Abstract
The COVID-19 pandemic causes a severe threat to human lives worldwide. Convalescent plasma as supportive care for COVID-19 is critical in reducing the death rate and staying in hospitals. Designing an efficient supply chain network capable of managing convalescent plasma in this situation seems necessary. Although many researchers investigated supply chains of blood products, no research was conducted on the planning of convalescent plasma in the supply chain framework with specific features of COVID-19. This gap is covered in the current work by simultaneous regular and convalescent plasma flow in a supply chain network. Besides, due to the growing importance of environmental problems, the resulting carbon emission from transportation activities is viewed to provide a green network. In other words, this study aims to plan the integrated green supply chain network of regular and convalescent plasma in the pandemic outbreak of COVID-19 for the first time. The presented mixed-integer multi-objective optimization model determines optimal network decisions while minimizing the total cost and total carbon emission. The Epsilon constraint method is used to handle the considered objectives. The model is applied to a real case study from the capital of Iran. Sensitivity analyses are carried out, and managerial insights are drawn. Based on the obtained results, product demand impacts the objective functions significantly. Moreover, the systems' total carbon emission is highly dependent on the flow of regular plasma. The results also reveal that changing transportation emission unit causes significant variation in the total emission while the total cost remains fixed.
Similar content being viewed by others
1 Introduction
Severe respiratory syndrome coronavirus 2 (SARS-CoV-2), known as COVID-19, is an acute respiratory disease in which, regarding the physiological characteristics of people, the mortality rate is estimated at 1 to 5 percent (Ibarra-Vega, 2020). On March 11, 2020, World Health Organization recognized COVID-19 as a pandemic (Tison et al., 2020). About 549 million people have been diagnosed with the disease worldwide by June 2022.Footnote 1 The high mortality rate of this disease and the pandemic’s resulting human and economic impact prompted the expert to investigate efficient cures for COVID-19. Before the approval of vaccines, various drugs and substances such as convalescent plasma, RamedSivir, and Favipiravir were used to reduce to control the virus (Abolghasemi et al., 2020; Lauer et al., 2020). Although the invention of some vaccines by lead developer companies such as Pfizer, Moderna, and AstraZeneca was a significant step toward controlling COVID-19, the accessibility and availability of these vaccines are not guaranteed according to limited production capacity and some other reasons. As an example, Africa had delivered almost 34 doses of vaccine per 100 persons as of April 2022. This vaccination rate is significantly lower than the global average, with 145 vaccinations per 100 people.Footnote 2
Due to that, and in the current situation, convalescent plasma still has a significant role as supportive care for the control and treatment of Covid-19. Convalescent plasma is collected from the recovered patient with a prespecified antibody level that can be used for other patients (Abolghasemi et al., 2020). The history of successful convalescent plasma therapy backs up more than 100 years ago for infectious diseases, including SARS, MERS, Ebola, etc. (Cheraghali et al., 2020; Zhang et al., 2020). Researchers confirmed the efficiency of convalescent plasma for COVID-19 treatment and stated that using recovered patients' plasma could reduce the death rate and stay time in hospitals (Chen et al., 2020).
Although various research works were published on the medical aspects of convalescent plasma, there is a lack of attention to how convalescent plasma should be managed. There is a limited supply of convalescent plasma as the recovered patients from Covid-19 are the only source of the donation. On another side, the high virus transmission rate causes more people to become infected. Hence, there is a need to design and develop a system for the efficient management of convalescent plasma (Asadpour et al., 2021; Wendel et al., 2021).
A research line is related to the blood supply chain to manage regular blood products. Pirabán et al. (2019) defined the blood supply chain as a network that operates blood products' flow from donors to patients through some echelons. Previous authors investigated various blood supply chains for different blood products such as red blood cells, platelet, and regular plasma under different real-world assumptions (Beliën & Forcé, 2012; Osorio et al., 2015; Pirabán et al., 2019). Despite the publication of many papers in this field, no work analyzed a blood supply chain in pandemic situations while considering the flow of convalescent plasma. The critical importance of convalescent plasma and the blood supply chain's capability in managing blood products motivated the current research to present a plasma supply chain network in the condition of the COVID-19 pandemic.
On the other hand, there is a lack of attention to the plasma supply chain in the literature (Dehghani Ashkezari & Yaghoubi, 2020; Hosseini-Motlagh et al., 2020a). Regular plasma flow is also considered in the presented network to bring the problem closer to reality. In other words, the goal is to cover the gaps simultaneously by offering a model for the multi-product plasma supply chain during COVID-19.
From an environmental viewpoint, there is a growing concern about carbon emissions in the industrial and service sectors (Fallahi et al., 2022a). There is a strictly increasing trend in greenhouse gas emissions where the global carbon emission has risen from 25 to 36 billion in recent two decades.Footnote 3 The global direction of carbon emission from 1940 to 2021 is shown in Fig. 1.
The annual carbon emission from 1940 to 2021 (www.statista.com)
This issue also impacted the supply chains and caused academics and practitioners' attention to design environmentally friendly supply chains. The concerns about carbon emission in health care supply chains and pollution reduction are reminded for health care managers during the COVID-19 pandemic (Goodarzian et al., 2021b). Recent studies in the medical field prove the significant role of carbon emission and environmental pollution on the severity of COVID-19 (Chen et al., 2022; Fattorini & Regoli, 2020; Frontera et al., 2020; Wu et al., 2020). The results of these studies, which are performed in different countries, show that air pollution increases the infection and mortality rate of COVID-19. Therefore, it can be concluded that the importance of carbon emission not only is not reduced during this pandemic but also is intensified and may impact the prevalence of the disease. The importance of carbon emission in the COVID-19 pandemic is so high that several recent research on health care supply chain planning investigated and addressed it (Goodarzian et al., 2021a, 2021b; Govindan et al., 2021).
In the current work, the resulting emission from the proposed plasma supply chain's activities is taken into account to address the mentioned concerns. Besides, our work is the first research investigating carbon emission in a plasma supply chain. We attempt to reduce carbon emission as much as possible by considering the total carbon emission as one of the objective functions of the proposed model. In summary, this work aims to address five research questions (RQ) as follows:
-
RQ 1: What is the optimal design of a green plasma supply chain during the outbreak of the COVID-19 pandemic?
To answer RQ 1, we develop a mixed-integer linear programming model to determine the location, allocation, motivational programs, and inventory decisions of the considered plasma supply chain network during the COVID-19 pandemic. The operational limitations of the studied network are formulated as the problem constraints. This model is presented by considering certainty for the parameters.
-
RQ 2: How to deal with the arising carbon emission concerns during the COVID-19 pandemic?
To answer RQ 2, we consider the total carbon emission from the transportation activities of the systems as an objective function of the problem along with the total cost. We formulate the problem as a bi-objective mathematical model.
-
RQ 3: How to deal with the total cost and total carbon emission as the conflicting objective of the problem simultaneously?
We utilize the epsilon constraint method as an efficient approach to deal with the considered economic and environmental objectives. This method can be easily applied and converts the problem to a single-objective problem by considering the more important goal as the objective function. This way, the less important objective is bounded as a problem constraint. After, the set of Pareto solutions is obtained using different upper bounds for the constrained objective function. This method enables the decision-makers to obtain a set of non-dominated solutions and selects the desired solution from this set.
-
RQ 4: What is the approach to solving the developed bi-objective mixed-integer linear mathematical model?
To answer RQ 4, we propose the GAMS programming environment and CPLEX commercial solver, one of the most powerful tools in solving the mixed-integer linear programming models.
-
RQ 5: How is the performance and applicability of the model?
To answer RQ 5, we apply the model to a case study and solve the model for the collected data of this supply chain network. In this regard, first, the Pareto front is calculated using the epsilon constraint method. After that one of the Pareto solutions is selected, and further analyses are performed on that solution.
The rest of the paper is organized as follows: Sect. 2 provides a literature review of the various aspects of this work. Section 3 defines the problem, and a multi-objective mathematical model is developed for the considered plasma supply chain network during the outbreak of the COVID-19 pandemic. Section 4 describes the epsilon constraint method as a solution methodology for handling the multi-objective model. In Sect. 5, a real-world case study from the plasma supply chain of the capital of Iran during COVID-19 is solved to evaluate the model. Section 6 provides the paper's conclusion with some suggestions for the extension of this work.
2 Literature review
This section reviews the literature of the present work's central aspects to highlight the gaps and specify current work contributions. We divide the literature review into three subsections: supply chain planning of blood products, supply chain planning during the COVID-19 pandemic, and green supply chain planning.
2.1 Blood-product supply chain planning
In the field of supply chain planning for blood products, Nagurney et al. (2012) presented the first mathematical programming model for a comprehensive supply chain network focusing on the general aspect of whole blood. Nagurney and Masoumi (2012) extended the previous work by formulating the capacity of network arcs as decision variables. Although this work's extension was significant progress in this research direction, the network was elementary and did not consider other important features.
Pierskalla (2005) investigated a simulation optimization problem for various decisions such as scheduling, location, allocation, and distribution in decentralized and centralized blood bank systems. A combination of stochastic dynamic programming and simulation approaches was proposed by Van Dijk et al. (2009) for a platelet production planning problem. Duan and Liao (2014) studied a red blood cells inventory system with ABO group compatibility and shelf-life features. Jabbarzadeh et al. (2014) formulated a supply chain network optimization problem under the pre-disaster and post-disaster assumptions, where a robust optimization method for this location-allocation problem was proposed. Zahiri and Pishvaee (2017) suggested a blood supply chain model considering different blood groups' compatibility. The goal was to minimize the total cost and the maximum unsatisfied demand under uncertainty.
Gunpinar and Centeno (2016) focused on routing aspects of the blood supply chain network by presenting an integer programming model for optimal routing of mobile collection centers. The objectives were minimizing the number of collection centers and total traveled distance. Hosseinifard and Abbasi (2018) investigated the hospital inventory centralization and transshipment impact on the performance of a two-echelon blood supply chain network. They found positive results from inventory centralization, where the performance would enhance from 21 to 40%. Khalilpourazari and Khamseh (2019) discussed disruption in blood supply chain networks in a bi-objective model with different transportation modes. The goal was to minimize the systems' total cost and delivery time. Yousefi Nejad Attari et al. (2019) proposed an integrated method considering stochastic programming, robust optimization, and epsilon constraint to deal with some combination of uncertainty in blood supply chain networks. The donation uncertainty was formulated by stochastic scenarios, while the polyhedral uncertainty sets were considered for patients' uncertainty demand. Khalilpourazari et al. (2020) worked on blood supply chain planning in disaster conditions. They proposed using a helicopter to transform blood to disaster points and return injured people from this point to safe medical centers. Using neural learning for decision-making was also discussed. The paper of Hosseini-Motlagh et al. (2020b) was the first research in the supply chain of blood products that investigated a stimulation program to motivate volunteer donors of the blood supply chain. Fallahi et al. (2021b) presented a mathematical model to formulate a closed-loop blood supply chain network considering the quality of the transportation equipment.
