Abstract
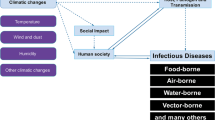
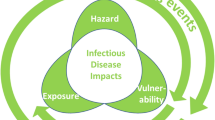
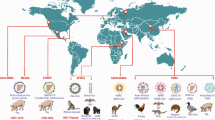
The world has experienced perceptible climate change for the past 100 years. Global warming enhances the rapid spread of mosquito-borne diseases resulting in unknown consequences in the future. The global economic development, increased urbanization, and climate change have significantly increased the mosquito-borne disease transmission pattern and dynamics. In India, mosquito-borne diseases have been a core public issue for decades. Hence, mosquito control is primordial for preventing the transmission of malaria, lymphatic filariasis, dengue fever, Yellow fever, Zika virus infection, West Nile fever, and chikungunya virus infection in the human population. The mosquito control strategies based on ecology have received much more attention during the 1960s, as chemical pesticides induce negative impacts on human health and the ecosystem. Most of the current approaches in mosquito control have several limitations related to the development of insecticide resistance, lack of long-term sustainability, and negative impacts on the ecosystem and the environment. This review offers invaluable insights into severe mosquito-borne diseases, various vector control strategies, and the influence of climate change in mosquito-borne disease transmission.









Similar content being viewed by others
References
Abad-Franch, F., Zamora-Perea, E., Luz, S. L. (2017). Mosquito-disseminated insecticide for citywide vector control and its potential to block arbovirus epidemics: Entomological observations and modeling results from Amazonian Brazil. PLoS medicine, 14(1), e1002213.
Abouzied, E. M. (2017). Life table analysis of Culex pipiens under simulated weather conditions in Egypt. Journal of the American Mosquito Control Association, 33(1), 16–24.
Achee, N. L., Gould, F., Perkins, T. A., Reiner Jr, R. C., Morrison, A. C., Ritchie, S. A., et al. (2015). A critical assessment of vector control for dengue prevention. PLoS neglected tropical diseases, 9(5), e0003655.
Amicizia, D., Zangrillo, F., Lai, P. L., Iovine, M., Panatto, D. (2018). Overview of Japanese encephalitis disease and its prevention. Focus on IC51 vaccine (IXIARO®). Journal of preventive medicine and hygiene, 59(1), E99.
Aneesh, E. M., Anoopkumar, A. N., Siva Prasad, M. S., & Rebello, S. (2021). A phylogenomic and evolutionary perspectives of COVID-19. Journal of Communicable Diseases, 53(1), 1–8.
Aneesh, E., Vijayan, V. (2010). Laboratory selection of carbofuran tolerant line of Culex quinquefasciatus Say, the filarial vector at Mysore.
Anoopkumar, A., Aneesh, E. M. (2021). Environmental epidemiology and neurological manifestations of dengue serotypes with special inference on molecular trends, virus detection, and pathogenicity. Environment, Development and Sustainability, p.1–23.
Anoopkumar, A., Aneesh, E. M., & Sudhikumar, A. V. (2020a). Exploring the mode of action of isolated bioactive compounds by induced reactive oxygen species generation in Aedes aegypti: a microbes based double-edged weapon to fight against Arboviral diseases. International Journal of Tropical Insect Science, 40(3), 573–585.
Anoopkumar, A., Aneesh, E. M., & Sudhikumar, A. V. (2020b). Exploring the mode of action of isolated bioactive compounds by induced reactive oxygen species generation in Aedes aegypti: a microbes based double-edged weapon to fight against Arboviral diseases. International Journal of Tropical Insect Science, 40(3), 1–13.
Anoopkumar, A., Puthur, S., Rebello, S., & Aneesh, E. M. (2017a). Screening of a Few traditionally used Medicinal Plants for their Larvicidal Efficacy against Aedes aegypti Linn (Diptera: Culicidae), a Dengue Fever Vector.
Anoopkumar, A., Puthur, S., Varghese, P., Rebello, S., Aneesh, E. M. (2017b). Life cycle, bio-ecology and DNA barcoding of mosquitoes Aedes aegypti (Linnaeus) and Aedes albopictus (Skuse). The Journal of Communicable Diseases, 49(3): 32-41.
Anoopkumar, A., Rebello, S., Aneesh, E. M., Sindhu, R., Binod, P., Pandey, A., et al. (2020c). Use of Different Enzymes in Biorefinery Systems. Biorefinery Production Technologies for Chemicals and Energy, p. 357–368.
