulose, Its Structure and Applications, in Cellulose, P. Alejandro Rodríguez and E.E.M. María, Editors. 2019, IntechOpen: Rijeka. p. Ch. 4.
Qiao, A., et al. (2021). Advances in nano cellulose-based materials as adsorbents of heavy metals and dyes. Carbohydrate Polymers, 272, 118471.
Rana, A. K., et al. (2021). Water desalination using nano celluloses/cellulose derivatives based membranes for sustainable future. Desalination, 520, 115359.
Sabarish, R., & Unnikrishnan, G. (2018a). Polyvinyl alcohol/carboxymethyl cellulose/ZSM-5 zeolite biocomposite membranes for dye adsorption applications. Carbohydrate Polymers, 199, 129–140.
Sabarish, R., & Unnikrishnan, G. (2018b). PVA/PDADMAC/ZSM-5 zeolite hybrid matrix membranes for dye adsorption: Fabrication, characterization, adsorption, kinetics, and antimicrobial properties. Journal of Environmental Chemical Engineering, 6(4), 3860–3873.
Seddiqi, H., et al. (2021). Cellulose and its derivatives: Towards biomedical applications. Cellulose, 28(4), 1893–1931.
Shaheen, T. I., & Emam, H. E. (2018). Sono-chemical synthesis of cellulose nanocrystals from wood sawdust using Acid hydrolysis. International Journal of Biological Macromolecules, 107, 1599–1606.
Sun, F., et al. (2021). Conductive polyethersulfone membrane facilely prepared by simultaneous phase inversion method for enhanced anti-fouling and separation under low driven-pressure. Journal of Environmental Management, 297, 113363.
Torre-Celeizabal, A., Garea, A., & Casado-Coterillo, C. (2022). Chitosan: Polyvinyl alcohol-based mixed matrix sustainable coatings for reusing composite membranes in water treatment: Fouling characterization. Chemical Engineering Journal Advances, 9, 100236.
Vedula, S. S., & Yadav, G. D. (2022). Wastewater treatment containing methylene blue dye as pollutant using adsorption by chitosan lignin membrane: Development of membrane, characterization, and kinetics of adsorption. Journal of the Indian Chemical Society, 99(1), 100263.
Vincent, S., & Kandasubramanian, B. (2021). Cellulose nanocrystals from agricultural resources: Extraction and functionalization. European Polymer Journal, 160, 110789.
Voisin, H., et al. (2021). Versatile nano cellulose-anatase TiO2 hybrid nanoparticles in Pickering emulsions for the photocatalytic degradation of organic and aqueous dyes. JCIS Open, 3, 100014.
Wang, K., et al. (2022a). Preparation of ZnS superhydrophobic coating on 316L stainless steel with self-cleaning property and excellent stability. Colloids and Surfaces a: Physicochemical and Engineering Aspects, 633, 127871.
Wang, Q., et al. (2021). Novel amidoxime-functionalized SBA-15-incorporated polymer membrane-type adsorbent for uranium extraction from wastewater. Journal of Water Process Engineering, 43, 102316.
Wang, X., et al. (2022b). Bendable poly(vinylidene fluoride)/polydopamine/β-cyclodextrin composite electrospun membranes for highly efficient and bidirectional adsorption of cation and anion dyes from aqueous media. Composites Science and Technology, 219, 109256.
Yadav, D., Karki, S., & Ingole, P. G. (2022). Current advances and opportunities in the development of nanofiltration (NF) membranes in the area of wastewater treatment, water desalination, biotechnological and pharmaceutical applications. Journal of Environmental Chemical Engineering, 10(4), 108109.
Zhang, Z., et al. (2013). Effect of nano-particles-induced phase inversion on largely improved impact toughness of PVC/α-methyl styrene–acrylonitrile copolymer (α-MSAN)/CPE-matrix composites. Composites Science and Technology, 86, 122–128.
Zhou, S., et al. (2021). Self-cleaning loose nanofiltration membranes enabled by photocatalytic Cu-triazole MOFs for dye/salt separation. Journal of Membrane Science, 623, 119058.
Acknowledgements
This paper is based upon work supported by Science. Technology & Innovation Funding Authority (STDF) under a Grant (call1/Post Graduate Support Grant (PGSG).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elfiky, A.A.E.A., Mubarak, M.F., Keshawy, M. et al. Novel nanofiltration membrane modified by metal oxide nanocomposite for dyes removal from wastewater. Environ Dev Sustain (2023). https://doi.org/10.1007/s10668-023-03444-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10668-023-03444-1