As one of the few works exploring carbon emission in blood supply chains, Heidari-Fathian and Pasandideh (2018) proposed a multi-objective sustainable model to minimize the total cost and carbon emission in the blood supply chain. In addition, Mousavi et al. (2021) investigated a sustainable blood supply chain in which both the environmental and social criteria were considered. This problem was formulated as a stochastic green split pick-up VRP model with uncertainty in donation capacity and the decomposition ratio of blood.
Beliën and Forcé (2012) shed light on the blood-product supply chain literature by providing a comprehensive review paper for the first time. Various approaches and methods for blood supply chain planning, including mathematical programming, simulation, and queuing models, were discussed in this work. In addition to this article, some other works tried to investigate the features of past works and presented some review papers, e.g., Osorio et al. (2015), Pirabán et al. (2019), and Meneses et al. (2022).
Despite the appearance of significant research in this field, the supply chain of plasma products is rarely investigated in recent years, and the focus of previous works in the literature was on other blood products.
Dehghani Ashkezari and Yaghoubi (2020) presented a single-period plasma supply chain network model among the published work in this area. The whole blood and apheresis donation methods were possible, and they considered plasma demand from pharmaceutical companies and hospitals. Hosseini-Motlagh et al. (2020a) proposed another location-allocation model for the plasma supply chain. They seek to enhance the quality of delivered plasma to hospitals and pharmaceutical companies, focusing on frozen time, where data envelopment analysis (DEA) evaluates the candidate locations for plasma donation. More details on the state of the art of the problem are available in the related papers in the literature (Amiri et al., 2020).
As mentioned earlier, many researchers investigated the supply chain of blood products. However, this product's supply chain is rarely studied despite plasma's significant importance. Moreover, some features of blood supply chains, such as pandemic situations and the sustainability criteria, remain unaddressed, or there is a lack of attention.
2.2 Supply chain planning during the COVID-19 pandemic
The occurrence of the COVID-19 pandemic influenced all nodes (members) of various supply chains worldwide (Gunessee & Subramanian, 2020). This issue attracted scholars' and researchers' attention to study the traditional supply chain networks under the assumptions of the COVID-19 pandemic.
Tsao et al. (2021) discussed the impact of COVID-19 on the supply chains in the energy sector, where the challenge was many disruptions in the supply of renewable energies in the network. This problem was investigated by presenting a resilient network. Majumdar et al. (2020) provided explanatory research to find the reasons for the lack of social responsibility in India's clothing supply chain, where the motivation was the unemployment of many laborers during the COVID-19 pandemic. Chowdhury et al. (2021) provided the first systematic review paper focusing on the emerging studies about the impact of the COVID-19 pandemic on supply chains. Paul and Chowdhury (2020) developed a recovery model for manufacturing supply chains under high-demand products like toilet papers. This problem arises from the disruption caused by increasing the demand for products and decreasing the supply of raw materials from suppliers.
A portion of current works in supply chain planning during COVID-19 relates to the supply chain in the medical sector. Goodarzian et al. (2021b) presented the first integrated multi-objective sustainable medical supply chain that considers the features of medicines such as RamedSivir and Favipiravir for COVID-19 patients. They viewed both the social and environmental dimensions of sustainability in their model. Mehrotra et al. (2020) utilized a stochastic programming approach for managing ventilator inventory under the US's pandemic limitations. They proposed resource sharing as an effective strategy to mitigate the risk of disruption. Lozano Díez et al. (2020) formulated the risk of resulting disruption from the pandemic and its effect on the shortage quantity in a general drug supply chain. Zahedi et al. (2021b) applied an IoT system to a relief logistic network in the COVID-19 pandemic. The network focused on the suspectable cases of COVID-19, a prioritizing, and an allocating approach were discussed. The goal of the first approach was minimizing the maximum response time of ambulances, and the second approach aimed to minimize the critical response time.
Mosallanezhad et al. (2021a) addressed a bi-objective logistic network for personal protection equipment of medical centers in the COVID-19 pandemic. They suggested the total cost and the maximum unsatisfied demand as the objectives.
Kargar et al. (2020) pointed out that the prevalence of the COVID-19 pandemic caused more generations of medical waste and presented a reverse supply chain network for better waste management. Regarding the shortage of personal protective equipment, Rowan and Laffey (2020) analyzed this problem from the supply chain standpoint and provided some solutions for this problem.
Despite these studies' great contribution, none considered the supply chain of plasma products in COVID-19, which has a significant role in controlling this pandemic.
2.3 Green supply chain planning
The environmental concerns about global warming resulting from carbon emissions caused the concentration of research on green supply chain planning and created an exciting research area.
Sheu et al. (2005) attempted to deal with logistic problems in a green supply chain by presenting a linear programming model. In Garg et al. (2015) work, the closed-loop supply chain's environmental concerns were addressed, where they used a bi-objective mixed-integer nonlinear programming model for this problem. They considered different transportation modes in the distribution layer. Nia et al. (2015) extended a sustainable multi-item EOQ model for a two-echelon supply chain under various operational constraints. To enhance the performance, they utilized Vendor Managed Inventory (VMI) contract as the coordination mechanism of the supply chain. Centobelli et al. (2017) presented a recent systematic review of the green supply chains problem. Mirzapour Al-e-hashem et al. (2013) proposed a stochastic mathematical model for aggregate production planning in an environmentally responsible supply chain network. Another extension was proposed by Entezaminia et al. (2016), where they developed the formulation of aggregate production planning in a green reverse supply chain network.
Resat and Unsal (2019) investigated multi-objective sustainable supply chain networks in packaging industries. They utilized a two-stage method based on the Analytic Hierarchy Process (AHP) and mathematical programming to minimize the total time and cost of the sustainability factors. Abdi et al. (2020) applied a new approach to design a green supply chain network by considering simultaneous pick-up and split delivery. The network's environmental features were also addressed by using a green VRP model for the network. Mokhtari and Rezvan (2020) investigated a multi-product single vendor-multibuyer supply chain under a VMI contract with a limitation on the system's total carbon emission. A bi-objective sustainable supply chain for palm date was presented by Hamdi-Asl et al. (2021). The total cost and total carbon emission were considered the problem's objective functions. Hasani et al. (2021) developed a sustainable and resilient supply chain for a company in the medical industry. The goal was to make a trade-off between the network's carbon emission, resiliency, and profitability. Salehi-Amiri et al. (2022a) studied the application of IoT in a sustainable waste management system. This problem was formulated as two VRP sub-models. As some research on the green supply chain models for agricultural products, Salehi-Amiri et al. (2021) studied a closed-loop agriculture supply chain network focusing on the walnut product. Chouhan et al. (2021) presented a closed-loop structure for a sugarcane supply chain network. The environmental features of the sugarcane supply chain were also studied by Chouhan et al. (2022). They formulated the supply chain network under carbon tax regulation. Another agricultural closed-loop supply chain was studied by Salehi-Amiri et al. (2022b). In addition to the economic and environmental pillars, they considered the social responsibility of the network by formulating the number of job opportunities as one of the objective functions. The closed-loop supply chain network design is also investigated for marine products. For example, Mosallanezhad et al. (2021b) developed a mathematical model for shrimp closed-loop supply chain planning. A closed-loop fish supply chain network was investigated by Fasihi et al. (2021a). In this regard, the computational complexity of the fish closed-loop supply chain was addressed in Fasihi et al. (2021b) research. They used three efficient metaheuristics, MOKA, NSGA-II, and MOSA, as the solution approaches. Qiao et al. (2021) used the Stackelberg game approach for a supply chain coordination problem under emission-dependent demand and investment options to curb environmental impacts. The interested reader can refer to the relevant papers in the literature for more information on green supply chain planning problems (Fallahi et al., 2021a; Boronoos et al., 2021; Daneshdoost et al., 2022; Khan et al., 2021; Rajak et al., 2022; Zahedi et al., 2021a). The summary of reviewed literature is provided in Table 1. Table 2 contains the abbreviations used in Table 1.
3 Research gap
Regarding the reviewed literature, despite the publication of a wide range of research in the blood supply chain, there is a lack of attention to managing convalescent plasma in pandemic situations like COVID-19. The convalescent plasma shows great efficiency in treating infected people in the epidemic and pandemic outbreak of several diseases. Therefore, it seems necessary to investigate the presence of this product in a supply chain framework. Besides, the regular plasma supply chain is rarely addressed and discussed. Finally, despite many carbons emission-related problems, the green blood products supply chain design, especially the plasma, is rarely addressed. The importance of carbon emission and environmental pollution not only reduced in COVID-19 but also intensified regarding the impact of this phenomenon on the disease severity of infected people.
Our current work tries to cover the above gaps. It is the first research that addresses the flow of convalescent plasma as a critical substance for treating COVID-19 disease alongside the flow of plasma in a multi-product plasma supply chain. We try to present a closer problem to real-world assumptions by considering various features of regular and convalescent plasma, the COVID-19 pandemic, and carbon emissions from the supply chain's activity. The main contributions of the present work can be listed as follows:
-
We develop a mixed-integer linear programming model to design a plasma supply chain network during the COVID-19 pandemic. In this model, we try to consider several specific assumptions about the pandemic, which have not been considered so far in the literature. The presence of convalescent plasma recovered patients, and the medical centers for COVID-19 patients as the special supply and demand point, the motivational programs for the recovered patients, and the transshipment of convalescent plasma are some of these assumptions which have not been addressed in the literature. Therefore, the newly developed model is a generalized framework of the previous works for plasma supply chain network design.
-
We consider the plasma supply chain network's carbon emission and the total cost and present the problem as a bi-objective model. Carbon emission is rarely addressed in the blood supply chain, and no work in the literature considers the presence of carbon emission in the plasma supply chain. Due to the intensified emission concern and the impact of pollution on the state of COVID-19 patients, we address the carbon emission from transportation activities. We aim to help the decision-makers to select the desired solution.
-
We investigate the applicability and efficiency of the mathematical model by applying it to the plasma supply chain network of the capital of Iran during the COVID-19 pandemic, as one of the largest cities in the Middle East. In this way, several analyses are performed, and insights are drawn that can help the decision-makers manage the studied network.
4 Problem description and formulation
This section first defines the proposed supply chain network for managing plasma and convalescent plasma under COVID-19 limits. After that a mixed-integer linear mathematical programming model is proposed for the desired network.
4.1 Problem definition
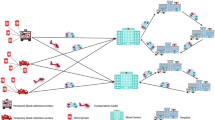
A four-echelon multi-product multi-period network is developed to determine the optimal location, allocation, production, motivational programs, and inventory control decisions of the considered plasma supply chain during the COVID-19 pandemic.