Anoopkumar, A., Rebello, S., Devassy, E., Raj, K. K., Puthur, S., Aneesh, E. M., et al. (2020d). Phytoextraction of Heavy Metals. Methods for Bioremediation of Water and Wastewater Pollution (pp. 267–276). Cham: Springer.
Anoopkumar, A., Rebello, S., Sudhikumar, A. V., Puthur, S., Aneesh, E. M. (2020e). A novel intervention on the inhibiting effects of Catunaregam spinosa induced free radical formation and DNA damage in Aedes aegypti (Diptera: Culicidae): a verdict for new perspectives on microorganism targeted vector control approach. International Journal of Tropical Insect Science, 40(4), 989-1002.
Anoopkumar, A., Siva Prasad, M., Rebello, S., Sini Francis, C., Aneesh, E. M. (2021). An Assessment of ITS rDNA PCR-based molecular identification, and characterization of fungal endophytes isolated from Hypericum japonicum. Plant Biosystems-An International Journal Dealing with all Aspects of Plant Biology, https://doi.org/10.1080/11263504.2021.1887958.
Ault, S. K. (1994). Environmental management: a re-emerging vector control strategy. The American journal of tropical medicine and hygiene, 50(6_Suppl), 35–49.
Benelli, G. (2016). Plant-mediated biosynthesis of nanoparticles as an emerging tool against mosquitoes of medical and veterinary importance: a review. Parasitology Research, 115(1), 23–34.
Brower, V. (2001). Vector-borne diseases and global warming: are both on an upward swing?: Scientists are still debating whether global warming will lead to a further spread of mosquitoes and the diseases they transmit. EMBO Reports, 2(9), 755–757.
Caminade, C., Medlock, J. M., Ducheyne, E., McIntyre, K. M., Leach, S., Baylis, M., et al. (2012). Suitability of European climate for the Asian tiger mosquito Aedes albopictus: recent trends and future scenarios. Journal of the Royal Society Interface, rsif20120138.
Capinha, C., Rocha, J., & Sousa, C. A. (2014). Macroclimate determines the global range limit of Aedes aegypti. EcoHealth, 11(3), 420–428.
CDC (2019). CHAPTER 4 Travel-Related Infectious Diseases. https://wwwnc.cdc.gov/travel/yellowbook/2020/travel-related-infectious-diseases/dengue. Last accessed on 25–10–2020.
CDC (2020a). Environmental Hazards & Other Noninfectious Health Risks. center for disease control and prevention, chapter 3. https://wwwnc.cdc.gov/travel/yellowbook/2020/noninfectious-health-risks/mosquitoes-ticks-and-other-arthropods. Last Accessed on 25–10–2020.
CDC (2020b). Malaria's Impact Worldwide. Global Health, Division of Parasitic Diseases and Malaria. https://www.cdc.gov/malaria/malaria_worldwide/impact.html#:~:text=Africa%20is%20the%20most%20affected,cause%20severe%20malaria%20and%20death. Last accessed on 23–10–2020.
Chandler, D., Bailey, A. S., Tatchell, G. M., Davidson, G., Greaves, J., & Grant, W. P. (2011). The development, regulation and use of biopesticides for integrated pest management. Philosophical Transactions of the Royal Society b: Biological Sciences, 366(1573), 1987–1998.
Chareonviriyaphap, T., Bangs, M. J., Suwonkerd, W., Kongmee, M., Corbel, V., & Ngoen-Klan, R. (2013). Review of insecticide resistance and behavioral avoidance of vectors of human diseases in Thailand. Parasites & Vectors, 6(1), 280.
Chen, L. H., & Wilson, M. E. (2020). Yellow fever control: Current epidemiology and vaccination strategies. Tropical Diseases, Travel Medicine and Vaccines, 6(1), 1–10.
Chianese, A., Stelitano, D., Astorri, R., Serretiello, E., Della Rocca, M. T., Melardo, C., et al. (2019). West Nile virus: an overview of current information. Translational Medicine Reports, 3(1).
Christophers, S. (1960a). Aedes aegypti (L.) the yellow fever mosquito: its life history, bionomics and structure. Aëdes aegypti (L.) the Yellow Fever Mosquito: its Life History, Bionomics and Structure.
Christophers, S. R. (1960b). Aedes aegypti: the yellow fever mosquito. CUP Archive.
Couret, J., & Benedict, M. Q. (2014). A meta-analysis of the factors influencing development rate variation in Aedes aegypti (Diptera: Culicidae). BMC Ecology, 14(1), 3.
Coutinho-Abreu, I. V., Zhu, K. Y., & Ramalho-Ortigao, M. (2010). Transgenesis and paratransgenesis to control insect-borne diseases: Current status and future challenges. Parasitology International, 59(1), 1–8.