In this network, there are two groups of donors at the first layer: the first group is regular donors (Hosseini-Motlagh et al., 2020a). In contrast, the second is the recovered patients from the COVID-19 disease with a predefined virus antibody level (Chen et al., 2020). Motivational programs are efficient methods used to motivate people to blood donation and boost the supply side of blood supply chains (Hosseini-Motlagh et al., 2020b). These programs have received much attention during COVID-19 to motivate recovered patients to donate their convalescent plasma in different countries (Bloch et al., 2021) (behdasht.gov.ir). We consider three categories of motivational programs: medical credits, advertisement, and educational courses for the recovered donors in the presented network. These programs positively correlate with each recovered group's convalescent plasma donation capacity, and there is a limited available budget for them.
Both groups of donors should refer to collection centers for blood donation. The state of the COVID-19 pandemic is not stable and may change in different areas. Hence, we consider mobile collection centers as facilities for collecting convalescent plasma from recovered donors. The location of these mobile centers can be changed in each period (Bloch et al., 2021). Besides, fixed collection centers are assumed for plasma collection. Logically, the location decision of these fixed centers is strategic and remains fixed prior, during, and past the pandemic. The location of mobile collection centers in each period and the overall location of fixed collection centers should be determined from a prespecified set of candidate locations.
There are two donation modes: apheresis donation and whole blood donation to collect blood from donors (Dehghani Ashkezari & Yaghoubi, 2020). The collection of convalescent plasma is similar to other products and is performed by these methods (Bloch et al., 2021). In apheresis donation, the plasma is extracted directly from the donor's body. In contrast, in whole blood donation, the whole blood is withdrawn, and the plasma is separated through some process operations (Dehghani Ashkezari & Yaghoubi, 2020). More plasma is attainable by the apheresis donation method, while whole blood donation is cheaper (Osorio et al., 2018). Both whole blood and apheresis donation modes are considered for mobile and fixed collection centers. The collected (convalescent) whole blood/plasma is assumed to be transferred to processing facilities within less than 6 h (Dehghani Ashkezari & Yaghoubi, 2020; Mobasher et al., 2015).
There is a limited set of candidate locations for processing facilities, and the location of these facilities should be specified, similar to fixed collection centers (Samani et al., 2018). The received (convalescent) whole blood/plasma is processed in processing facilities to become usable for final consumption. The shelf life and perishability of both products are regarded from this layer. Convalescent plasma production and shelf life are similar to regular plasma (Bloch et al., 2021).
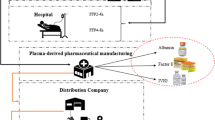
Pharmaceutical companies and hospitals are the two main demand points for regular plasma (Dehghani Ashkezari & Yaghoubi, 2020). Regular plasma is used in pharmaceutical companies to produce various plasma-based medicines such as human albumin. In hospital demand points, regular plasma is used to treat patients with burn, shock, hemophilia, etc. (Rytilä & Spens, 2006). Another special feature of the plasma supply chain during the COVID-19 pandemic is some medical centers that admit patients with COVID-19. These centers need convalescent plasma to treat their COVID-19 patients, and the produced convalescent plasma is transformed to these centers based on their needs. All three demand points are taken into account in the proposed model, and the flow of regular plasma and convalescent plasma is established based on their demand.
The high mortality rate of COVID-19 boosted the demand for convalescent plasma, and various inventory challenges appeared (Wendel et al., 2021). In our model, the possibility of convalescent plasma transshipment between processing facilities is regarded to restrict the shortage of this critical substance during the pandemic as much as possible. Regarding the related inventory challenges of convalescent plasma, a minimum safety-stock level is considered for this product in processing facilities.
Finally, the movement of mobile collection centers and product transportation is the primary source of carbon emissions in blood supply chains (Heidari-Fathian & Pasandideh, 2018; Mousavi et al., 2021). We try to incorporate sustainability in the supply chain of plasma and blood during the COVID-19 pandemic for the first time by taking into account these primary sources of pollution in the network and attempting to minimize the total carbon emission of the system as much as possible. Figure 2 presents the framework of the studied plasma supply chain network under the assumptions of the COVID-19 pandemic.
The main assumptions and decisions of the proposed plasma supply chain network for the COVID-19 pandemic are specified as follows:
4.2 Assumptions
The following assumptions are considered for the presented plasma supply chain:
-
The supply chain is a multi-echelon, multi-product, and multi-period network.
-
There are two groups of volunteers for donation in the network: The first group is the regular people who donate regular plasma. The second group comprises the recovered patients from COVID-19 disease that donate convalescent plasma.
-
There are two types of donation methods for donors and recovered donors, including apheresis and whole blood donation.
-
Two collection centers are fixed collection centers for regular volunteers and mobile collection centers for recovered volunteers.
-
Three motivational programs for recovered patients from COVID-19 motivate them to donate their convalescent plasma: Medical credits, advertisement, and educational courses.
-
There is a limited budget for the motivational programs of recovered donors from COVID-19.
-
There is a limited set of candidate locations for the location of mobile collection centers in each period.
-
There are limited sets of candidates, fixed collection centers, and processing facilities.
-
The perishability of plasma and convalescent plasma is considered due to the shelf-life parameter.
-
The transshipment of convalescent plasma between processing facilities is allowed to prevent shortage as much as possible.
-
The carbon emission causes by the movement of collection facilities and transportation of products in the network.
4.3 Decisions
The following decisions are made by solving the presented mathematical model:
4.3.1 Optimal motivational programs decisions
-
Assigned medical credit units to each recovered donor group in each period
-
Assigned advertisement units to each recovered donor group in each period
-
Assigned educational course units to each recovered donor group in each period
4.3.2 Optimal location decisions
-
Location of mobile collection centers in each period
-
Location of fixed collection centers
-
Location of processing facilities
4.3.3 Optimal allocation decisions
-
Allocation pattern of donors to fixed collection centers and received quantity of regular whole blood/plasma in each period
-
Allocation pattern of recovered donors to mobile collection centers and received quantity of convalescent whole blood/plasma in each period
-
Allocation pattern of fixed collection centers to processing facilities and transformed quantity of regular whole blood/plasma in each period
-
Allocation pattern of mobile collection centers to processing facilities and transformed quantity of convalescent whole blood/plasma in each period
-
Allocation pattern of processing facilities to pharmaceutical companies and transformed quantity of regular plasma in each period
-
Allocation pattern of processing facilities to hospitals and transformed quantity of regular plasma in each period
-
Allocation pattern of processing facilities to medical centers for COVID-19 patients and transformed quantity of convalescent plasma in each period
-
Allocation pattern between processing facilities and transshipment quantity of convalescent plasma in each period
4.3.4 Optimal production decisions
-
Production quantity of regular plasma in each processing facility in each period
-
Production quantity of convalescent plasma in each processing facility in each period
4.3.5 Optimal inventory decisions
-
Inventory level of regular plasma in each processing facility in each period
-
Inventory level of convalescent plasma in each processing facility in each period
-
Shortage quantity of regular plasma in each pharmaceutical company in each period
-
Shortage quantity of regular plasma in each hospital in each period
-
Shortage quantity of convalescent plasma in each medical center for COVID-19 patients in each period
-
Expired quantity of regular plasma in each pharmaceutical company in each period
-
Expired quantity of regular plasma in each hospital in each period
-
Expired quantity of convalescent plasma in each medical center for COVID-19 patients in each period
4.4 Mathematical modeling
A mixed-integer multi-objective model is developed in this section to formulate the considered plasma supply chain network. The model contains the following notations, parameters, and decision variables.
4.4.1 Notations
The indices, parameters, and decision variables are defined as follows:
Sets and indices | |
|---|---|
\(I\) | Set of regular donors; indexed by \(i(i\in I)\) |
\(J\) | Set of recovered donors from COVID-19 disease; indexed by \(j(j\in J)\) |
\(R\) | Set of donation methods; indexed by \(r(r\in R)\) |
\(M\) | Set of candidate locations for mobile collection centers; indexed by \(m,{m}_{1},{m}_{2}(m,{m}_{1},{m}_{2}\in M)\) |
\(F\) | Set of candidate locations for fixed collection centers; indexed by \(f(f\in F)\) |
\(P\) | Set of candidate locations for processing facilities; indexed by \(p,{p}^{^{\prime}}(p,{p}^{^{\prime}}\in P)\) |
\(E\) | Set of pharmaceutical companies; indexed by \(e(e\in E)\) |
\(H\) | Set of hospitals; indexed by \(h(h\in H)\) |
\(C\) | Set of medical centers for COVID-19 patients; indexed by \(c(c\in C)\) |
\(T\) | Set of periods; indexed by \(t,{t}^{^{\prime}}(t,{t}^{^{\prime}}\in T)\) |
Parameters | |
|---|---|
\({crcs}_{t}\) | The unit cost of a medical credit for motivation of convalescent plasma donors in period \(t\) |
\({adcs}_{t}\) | The unit cost of an advertisement for motivation of convalescent plasma donors in period \(t\) |
\({edcs}_{t}\) | The unit cost of an educational course for motivation of convalescent plasma donors in period \(t\) |
\({crcf}_{t}\) | Correlation factor of convalescent plasma donation capacity of a recovered group and a medical credit unit in period \(t\) |
\({adcf}_{t}\) | Correlation factor of convalescent plasma donation capacity of a recovered group and an advertisement unit in period \(t\) |
\({edcf}_{t}\) | Correlation factor of convalescent plasma donation capacity of a recovered group and an educational course unit in period \(t\) |
\(BMOT\) | The maximum available budget for motivational programs |
\({dcnm}_{irt}\) | Maximum possible donation of regular donors group \(i\) through donation method \(r\) (regular whole blood/plasma) in period \(t\) |
\({dccm}_{jrt}\) | Maximum possible donation of recovered donors \(j\) through donation method \(r\) (convalescent whole blood/ plasma) in period \(t\) |
\({lcfc}_{f}\) | The location cost of fixed collection center \(f\) |
\({lcmm}_{{m}_{1}{m}_{2}t}\) | Moving cost of each mobile collection center from node \({m}_{1}\) to node \({m}_{2}\) in period \(t\) |
\({cnfm}_{ifrt}\) | Unit collection cost of a regular whole blood/plasma through donation method r from donor group \(i\) at fixed collection center \(f\) in period \(t\) |
\({ccmm}_{jmrt}\) | Unit collection cost of a convalescent whole blood/plasma through donation method r from recovered donor group \(j\) at mobile collection center \(m\) in period \(t\) |
\({fcnm}_{fr}\) | The capacity of fixed collection center \(f\) for collecting regular whole blood/plasma through donation method \(r\) |
\({mccm}_{mr}\) | The capacity of mobile collection center \(m\) for collecting convalescent whole blood/plasma through donation method \(r\) |
\({lcpc}_{p}\) | The location cost of processing facility \(p\) |
\({pctp}_{p}\) | Collection capacity of processing facility \(p\) |
\({mnfp}_{fpt}\) | Unit transportation cost of a regular whole blood/plasma from fixed collection center \(f\) to processing facility \(p\) in period \(t\) |
\({mcmp}_{mpt}\) | Unit transportation cost of a convalescent whole blood/plasma from mobile collection center \(m\) to processing facility \(p\) in period \(t\) |
\({pnmp}_{rpt}\) | The unit production cost of a regular whole blood/plasma collected through donation method \(r\) in production facility \(p\) in period \(t\) |
\({pcmp}_{rpt}\) | The unit production cost of a convalescent whole blood/plasma collected through donation method \(r\) in production facility \(p\) in period \(t\) |
\({cppm}_{rp}\) | The unit production rate for a convalescent/regular whole blood/plasma collected through donation method \(r\) in processing facility \(p\) |
\({hcnp}_{pt}\) | Unit holding cost of a regular plasma in processing facility \(p\) in period \(t\) |
\({hccp}_{pt}\) | Unit holding cost of convalescent plasma in processing facility \(p\) in period \(t\) |
\({innp}_{p}\) | Total available space of processing facility \(p\) for storing regular plasma |
\({incp}_{p}\) | Total available space of processing facility \(p\) for storing convalescent plasma |
\(pshl\) | Shelf life of a convalescent/regular plasma |
\({mnpe}_{pet}\) | Unit transportation cost of a regular plasma from processing facility \(p\) to pharmaceutical company \(e\) in period \(t\) |
\({mnph}_{pht}\) | Unit transportation cost of a regular plasma from processing facility \(p\) to hospital \(h\) in period \(t\) |
\({mcpc}_{pct}\) | Unit transportation cost of convalescent plasma from processing facility \(p\) to medical center for COVID-19 patients \(c\) in period \(t\) |
\({shne}_{et}\) | Unit shortage cost of a regular plasma in pharmaceutical company \(e\) period \(t\) |
\({shnh}_{ht}\) | Unit shortage cost of a regular plasma in hospital \(h\) period \(t\) |
\({shcc}_{ct}\) | Unit shortage cost of convalescent plasma in medical center for COVID-19 patients \(c\) in period \(t\) |
\({exne}_{et}\) | Unit expiration cost of a regular plasma in pharmaceutical company \(e\) in period \(t\) |
\({exnh}_{ht}\) | Unit expiration cost of a regular plasma in hospital \(h\) in period \(t\) |
\({excc}_{ct}\) | Unit expiration cost of convalescent plasma in medical center for COVID-19 patients \(c\) in period \(t\) |
\({dmne}_{et}\) | The demand of pharmaceutical company \(e\) for regular plasma in period \(t\) |
\({dmnh}_{ht}\) | The demand of hospital \(h\) for regular plasma in period \(t\) |
\({dmcc}_{ct}\) | The demand of medical center for COVID-19 patients \(c\) for convalescent plasma in period \(t\) |
\({sscp}_{pt}\) | Safety-stock level of convalescent plasma in processing facility \(p\) in period \(t\) |
\({mccp}_{p{p}^{^{\prime}}t}\) | Unit transshipment cost of convalescent plasma from processing facility \(p\) to processing facility \({p}^{^{\prime}}\) in period \(t\) |
\({cemm}_{{m}_{1}{m}_{2}}\) | Unit carbon emission to move a mobile collection center from node \({m}_{1}\) to node \({m}_{2}\) |
\({cefp}_{fp}\) | Unit carbon emission to transport a regular whole blood/plasma from fixed collection center \(f\) to processing facility \(p\) |
\({cemp}_{mp}\) | Unit carbon emission to transport a convalescent whole blood/ plasma from mobile collection center \(m\) to processing facility \(p\) |
\({cepe}_{pe}\) | Unit carbon emission to transport a regular plasma from processing facility \(p\) to pharmaceutical company \(e\) |
\({ceph}_{ph}\) | Unit carbon emission to transport a regular plasma from processing facility \(p\) to hospital \(h\) |
\({cepc}_{pc}\) | Unit carbon emission to transport a convalescent plasma from processing facility \(p\) to a medical center for COVID-19 patients \(c\) |
\({cecp}_{p{p}^{^{\prime}}}\) | Unit carbon emission to transport a convalescent plasma from processing facility \(p\) to processing facility \({p}^{^{\prime}}\) in period \(t\) |
\(M\) | A big number |
Decision variables | |
|---|---|
\({lcf}_{f}\) | Equals one if fixed collection center \(f\) is located; 0 otherwise |
\(lc{m}_{{m}_{1}{m}_{2}t}\) | Equals one if mobile collection center located in the node \({m}_{1}\) in period \(t-1\) move to the location \({m}_{2}\) in period \(t\); 0 otherwise |
\(a{df}_{ifrt}\) | Equals one if regular donor group \(i\) is allocated to fixed collection center \(f\) for donation through donation method \(r\) in period \(t\); 0 otherwise |
\(a{dm}_{jmrt}\) | Equals one if recovered donor group \(j\) is allocated to mobile collection center \(m\) for donation through donation method \(r\) in period \(t\); 0 otherwise |
\({lcp}_{p}\) | Equals one if processing facility \(p\) is located; 0 otherwise |
\(a{fp}_{fprt}\) | Equals one if fixed collection center \(f\) is allocated to production facility \(p\) for transport collected regular whole blood/plasma through donation method \(r\) in period \(t\); 0 otherwise |
\(a{mp}_{mprt}\) | Equals one if mobile collection center \(m\) is allocated to production facility \(p\) for transport collected convalescent whole blood/plasma through donation method \(r\) in period \(t\); 0 otherwise |
\(a{pe}_{pet}\) | Equals one1 if production facility \(p\) is allocated to pharmaceutical company \(e\) for transporting regular plasma in period \(t\); 0 otherwise |
\(a{ph}_{pht}\) | Equals one1 if production facility \(p\) is allocated to hospital \(h\) for transporting regular plasma in period \(t\); 0 otherwise |
\(a{pc}_{pct}\) | Equals one if production facility \(p\) is allocated to a medical center for COVID-19 patients \(c\) for transport convalescent plasma in period \(t\); 0 otherwise |
\({mcr}_{jt}\) | Assigned medical credit units to motivate recovered donor group \(j\) in period \(t\) |
\({mad}_{jt}\) | Assigned advertisement units to motivate recovered donor group \(j\) in period \(t\) |
\({med}_{jt}\) | Assigned educational course units to motivate recovered donor group \(j\) in period \(t\) |
\(c{df}_{ifrt}\) | Quantity of collected regular whole blood/plasma through donation method r from donor group \(i\) at fixed collection center \(f\) in period \(t\) |
\(c{dm}_{jmrt}\) | Quantity of collected convalescent whole blood/plasma through donation method r from recovered donor group \(j\) at mobile collection center \(m\) in period \(t\) |
\({tfp}_{fprt}\) | Quantity transformed regular whole blood/plasma from fixed collection center \(f\) to processing facility \(p\), collected through donation method \(r\) in period \(t\) |
\({tmp}_{mprt}\) | Quantity transformed convalescent whole blood/plasma from mobile collection center \(m\) to processing facility \(p\), collected through donation method \(r\) in period \(t\) |
\({ppn}_{rpt}\) | Production quantity of regular plasma from received regular whole blood/plasma that collected through donation method \(r\) in processing facility \(p\) in period \(t\) |
\({ppc}_{rpt}\) | Production quantity of convalescent plasma from received convalescent whole blood/plasma that collected through donation method \(r\) in processing facility \(p\) in period \(t\) |
\({inp}_{pt}\) | Inventory level of regular plasma in processing facility \(p\) in period \(t\) |
\({icp}_{pt}\) | Inventory level of convalescent plasma in processing facility \(p\) in period \(t\) |
\({qne}_{pet{t}^{^{\prime}}}\) | Quantity of transported regular plasma from processing facility \(p\) in period \(t\) to pharmaceutical company \(e\) that consumed in the period \({t}^{^{\prime}}\) |
\({sne}_{et}\) | Shortage quantity of regular plasma in pharmaceutical company \(e\) in period \(t\) |
\({ene}_{et}\) | Expired quantity of regular plasma in pharmaceutical company \(e\) in period \(t\) |
\({qnh}_{pht{t}^{^{\prime}}}\) | Quantity of transported regular plasma from processing facility \(p\) in period \(t\) to hospital \(h\) that consumed in the period \({t}^{^{\prime}}\) |
\({snh}_{ht}\) | Shortage quantity of regular plasma in hospital \(h\) in period \(t\) |
\({enh}_{ht}\) | Expired quantity of regular plasma in hospital \(h\) in period \(t\) |
\({qcc}_{pct{t}^{^{\prime}}}\) | Quantity of transported convalescent plasma from processing facility \(p\) in period \(t\) to a medical center for COVID-19 patient \(c\) that consumed in the period \({t}^{^{\prime}}\) |
\({scc}_{ct}\) | Shortage quantity of convalescent plasma in medical center for COVID-19 patients \(c\) in period \(t\) |
\({ecc}_{ct}\) | Expired quantity of convalescent plasma in medical center for COVID-19 patients \(c\) in period \(t\) |
\({tcp}_{p{p}^{^{\prime}}t}\) | Transshipment quantity of convalescent plasma between processing facility \(p\) and COVID-19 medical center \({p}^{^{\prime}}\) in period \(t\) |
4.4.2 The model
The first objective function of the model to minimize is:
Objective function (1) minimizes the total cost of integrated network including movement cost of mobile collection centers, collection cost of regular plasma from regular donors, collection cost of convalescent plasma from recovered donors, location cost of processing facilities, location cost of fixed collection centers, production cost of regular plasma, production cost of convalescent plasma, inventory holding cost of regular plasma in processing facilities, inventory holding cost of convalescent plasma in processing facilities, transportation cost between fixed collection centers and processing facilities, transportation cost between mobile collection centers and processing facilities, transportation cost between processing facilities and pharmaceutical companies, transportation cost between processing facilities and hospitals, transportation cost between processing facilities and medical centers for COVID-19 patients, convalescent plasma transshipment cost between processing facilities, regular plasma expiration cost in pharmaceutical companies, regular plasma expiration cost in hospitals, convalescent plasma expiration cost in medical centers for COVID-19 patients, regular plasma shortage cost in pharmaceutical companies, regular plasma shortage cost in hospitals, convalescent plasma shortage cost in medical centers for COVID-19 patients, and the cost of motivational programs for recovered donors.