Cox, F. E. (2010). History of the discovery of the malaria parasites and their vectors. Parasites & Vectors, 3(1), 5.
de Azevedo Marques, E. T., Dhalia, R., & Maciel Filho, R. (2019) 'Dna vaccine against virus of yellow fever'. Google Patents.
de Freitas, C. S., Higa, L. M., Sacramento, C. Q., Ferreira, A. C., Reis, P. A., Delvecchio, R., et al. (2019). Yellow fever virus is susceptible to sofosbuvir both in vitro and in vivo. PLoS neglected tropical diseases, 13(1), e0007072.
De Silva, P. M., & Marshall, J. M. (2012). Factors contributing to urban malaria transmission in sub-Saharan Africa: a systematic review. Journal of tropical medicine, 2012(1), 10.
Dénes, A., Ibrahim, M. A., Oluoch, L., Tekeli, M., & Tekeli, T. (2019). Impact of weather seasonality and sexual transmission on the spread of Zika fever. Scientific Reports, 9(1), 1–10.
Epstein, P. R. (2001). Climate change and emerging infectious diseases. Microbes and Infection, 3(9), 747–754.
Fang, Y., & Zhang, Y. (2019). Lessons from lymphatic filariasis elimination and the challenges of post-elimination surveillance in China. Infectious Diseases of Poverty, 8(1), 66.
Fischer, D., Thomas, S. M., Suk, J. E., Sudre, B., Hess, A., Tjaden, N. B., et al. (2013). Climate change effects on Chikungunya transmission in Europe: Geospatial analysis of vector’s climatic suitability and virus’ temperature requirements. International Journal of Health Geographics, 12(1), 51.
Foy, B. D., Kobylinski, K. C., Foy, J. L. C., Blitvich, B. J., da Rosa, A. T., Haddow, A. D., et al. (2011). Probable non–vector-borne transmission of Zika virus, Colorado, USA. Emerging Infectious Diseases, 17(5), 880.
Ganesan, V. K., Duan, B., & Reid, S. P. (2017). Chikungunya virus: Pathophysiology, mechanism, and modeling. Viruses, 9(12), 368.
Ganeshkumar, P., Murhekar, M. V., Poornima, V., Saravanakumar, V., Sukumaran, K., Anandaselvasankar, A., et al. (2018). Dengue infection in India: A systematic review and meta-analysis. PLoS neglected tropical diseases, 12(7), e0006618.
Gratz, N., Pal, R. (1988). Malaria vector control: larviciding. Malaria: Principles and practices of malariology, p. 1213–1226.
Guarner, J., Shieh, W.-J., Hunter, S., Paddock, C. D., Morken, T., Campbell, G. L., et al. (2004). Clinicopathologic study and laboratory diagnosis of 23 cases with West Nile virus encephalomyelitis. Human Pathology, 35(8), 983–990.
Hasan, S., Saeed, S., Panigrahi, R., & Choudhary, P. (2019). Zika virus: a global public health menace: a comprehensive update. Journal of International Society of Preventive & Community Dentistry, 9(4), 316.
Hemingway, J., & Ranson, H. (2000). Insecticide resistance in insect vectors of human disease. Annual Review of Entomology, 45(1), 371–391.
Houghton, J. T. (1996). Climate change 1995: The science of climate change: Contribution of working group I to the second assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press.
Katz, T. M., Miller, J. H., & Hebert, A. A. (2008). Insect repellents: historical perspectives and new developments. Journal of the American Academy of Dermatology, 58(5), 865–871.
Kearney, M., Porter, W. P., Williams, C., Ritchie, S., & Hoffmann, A. A. (2009). Integrating biophysical models and evolutionary theory to predict climatic impacts on species’ ranges: the dengue mosquito Aedes aegypti in Australia. Functional Ecology, 23(3), 528–538.
Kobayashi, M., Nihei, N., & Kurihara, T. (2002). Analysis of northern distribution of Aedes albopictus (Diptera: Culicidae) in Japan by geographical information system. Journal of Medical Entomology, 39(1), 4–11.
Kumar, A., Valecha, N., Jain, T., Dash, A. P. (2007). Burden of malaria in India: retrospective and prospective view. The American journal of tropical medicine and hygiene, 77(6_Suppl), p. 69–78.
Laumann, V. (2010). Environmental strategies to replace DDT and control malaria. PAN Germany.