The second objective function to minimize is the total carbon emission:
The second objective (2) goal is to minimize the total carbon emission in the plasma supply chain network. This consists of the resulted carbon emission from the movement of mobile collection centers, the transformation of flow from fixed collection centers to processing facilities, the transformation of flow from mobile collection centers to processing facilities, transshipment between processing facilities, the transformation of flow from processing facilities to pharmaceutical companies and hospitals, and transformation from processing facilities to medical centers for COVID-19 patients.
The constraints of the mathematical formulation are:
There should not be more than one mobile collection center in a candidate location. Constraint (3) puts an upper bound on the number of mobile collection centers in a location. It ensures that only one mobile collection center is located in a specific node in each period.
Constraints (4) and (5) provide that one donation method (apheresis or whole blood) is used for each group of regular donors and recovered donors in the considered fixed and mobile collection centers in a period.
Constraint (6) implies that the relocation of a mobile collection center in a period is possible for the facilities located in the previous period.
The flow of regular plasma and convalescent plasma between two facilities in the supply chain is possible when the considered facilities are allocated in a period. Constraints (7) and (8) are the allocation constraints of regular donors and recovered donors to the fixed and mobile collection centers. The possibility of flow to the allocated collection centers and processing facilities is limited via constraints (9) and (10). Constraints (11)–(13) are the allocation constraints between the processing facilities and the demand points.
Constraints (14) and (15) limit the collection capacity of each fixed collection center and mobile collection center for collecting the donated regular/convalescent whole blood/plasma through each donation method in each period.
Constraint (16) shows the maximum donation capacity of each group for regular plasma donation in a period, where the donation capacity of recovered groups considering the impact of motivational programs in each period is expressed via constraint (17).
Constraints (18)–(26) enforce that the flow of convalescent and regular plasma is possible only for the established facilities in each period. Constraints (18) and (19) ensure that the regular donor and recovered donors only refer to the established fixed collection centers and located mobile collection centers. Constraints (20) and (21) limit the flow of regular plasma between the established fixed collection centers and process facilities. Similarly, constraints (22) and (23) are the flow restriction constraints of convalescent plasma between the located mobile collection centers and the established processing centers. Constraints (24)–(26) state that the transformation of regular and convalescent plasma is possible only from the established processing centers to the demand points.
All the received regular whole blood/plasma from the regular donors in the fixed collection centers should be transformed to the processing centers. Constraints (27) and (28) control the inflow and outflow of received regular whole blood/plasma in fixed collection centers in each period.
The flow constraint of received convalescent whole blood/plasma in mobile collection centers in each period is formulated by Eqs. (29) and (30). Regarding these equations, the total convalescent whole blood/plasma delivered to the processing centers should equal the received quantity from the recovered donors in each period.
The total received and convalescent plasma from the collection centers (fixed and mobile) should be equal to or less than the processing centers' storage capacity. Constraint (31) limits the capacity of each processing facility in each period.
The production and transformation of received regular plasma and convalescent plasma in each processing facility in each period are shown by constraints (32) and (33). The total production is obtained by multiplying the received regular and convalescent plasma by the production rate of each item.
In the processing centers, the inflow of convalescent plasma should equal this product's outflow for each period. Constraints (34) is the inventory balance equation of convalescent plasma in the processing facilities in each period.
The balance of regular plasma inventory in processing facilities in each period is limited by constraints (35). These constraints specify the inflow and outflow of regular plasma in processing centers in each period.
Constraint (36) ensures that the minimum safety stock of convalescent plasma in an established process center is provided in each period. In other words, the convalescent plasma inventory level should be equal to or more than the specified safety stock.
The storage capacity of processing centers is limited for regular and convalescent plasma in each period. Constraints (37) and (38) demonstrate that each processing facility's stored inventory of plasma and convalescent plasma should be less than the storage capacity for each product in each period.
If the product delivery be less than the demand rate, a shortage occurs. Constraints (39) and (40) calculate the shortage quantity of regular plasma in each pharmaceutical company and hospital in each period, respectively. The shortage of convalescent plasma in each medical center for COVID-19 patients in each period is formulated by Eq. (41).
If the time interval between the production and delivery of plasma or convalescent plasma unit is more than the shelf life that unit would be expired. Constraints (42)–(44) reveal the expiration of plasma and convalescent plasm in pharmaceutical companies, hospitals, and medical centers for COVID-19 patients in each period, based on the shelf life of plasma products.
Constraint (45) considers the limits on the maximum available budget for motivational programs for recovered donors in the planning horizon.
Constraints (46) and (47) present the types of decision variables.
5 The solution approach
In many real-world situations, the decision-makers usually deal with different goals and intend to trade-off multi-objectives in their problem (Mokhtari & Hasani, 2017). In this condition, the classic optimization approaches are not practical. Therefore, various approaches and methods are discussed for optimizing multi-objective optimization problems.
One of the main approaches for handling different objective functions in an optimization problem is the epsilon constraint method which tries to construct a single-objective model by converting other objectives to model constraints with predefined bounds (Haimes, 1971). By changing the bounds of the constraints, Pareto solutions are obtained, and the decision-maker can select the preferred solution based on their preference.
Consider the following model as a general multi-objective problem:
Subject to:
The epsilon constraint method transforms the objective functions of this problem into a single objective as:
Subject to:
where \({f}_{h}\left(x\right)\) is the hth objective function preferred to others, \(R\) is the total number of objective functions of the problem, and \({\varepsilon }_{r}\) is considered the upper bound on the rth objective function. For solving the proposed plasma supply chain network during the COVID-19 pandemic, the solving procedure of the epsilon constraint method can be summarized in 4 steps:
-
Step 1 Obtain a payoff table by solving the model with each objective function separately to find each objective function's best and worst value.
-
Step 2 Select \(TC\) (total cost of the supply chain) or \(TE\) (total emission of the supply chain) as the objective function of the model and let the other be the model constraint.
-
Step 3 Calculate the range between the best and worst value of the bounded objective function, and divide the range to \({\varepsilon }_{1 },{\varepsilon }_{2 },\dots ,{\varepsilon }_{k}\)
Using the obtained information from the above steps, establish the single-objective model as:
$$\mathrm{Min} \, Z=\mathrm{TC} \, (\mathrm{TE})$$(55)Subject to:
$$\mathrm{TE} \, \left(\mathrm{TC}\right)\le \varepsilon$$(56)$$W_{{\text{o}}} \le \varepsilon \le {\text{BE}}$$(57)where \(\mathrm{BE}\) and \(W\mathrm{o}\) are the best and worst obtained values from the payoff table for the constrained objective function.
-
Step 4 Solve the resulted problem in the previous section with different epsilon values to find the Pareto solutions.
6 Case description
In this section, a real-world case study from Tehran, the capital of Iran, is solved, to illustrate the efficiency and performance of the proposed multi-objective model in plasma supply chain planning during the COVID-19 pandemic.
Tehran is selected due to its high importance in three main dimensions of current research:
-
Regarding the official reports, 2,739,875 cases were identified in Iran until May 15, 2021, and Iran is among the top 15 countries with high identified COVID-19 patients. The high population and various socioeconomic and cultural activities in Tehran have caused many people to be infected at any peak of the disease, which caused growing concerns about managing COVID-19.
-
Tehran accounts for about 24% of Iran's supply and 27% of its total blood demand. There is high demand in Tehran, considering particular regular plasma products. For example, a recent global survey confirmed that Iran is one of the countries with a high rate of hemophilia, about 12,000 per year. In this respect, Tehran is among the top three cities that need regular plasma to treat patients (Hosseini-Motlagh et al., 2020a).
-
Tehran is facing various environmental and air pollution problem which is caused by carbon emissions. Official statistics from the Tehran Municipal Air Quality Control company state that there were 123 days in the previous year that the air in Tehran was unhealthy due to greenhouse gas emissions (airnow.tehran.ir).
Tehran metropolis is the most populated city in Iran, with an 8,693,706 population according to a census in 2016 (Samani & Hosseini-Motlagh, 2019; Samani et al., 2019). Tehran has 22 municipal districts, and the disparity between these districts and their population is shown in Fig. 3.
Iranian Blood Transfusion Organization (IBTO) manages the supply chain of regular and convalescent plasma in Tehran. The data of our case study are gathered from three primary sources as (1) the opinion of experts in the field, (2) IBTO and Tehran municipality documents, and (3) relevant published studies (Dehghani Ashkezari & Yaghoubi, 2020; Hosseini-Motlagh et al., 2020a; Nikpouraghdam et al., 2020; Samani et al., 2019; Yousefi Nejad Attari et al., 2017). Noteworthy, the dimension of the parameters of the model is presented in Table 3.
As mentioned, Tehran metropolitan has 22 municipal districts. Each city district is considered one group that can donate regular and convalescent plasma donors. The donation capacities of the districts are estimated based on their population. The geographical coordination of the districts and the estimated donation in a month are provided in Table 4.
There are two fixed collection centers in districts 5 and 6 to donate regular plasma in Tehran. To increase the collection capacity of the network, three more fixed centers in areas 1, 2, and 4 have been considered candidate points for donation. Since the beginning of the pandemic, the IBTO has been deemed the Vesal center and a center in district two as temporary centers for receiving recovery plasma. Two new candidate locations for locating new mobile centers exist in districts 1 and 3. Tehran's current plasma supply chain works with one processing facility, i.e., the Vesal center. Establishing more processing facilities is necessary due to the high demand rate in Tehran. Three other points in districts 14, 19, and 22 are assumed as the candidate locations for establishing new processing facilities. There are ten medical centers for COVID-19 patients in Tehran. The features of these medical centers are provided in Table 5.
Currently, there is a demand for regular plasma from eight hospitals in Tehran. The detailed properties of these hospitals are shown in Table 6.
Finally, one pharmaceutical company in Tehran utilized regular plasma to produce some plasma-based drugs. The properties of this single-center are presented in Table 7.
The geographical location of the established and candidate facilities and the demand points in Tehran's current plasma supply chain is schematically provided in Fig. 4. The transportation cost between facilities is estimated at 0.22 Million Rials per kilometer. The distance between each pair of nodes is calculated using the following distance formula (Jabbarzadeh et al., 2014):
where \(Lat\) and \(Long\) are the geographical latitude and longitude, respectively, and should be converted to radian by multiplying \(\frac{\pi }{180}\). The carbon emission coefficients of transportation between nodes are calculated regarding the travel distance and the vehicle's load as 0.062 g/kilogram/ton. The location cost for the established facilities is set to zero, and the total available budget for motivational programs for recovered donors is assumed to be about 500 Million Rials. This case study is analyzed for three months planning horizon.
6.1 Implementation
Tehran's green plasma supply chain case study is coded in the GAMS programming environment on a laptop with a Core i7 CPU 2.6 GHz and 8 GB RAM, where the solver is CPLEX. The corresponding payoff Table 8 shows the obtained results where the value in this table is calculated by optimization of each objective function separately.
As seen, the minimum value for the total emission function is zero, which is the case where there is no flow of plasma and convalescent plasma in the network. In contrast, the maximum amount of carbon emission has happened when there is no attention to the environmental concerns, and the total cost is considered the network's objective.
The total cost function is considered the objective function, and the total emission function is bounded in model constraints with different upper bounds, implementing the epsilon constraint method. The obtained Pareto solutions are reported in Table 9.
Regarding the provided results in Table 9, the dimension of the problem is not increased by changing the bound for the total carbon emission, and the computational time is similar. Moreover, the gap between the obtained solutions is zero, which confirms that the CPLEX solver is able to reach the global optimum solutions for this case study.
The Pareto frontier is also drawn in Fig. 5, representing the total cost against the total carbon emission. As evident, the total cost behaves against the total carbon emission. The rationale for this behavior is that while increasing the flow of regular and convalescent plasma reduces the shortage quantity and the system's overall cost; it hurts the system's total carbon emission by increasing transportation activities.
For further analysis of the results, the 8th Pareto solution (\(\varepsilon =79066.94 , {TC}^{*}=53014784.77\)) is selected. For this solution, established mobile collection centers, fixed collection centers, and processing facilities are shown in Tables 10, 11 and 12. As seen, the current network states require locating all candidate mobile and fixed collection centers. In addition, there is a need to build a new processing facility in district 4.
Such a need for establishing new facilities shows the pandemic's imposed load and proves the necessity of proper planning by considering the special features of COVID-19.
The assignment pattern of supply chain facilities, including regular donors and recovered donors, to the related collection centers, collection centers to processing facilities, and processing facilities to demand points, is presented in Tables 13, 14, 15, 16, 17, 18 and 19. Note that the donation method \(r=1\) shows the whole blood donation method, and \(r=2\) is the apheresis method. Besides, m = 1 to m = 4 offers the mobile collection centers 1, 2, 3, and Vesal, and \(f=1\) to \(f=5\) are the indices of the fixed collection centers in districts 1, 2, 4,5, and 6. The processing facilities Vesal, district 4, district 19, and district 22 are indexed by \(p=1\) to 4. The indices of the demand points are according to the mentioned number in Tables 5, 6 and 7.
The total cost is divided into its main parts to provide more insights into the cost performance of the network. The results are shown in Table 20. As seen in this table, the limited supply of convalescent plasma, high demand in the COVID-19 pandemic, and the high unit shortage cost of these products have led to a large part of the chain's total cost.
The percentage of each component is also graphically shown on a pie chart in Fig. 6. The shortage cost of products encompasses approximately 55% of the network cost. The donation cost accounts for about 40% of the total cost and ranks second. As can be seen, a small portion of the total cost is caused by transshipment cost, and this is because only a small portion of the convalescent plasma flow is transshipped in the case study’s network.
The total carbon emission of the supply plasma chain is divided into sub-elements, and the results are provided in Table 21. We can conclude that the volume flow of the regular plasma causes a significant portion of the emitted carbon. As all the mobile collection centers remain fixed during the planning horizon, there is no carbon emission from the movement of these facilities.
The schematic representation of the total carbon emission components and the relevant percentage is shown in Fig. 7.
The role of each donation method is analyzed by comparing the collected quantity of each product through these methods in Fig. 8. Interestingly, most of the convalescent plasma volume is received through the whole blood method. In contrast, the total amount of received regular plasma through the apheresis method is higher than in the whole blood method. From this, it can concluded that none of these methods are superior to each other, and each may be selected regarding the features of the network.
The role of each fixed and mobile center in the collection of products is investigated to see which center is more active in the collection process from the donors. The results in Fig. 9 show that the established fixed collection center in district 5 and the mobile collection center in district 2 receive a higher amount of plasma and convalescent plasma, respectively.
The production portion of each product in the processing facilities is different, as demonstrated in Fig. 10. The Vesal center is more active in the production of convalescent plasma. Furthermore, more regular plasma should be produced in the production facility in district 2, compared to the Vesal center.
The state of demand satisfaction in the medical centers for COVID-19 patients is shown in Fig. 11, where the total shortage and the service level are presented. The limited donation of convalescent plasma made a huge impact on these demand points, and many of them are incapable of meeting the demand of their patients. As can be seen, there is a high volume of convalescent plasma shortages in most of these centers. Seven out of ten available medical centers for COVID-19 patients are unable to meet their demand, which shows the reason for the high shortage cost of the network.
The demand points for regular plasma can meet more demand than medical centers for COVID-19 patients. Due to the importance of the hospitals, more plasma is delivered to them in these conditions, and pharmaceutical companies consume the remaining plasma. As presented in Fig. 12, all hospitals can meet their demand, and there is a shortage only for a single pharmaceutical company in the network. The average service level of the demand points for regular plasma is about 95.8 percent. Here, all hospitals are capable of meeting their demand for regular plasma. The reason for such a high service level is the sufficient flow of regular plasma in the network.
6.2 Sensitivity analyses
Sensitivity analyses are performed on some basic input parameters of the model, including the supply and demand of plasma and convalescent plasma, to obtain more insights into the performances of the presented model. The impact of parameter variation in the −30% to + 30% interval is investigated on the system's total cost and total carbon emission in the implemented analysis.
The demand for convalescent plasma may change during the pandemic. Some reasons, such as national social distancing or shutdowns, may reduce the rate of disease. In contrast, ignoring the alerts, making trips, and normalizing the activities may increase the infection rate. Each increase in the rate of infected people increases the demand for convalescent plasma. The impact of changing the demand for convalescent plasma is illustrated in Fig. 13. A slight increase trend can be observed in the total emission of the network. However, the system's total cost is much dependent on this parameter. Causing more demand in a limited supply situation results in more shortage and overall cost of the system.
The changing trends of the total cost and total carbon emission, when the demand for regular plasma change is the same as the demand for convalescent plasma, are demonstrated in Fig. 14. Demand increasing in the condition of limited supply imposes more shortage on the system, and the overall cost will be increased. In this figure, the positive relation of the parameter with the objective functions can be seen. However, the primary difference is that the demand for regular plasma significantly impacts the supply chain's total cost and total carbon emission.
According to Fig. 15, increasing the donation of the convalescent plasma reduces the total cost of the network to a reasonable level. When the donation rate rises, more infected patients can be treated, and as a result, the shortage cost and total cost of the system are decreased. Moreover, the more convalescent plasma flow positively correlates with the total carbon emission. This can be rationalized by the fact that the more flow of convalescent plasma in the network, the more carbon emission results from the transportation activities.
Contrary to the supply of convalescent plasma, variation in the supply of regular plasma in the considered interval has no meaningful effect on the objective functions of the system. This is illustrated in Fig. 16. This can be the result of several factors, such as the low shortage cost of regular plasma in the pharmaceutical company and the overall lower demand rate for the regular plasma product. In other words, when there is an adequate flow in the network, there is no need to receive more plasma from the donors.
Finally, we analyze the impact of changing the unit carbon emission multiplier of nodes on the objective functions of the network. The sensitivity analysis result of this parameter is graphically shown in Fig. 17.
As can be seen, reducing the unit carbon emission can significantly reduce the total carbon emission of the systems. In contrast, the parameters do not have any impact on the total cost of the system. The unit carbon emission parameters can be controlled by the type of vehicles used or investment in the other infrastructures of the network.
In the end, the summary of computational results is presented in Table 22 to provide better insights into the performance of Tehran's plasma supply chain network for managing this network during the COVID-19 pandemic.
7 Conclusions
This research presented a four-echelon multi-objective multi-product multi-period model to design a plasma supply chain network under the COVID-19 pandemic. The aim was simultaneous incorporation of convalescent plasma as a critical subsistence for treatment of infected patients and regular plasma, which is required by therapy and pharmacy sectors, as well as some other features of the COVID-19 pandemic in a plasma supply chain network for the first time. The arising environmental concerns from carbon emissions attracted attention in recent years. Hence, the resulted carbon emission of network activities was also considered to design a green supply chain. The objectives of the mixed-integer linear model were to minimize both the total cost and total carbon emission. A wide range of decisions, including location, allocation, production, inventory, and motivational programs, can be made by solving this model. The epsilon constraint method is adapted to make a trade-off between the objectives and handle the multi-objective nature of the problem. A real-world case study was investigated to examine the applicability and efficiency of the model. The exact Pareto solutions were obtained through solving the model by the CPLEX solver in the GAMS programming environment. A sensitivity analysis was carried out to get insights into the impact of some crucial parameters. The results showed that the shortage of plasma and convalescent plasma imposes a considerable cost on the network. In addition, a significant portion of carbon emission is imposed by the flow of regular plasma from the collection centers to the processing facilities. The sensitivity analysis results demonstrated that both objective functions are affected negatively by the increasing demand for products. The total cost increase is mainly due to the limited supply sources and the shortage cost. Here, the total carbon emission is increased because of increasing the transportation flow in the network.
This is not the endpoint, and various enhancement and development options exist. Researchers can investigate the following recommendations to extend the current work:
-
I.
Considering uncertainty in some model parameters such as the donation and demand (Samani et al., 2018). This research assumed a high degree of certainty for the donation and demand of the network. The pandemic status may not remain fixed, and the number of infected and recovered people can violate. Moreover, the donation and demand for regular plasma may not have a regular pattern. The extension of the proposed model under uncertainty can efficiently address these concerns.
-
II.
Discussing the impact of investment on carbon emission reduction (Lou et al., 2015). Investment in the network’s infrastructures, such as transportation modes, can significantly reduce carbon emissions. Therefore, an investigation of the problem with emission investment seems interesting.
-
III.
Developing the model under different carbon emission regulations, including cap-and-trade and carbon tax (Huang et al., 2020). Several countries use these regulations to curb carbon emissions. These policies can affect the optimal decision of the network. Reformulating the problem under these environmental policies is another suggestion for future works.
-
IV.
Presenting solution methods such as benders decomposition, Lagrangian relaxation, and metaheuristics for solving the problem for large-size networks (Fallahi et al., 2022b). The presented model is solved for Tehran’s case study via a commercial solver. However, there may be more extensive supply chain networks that the commercial solver is not efficient in solving the problem. More advanced solution approaches can assist the managers in decision-making for such networks.
Data availability and materials
Not applicable.
References
Abdi, A., Abdi, A., Akbarpour, N., Amiri, A. S., & Hajiaghaei-Keshteli, M. (2020). Innovative approaches to design and address green supply chain network with simultaneous pick-up and split delivery. Journal of Cleaner Production, 250, 119437.
Abolghasemi, H., Eshghi, P., Cheraghali, A. M., Fooladi, A. A. I., Moghaddam, F. B., Imanizadeh, S., Maleki, M. M., Ranjkesh, M., Rezapour, M., Bahramifar, A., & Einollahi, B. (2020). Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: Results of a multicenter clinical study. Transfusion and Apheresis Science, 59(5), 102875.
Amiri, S. A. H. S., Zahedi, A., Kazemi, M., Soroor, J., & Hajiaghaei-Keshteli, M. (2020). Determination of the optimal sales level of perishable goods in a two-echelon supply chain network. Computers and Industrial Engineering, 139, 106156.
Asadpour, M., Boyer, O., & Tavakkoli-Moghaddam, R. (2021). A blood supply chain network with backup facilities considering blood groups and expiration date: A real-world application. International Journal of Engineering, 34(2), 470–479.
Beliën, J., & Forcé, H. (2012). Supply chain management of blood products: A literature review. European Journal of Operational Research, 217(1), 1–16.
Bloch, E. M., Goel, R., Wendel, S., Burnouf, T., Al-Riyami, A. Z., Ang, A. L., DeAngelis, V., Dumont, L. J., Land, K., Lee, C. K., & Oreh, A. (2021). Guidance for the procurement of COVID-19 convalescent plasma: differences between high-and low-middle-income countries. Vox Sanguinis, 116(1), 18–35.
Boronoos, M., Mousazadeh, M., & Torabi, S. A. (2021). A robust mixed flexible-possibilistic programming approach for multi-objective closed-loop green supply chain network design. Environment, Development and Sustainability, 23(3), 3368–3395.
Centobelli, P., Cerchione, R., & Esposito, E. (2017). Environmental sustainability in the service industry of transportation and logistics service providers: Systematic literature review and research directions. Transportation Research Part D: Transport and Environment, 53, 454–470.
Chen, C., Wang, J., Kwong, J., Kim, J., van Donkelaar, A., Martin, R. V., Hystad, P., Su, Y., Lavigne, E., Kirby-McGregor, M., & Kaufman, J. S. (2022). Association between long-term exposure to ambient air pollution and COVID-19 severity: a prospective cohort study. CMAJ, 194(20), E693–E700.
Chen, L., Xiong, J., Bao, L., & Shi, Y. (2020). Convalescent plasma as a potential therapy for COVID-19. The Lancet Infectious Diseases, 20(4), 398–400.
Cheraghali, A. M., Abolghasemi, H., & Eshghi, P. (2020). Management of COVID-19 virus infection by convalescent plasma. Iranian Journal of Allergy, Asthma and Immunology. https://doi.org/10.18502/ijaai.v19i(s1.r1).2847
Chouhan, V. K., Khan, S. H., & Hajiaghaei-Keshteli, M. (2021). Metaheuristic approaches to design and address multi-echelon sugarcane closed-loop supply chain network. Soft Computing, 25(16), 11377–11404.
Chouhan, V. K., Khan, S. H., & Hajiaghaei-Keshteli, M. (2022). Sustainable planning and decision-making model for sugarcane mills considering environmental issues. Journal of Environmental Management, 303, 114252.
Chowdhury, P., Paul, S. K., Kaisar, S., & Moktadir, M. A. (2021). COVID-19 pandemic related supply chain studies: a systematic review. Transportation Research Part E: Logistics and Transportation Review, 102271.
Daneshdoost, F., Hajiaghaei-Keshteli, M., Sahin, R., & Niroomand, S. (2022). Tabu search based hybrid meta-heuristic approaches for schedule-based production cost minimization problem for the case of cable manufacturing systems. Informatica, 1–24.
Dehghani Ashkezari, H., & Yaghoubi, S. (2020). Designing an integrated blood plasma supply chain under uncertainty demand of both therapy and medicine. Journal of Industrial and Systems Engineering, 12(4), 252–268.
Duan, Q., & Liao, T. W. (2014). Optimization of blood supply chain with shortened shelf lives and ABO compatibility. International Journal of Production Economics, 153, 113–129.
Entezaminia, A., Heydari, M., & Rahmani, D. (2016). A multi-objective model for multi-product multi-site aggregate production planning in a green supply chain: Considering collection and recycling centers. Journal of Manufacturing Systems, 40, 63–75.
Fallahi, A., Azimi-Dastgerdi, M., & Mokhtari, H. (2021a). A Sustainable Production-Inventory Model Joint with Preventive Maintenance and Multiple Shipments for Imperfect Quality Items. Scientia Iranica. https://doi.org/10.24200/sci.2021.55927.4475
Fallahi, A., Bani, E. A., & Niaki, S. T. A. (2022a). A constrained multi-item EOQ inventory model for reusable items: Reinforcement learning-based differential evolution and particle swarm optimization. Expert Systems with Applications, 207, 118018.
Fallahi, A., Mahnam, M., & Niaki, S. T. A. (2022b). Direct aperture optimization for intensity modulated radiation therapy: Two calibrated metaheuristics and liver cancer case study. International Journal of Industrial Engineering and Production Research, 33(2), 1–14.
Fallahi, A., Mokhtari, H., & Niaki, S. T. A. (2021b). Designing a closed-loop blood supply chain network considering transportation flow and quality aspects. Sustainable Operations and Computers, 2, 170–189.
Fasihi, M., Tavakkoli-Moghaddam, R., Najafi, S. E., & Hajiaghaei-Keshteli, M. (2021a). Developing a bi-objective Mathematical Model to Design the Fish Closed-loop Supply Chain. International Journal of Engineering, 34(5), 1257–1268.
Fasihi, M., Tavakkoli-Moghaddam, R., Najafi, S. E., & Hajiaghaei, M. (2021b). Optimizing a bi-objective multi-period fish closed-loop supply chain network design by three multi-objective meta-heuristic algorithms. Scientia Iranica. https://doi.org/10.24200/sci.2021.57930.5477
Fattorini, D., & Regoli, F. (2020). Role of the chronic air pollution levels in the Covid-19 outbreak risk in Italy. Environmental Pollution, 264, 114732.
Frontera, A., Cianfanelli, L., Vlachos, K., Landoni, G., & Cremona, G. (2020). Severe air pollution links to higher mortality in COVID-19 patients: The “double-hit” hypothesis. Journal of Infection, 81(2), 255–259.
Garg, K., Kannan, D., Diabat, A., & Jha, P. (2015). A multi-criteria optimization approach to manage environmental issues in closed loop supply chain network design. Journal of Cleaner Production, 100, 297–314.
Goodarzian, F., Ghasemi, P., Gunasekaren, A., Taleizadeh, A. A., & Abraham, A. (2021a). A sustainable-resilience healthcare network for handling COVID-19 pandemic. Annals of Operations Research, 312, 761–825.
Goodarzian, F., Taleizadeh, A. A., Ghasemi, P., & Abraham, A. (2021b). An integrated sustainable medical supply chain network during COVID-19. Engineering Applications of Artificial Intelligence, 100, 104188.
Govindan, K., Nasr, A. K., Mostafazadeh, P., & Mina, H. (2021). Medical waste management during coronavirus disease 2019 (COVID-19) outbreak: A mathematical programming model. Computers and Industrial Engineering, 162, 107668.
Gunessee, S., & Subramanian, N. (2020). Ambiguity and its coping mechanisms in supply chains lessons from the Covid-19 pandemic and natural disasters. International Journal of Operations and Production Management., 40, 1201–1223.
Gunpinar, S., & Centeno, G. (2016). An integer programming approach to the bloodmobile routing problem. Transportation Research Part E: Logistics and Transportation Review, 86, 94–115.
Haimes, Y. (1971). On a bicriterion formulation of the problems of integrated system identification and system optimization. IEEE Transactions on Systems, Man, and Cybernetics, 1(3), 296–297.
Hamdi-Asl, A., Amoozad-Khalili, H., Tavakkoli-Moghaddam, R., & Hajiaghaei-Keshteli, M. (2021). Toward sustainability in designing agricultural supply chain network A case study on palm date. Scientia Iranica. https://doi.org/10.24200/sci.2021.58302.5659
Hasani, A., Mokhtari, H., & Fattahi, M. (2021). A multi-objective optimization approach for green and resilient supply chain network design: A real-life Case Study. Journal of Cleaner Production, 278, 123199.
Heidari-Fathian, H., & Pasandideh, S. H. R. (2018). Green-blood supply chain network design: Robust optimization, bounded objective function & Lagrangian relaxation. Computers and Industrial Engineering, 122, 95–105.
Hosseini-Motlagh, S.-M., Gilani Larimi, N., & Oveysi Nejad, M. (2020a). A qualitative, patient-centered perspective toward plasma products supply chain network design with risk controlling. Operational Research, 22, 779–824.
Hosseini-Motlagh, S.-M., Samani, M. R. G., & Cheraghi, S. (2020b). Robust and stable flexible blood supply chain network design under motivational initiatives. Socio-Economic Planning Sciences, 70, 100725.
Hosseinifard, Z., & Abbasi, B. (2018). The inventory centralization impacts on sustainability of the blood supply chain. Computers and Operations Research, 89, 206–212.
Huang, Y.-S., Fang, C.-C., & Lin, Y.-A. (2020). Inventory management in supply chains with consideration of Logistics, green investment and different carbon emissions policies. Computers and Industrial Engineering, 139, 106207.
Ibarra-Vega, D. (2020). Lockdown, one, two, none, or smart. Modeling containing covid-19 infection. A conceptual model. Science of the Total Environment, 730, 138917.
Jabbarzadeh, A., Fahimnia, B., & Seuring, S. (2014). Dynamic supply chain network design for the supply of blood in disasters: A robust model with real world application. Transportation Research Part e: Logistics and Transportation Review, 70, 225–244.
Kargar, S., Pourmehdi, M., & Paydar, M. M. (2020). Reverse logistics network design for medical waste management in the epidemic outbreak of the novel coronavirus (COVID-19). Science of the Total Environment, 746, 141183.
Khalilpourazari, S., & Khamseh, A. A. (2019). Bi-objective emergency blood supply chain network design in earthquake considering earthquake magnitude: A comprehensive study with real world application. Annals of Operations Research, 283(1), 355–393.
Khalilpourazari, S., Soltanzadeh, S., Weber, G.-W., & Roy, S. K. (2020). Designing an efficient blood supply chain network in crisis: Neural learning, optimization and case study. Annals of Operations Research, 289(1), 123–152.
Khan, S. A. R., Mathew, M., Dominic, P., & Umar, M. (2021). Evaluation and selection strategy for green supply chain using interval-valued q-rung orthopair fuzzy combinative distance-based assessment. Environment, Development and Sustainability, 1–33.
Lauer, S. A., Grantz, K. H., Bi, Q., Jones, F. K., Zheng, Q., Meredith, H. R., Azman, A. S., Reich, N. G., & Lessler, J. (2020). The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Annals of internal medicine, 172(9), 577–582.
Lou, G. X., Xia, H. Y., Zhang, J. Q., & Fan, T. J. (2015). Investment strategy of emission-reduction technology in a supply chain. Sustainability, 7(8), 10684–10708.
Lozano Díez, J. A., Marmolejo-Saucedo, J.-A., & Rodriguez-Aguilar, R. (2020). Designing a resilient supply chain: An approach to reduce drug shortages in epidemic outbreaks. EAI Endorsed Transactions on Pervasive Health and Technology, 6, 164260.
Majumdar, A., Shaw, M., & Sinha, S. K. (2020). COVID-19 debunks the myth of socially sustainable supply chain: A case of the clothing industry in South Asian countries. Sustainable Production and Consumption, 24, 150–155.
Mehrotra, S., Rahimian, H., Barah, M., Luo, F., & Schantz, K. (2020). A model of supply-chain decisions for resource sharing with an application to ventilator allocation to combat COVID-19. Naval Research Logistics (NRL), 67(5), 303–320.
Meneses, M., Santos, D., & Barbosa-Póvoa, A. (2022). Modelling the blood supply chain-from strategic to tactical decisions. European Journal of Operational Research. https://doi.org/10.1016/j.ejor.2022.06.005
Mirzapour Al-e-hashem, S. M. J., Baboli, A., & Sazvar, Z. (2013). A stochastic aggregate production planning model in a green supply chain: Considering flexible lead times, nonlinear purchase and shortage cost functions. European Journal of Operational Research, 230(1), 26–41.
Mobasher, A., Ekici, A., & Özener, O. Ö. (2015). Coordinating collection and appointment scheduling operations at the blood donation sites. Computers and Industrial Engineering, 87, 260–266.
Mokhtari, H., & Hasani, A. (2017). An energy-efficient multi-objective optimization for flexible job-shop scheduling problem. Computers and Chemical Engineering, 104, 339–352.
Mokhtari, H., & Rezvan, M. T. (2020). A single-supplier, multi-buyer, multi-product VMI production-inventory system under partial backordering. Operational Research, 20(1), 37–57.
Mosallanezhad, B., Chouhan, V. K., Paydar, M. M., & Hajiaghaei-Keshteli, M. (2021a). Disaster relief supply chain design for personal protection equipment during the COVID-19 pandemic. Applied Soft Computing, 112, 107809.
Mosallanezhad, B., Hajiaghaei-Keshteli, M., & Triki, C. (2021b). Shrimp closed-loop supply chain network design. Soft Computing, 25(11), 7399–7422.
Mousavi, R., Salehi-Amiri, A., Zahedi, A., & Hajiaghaei-Keshteli, M. (2021). Designing a supply chain network for blood decomposition by utilizing social and environmental factor. Computers and Industrial Engineering, 160, 107501.
Nagurney, A., & Masoumi, A. H. (2012). Supply chain network design of a sustainable blood banking system. Sustainable supply chains (pp. 49–72). Springer.
Nagurney, A., Masoumi, A. H., & Yu, M. (2012). Supply chain network operations management of a blood banking system with cost and risk minimization. Computational Management Science, 9(2), 205–231.
Nia, A. R., Far, M. H., & Niaki, S. T. A. (2015). A hybrid genetic and imperialist competitive algorithm for green vendor managed inventory of multi-item multi-constraint EOQ model under shortage. Applied Soft Computing, 30, 353–364.
Nikpouraghdam, M., Farahani, A. J., Alishiri, G., Heydari, S., Ebrahimnia, M., Samadinia, H., Sepandi, M., Jafari, N. J., Izadi, M., Qazvini, A., & Dorostkar, R. (2020). Epidemiological characteristics of coronavirus disease 2019 (COVID-19) patients in IRAN: A single center study. Journal of Clinical Virology, 127, 104378.
Osorio, A. F., Brailsford, S. C., & Smith, H. K. (2015). A structured review of quantitative models in the blood supply chain: A taxonomic framework for decision-making. International Journal of Production Research, 53(24), 7191–7212.
Osorio, A. F., Brailsford, S. C., & Smith, H. K. (2018). Whole blood or apheresis donations? A multi-objective stochastic optimization approach. European Journal of Operational Research, 266(1), 193–204.
Paul, S. K., & Chowdhury, P. (2020). A production recovery plan in manufacturing supply chains for a high-demand item during COVID-19. International Journal of Physical Distribution and Logistics Management, 51(2), 104–125.
Pierskalla, W. P. (2005). Supply chain management of blood banks. Operations research and health care (pp. 103–145). Springer.
Pirabán, A., Guerrero, W. J., & Labadie, N. (2019). Survey on blood supply chain management: Models and methods. Computers and Operations Research, 112, 104756.
Qiao, A., Choi, S., & Pan, Y. (2021). Multi-party coordination in sustainable supply chain under consumer green awareness. Science of the Total Environment, 777, 146043.
Rajak, S., Vimal, K., Arumugam, S., Parthiban, J., Sivaraman, S. K., Kandasamy, J., & Duque, A. A. (2022). Multi-objective mixed-integer linear optimization model for sustainable closed-loop supply chain network: A case study on remanufacturing steering column. Environment, Development and Sustainability, 24(5), 6481–6507.
Resat, H. G., & Unsal, B. (2019). A novel multi-objective optimization approach for sustainable supply chain: A case study in packaging industry. Sustainable Production and Consumption, 20, 29–39.
Rowan, N. J., & Laffey, J. G. (2020). Challenges and solutions for addressing critical shortage of supply chain for personal and protective equipment (PPE) arising from Coronavirus disease (COVID19) pandemic–Case study from the Republic of Ireland. Science of the Total Environment, 725, 138532.
Rytilä, J. S., & Spens, K. M. (2006). Using simulation to increase efficiency in blood supply chains. Management Research News, 29, 801–819.
Salehi-Amiri, A., Akbapour, N., Hajiaghaei-Keshteli, M., Gajpal, Y., & Jabbarzadeh, A. (2022a). Designing an effective two-stage, sustainable, and IoT based waste management system. Renewable and Sustainable Energy Reviews, 157, 112031.
Salehi-Amiri, A., Zahedi, A., Akbapour, N., & Hajiaghaei-Keshteli, M. (2021). Designing a sustainable closed-loop supply chain network for walnut industry. Renewable and Sustainable Energy Reviews, 141, 110821.
Salehi-Amiri, A., Zahedi, A., Gholian-Jouybari, F., Calvo, E. Z. R., & Hajiaghaei-Keshteli, M. (2022b). Designing a closed-loop supply chain network considering social factors; a case study on avocado industry. Applied Mathematical Modelling, 101, 600–631.
Samani, M. R. G., & Hosseini-Motlagh, S.-M. (2019). An enhanced procedure for managing blood supply chain under disruptions and uncertainties. Annals of Operations Research, 283(1), 1413–1462.
Samani, M. R. G., Hosseini-Motlagh, S.-M., Sheshkol, M. I., & Shetab-Boushehri, S.-N. (2019). A bi-objective integrated model for the uncertain blood network design with raising products quality. European Journal of Industrial Engineering, 13(5), 553–588.
Samani, M. R. G., Torabi, S. A., & Hosseini-Motlagh, S.-M. (2018). Integrated blood supply chain planning for disaster relief. International Journal of Disaster Risk Reduction, 27, 168–188.
Sheu, J.-B., Chou, Y.-H., & Hu, C.-C. (2005). An integrated logistics operational model for green-supply chain management. Transportation Research Part E: Logistics and Transportation Review, 41(4), 287–313.
Tison, G. H., Avram, R., Kuhar, P., Abreau, S., Marcus, G. M., Pletcher, M. J., & Olgin, J. E. (2020). Worldwide effect of COVID-19 on physical activity: A descriptive study. Annals of Internal Medicine, 173(9), 767–770.
Tsao, Y.-C., Thanh, V.-V., Lu, J.-C., & Wei, H.-H. (2021). A risk-sharing-based resilient renewable energy supply network model under the COVID-19 pandemic. Sustainable Production and Consumption, 25, 484–498.
Van Dijk, N., Haijema, R., Van Der Wal, J., & Sibinga, C. S. (2009). Blood platelet production: A novel approach for practical optimization. Transfusion, 49(3), 411–420.
Wendel, S., Land, K., Devine, D. V., Daly, J., Bazin, R., Tiberghien, P., Lee, C. K., Arora, S., Patidar, G. K., Khillan, K., & Smid, W. M. (2021). Lessons learned in the collection of convalescent plasma during the COVID-19 pandemic. Vox sanguinis, 116(8), 872–879.
Wu, X., Nethery, R. C., Sabath, M. B., Braun, D., & Dominici, F. (2020). Exposure to air pollution and COVID-19 mortality in the United States: A nationwide cross-sectional study. MedRxiv, 183, 108924.
Yousefi Nejad Attari, M., Pasandide, S. H. R., Agaie, A., & Akhavan Niaki, S. T. (2017). Presenting a stochastic multi choice goal programming model for reducing wastages and shortages of blood products at hospitals. Journal of Industrial and Systems Engineering, 10, 81–96.
Yousefi Nejad Attari, M., Pasandideh, S. H. R., & Akhavan Niaki, S. T. (2019). A hybrid robust stochastic programming for a bi-objective blood collection facilities problem (Case study: Iranian blood transfusion network). Journal of Industrial and Production Engineering, 36(3), 154–167.
Zahedi, A., Salehi-Amiri, A., Hajiaghaei-Keshteli, M., & Diabat, A. (2021a). Designing a closed-loop supply chain network considering multi-task sales agencies and multi-mode transportation. Soft Computing, 25(8), 6203–6235.
Zahedi, A., Salehi-Amiri, A., Smith, N. R., & Hajiaghaei-Keshteli, M. (2021b). Utilizing IoT to design a relief supply chain network for the SARS-COV-2 pandemic. Applied Soft Computing, 104, 107210.
Zahiri, B., & Pishvaee, M. S. (2017). Blood supply chain network design considering blood group compatibility under uncertainty. International Journal of Production Research, 55(7), 2013–2033.
Zhang, X., Li, X. I., Fang, P. A. N. G., Yan, D. U., Qiuzhen, Y. U. A. N., Minjuan, S. H. I., Jiping, L. I. U., Hui, M. A., & Bo, L. I. (2020). Convalescent plasma in the treatment of severe COVID-19: A systematic review and meta-analysis. Iranian Journal of Public Health, 49(11), 2022.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors conceived of the presented idea. Ali Fallahi and Seyed Alireza Mousavian Anaraki developed the theory and performed the computations. Hadi Mokhtari and Seyed Taghi Akhavan Niaki verified the analytical methods. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Ethical approval
This study has not been duplicate publication or submission elsewhere. The Local Ethics Committee approval was obtained.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fallahi, A., Mousavian Anaraki, S.A., Mokhtari, H. et al. Blood plasma supply chain planning to respond COVID-19 pandemic: a case study. Environ Dev Sustain 26, 1965–2016 (2024). https://doi.org/10.1007/s10668-022-02793-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10668-022-02793-7