Lowe, R., Ryan, S. J., Mahon, R., Van Meerbeeck, C. J., Trotman, A. R., Boodram, L.-L. G., et al. (2020). Building resilience to mosquito-borne diseases in the Caribbean. Plos Biology, 18(11), e3000791.
Malaria, U., & Ums, S. N. (2011) 'National Vector Borne Disease Control Programme'. Control.
Marten, G. (1986). Mosquito control by plankton tnanagetnent: The potential of indigestible green algae. Journal of Tropical Medicine and Hygiene, 89, 213–222.
Mores, C. N., Christofferson, R. C., Davidson, S. A. (2014). The role of the mosquito in a dengue human infection model. The Journal of infectious diseases, 209(suppl_2), S71-S78.
Musso, D., Roche, C., Robin, E., Nhan, T., Teissier, A., & Cao-Lormeau, V.-M. (2015). Potential sexual transmission of Zika virus. Emerging Infectious Diseases, 21(2), 359.
Narain, J. P., Dhariwal, A., & MacIntyre, C. R. (2017). Acute encephalitis in India: An unfolding tragedy. The Indian Journal of Medical Research, 145(5), 584.
Nguyen, C., Gray, M., Burton, T. A., Foy, S. L., Foster, J. R., Gendernalik, A. L., et al. (2019). Evaluation of a novel West Nile virus transmission control strategy that targets Culex tarsalis with endectocide-containing blood meals. PLoS neglected tropical diseases, 13(3), e0007210.
NHM (2019). Disease Control
NOAA (2020). Global Climate Report - May 2020. National Centers for Environmental Information. https://www.ncdc.noaa.gov/sotc/global/202005. Last accessed on October 25, 2020.
Oehler, E., Fournier, E., Leparc-Goffart, I., Larre, P., Cubizolle, S., Sookhareea, C., et al. (2015). Increase in cases of Guillain-Barré syndrome during a Chikungunya outbreak, French Polynesia, 2014 to 2015. Eurosurveillance, 20(48), 30079.
Ottesen, E., Duke, B., Karam, M., & Behbehani, K. (1997). Strategies and tools for the control/elimination of lymphatic filariasis. Bulletin of the World Health Organization, 75(6), 491.
Parry, M., Canziani, O., Palutikof, J., van der Linden, P. J., Hanson, C. E. (2007). Climate change 2007: impacts, adaptation and vulnerability. Cambridge University Press Cambridge.
Pascual, M., & Bouma, M. J. (2009). Do rising temperatures matter? Ecology, 90(4), 906–912.
Pearson, E. F., & Miles, W. (1980). Disinfection of mail in the United States. Bulletin of the History of Medicine, 54(1), 111.
Pesticides, W. (2006). their Application for the Control of Vectors and Pests of Public Health Importance. WHO.
Puthur, S., Anoopkumar, A., Rebello, S., Aneesh, E. M. (2018). Hypericum japonicum: a Double-Headed Sword to Combat Vector Control and Cancer. Applied biochemistry and biotechnology, 186(1), 1–11.
Puthur, S., Anoopkumar, A., Rebello, S., & Aneesh, E. M. (2019). Synergistic control of storage pest rice weevil using Hypericum japonicum and deltamethrin combinations: a key to combat pesticide resistance. Environmental Sustainability, 2(4), 411–417.
Puthur, S., Anoopkumar, A., Rebello, S., Aneesh, E. M., Sindhu, R., Binod, P., et al. (2021). Toxic Effects of Pesticides on Avifauna Inhabiting Wetlands. Sustainable Agriculture Reviews 47 (pp. 335–349). Cham: Springer.
Puthur, S., Raj, K. K., Anoopkumar, A., Rebello, S., Aneesh, E. M. (2020). Acorus calamus mediated green synthesis of ZnONPs: A novel nano antioxidant to future perspective. Advanced Powder Technology. 31(12), 4679-4682.
Rajagopal, R. (1977). Malathion resistance in Anopheles culicifacies in Gujarat. Indian Journal of Medical Research, 66(1), 27–28.
Ramzy, R. M., Goldman, A. S., & Kamal, H. A. (2005). Defining the cost of the Egyptian lymphatic filariasis elimination programme. Filaria Journal, 4(1), 7.
Rao, B. (1958). The national malaria control programme in India and the possibilities of eradication of malaria in India and the tropics. Bull Nat Soc Mal Mosq Dis, 6, 5–6.
Rebello, S., Anoopkumar, A., Aneesh, E. M., Sindhu, R., Binod, P., & Pandey, A. (2020a). Sustainability and life cycle assessments of lignocellulosic and algal pretreatments. Bioresource Technology, 301, 122678.
Rebello, S., Anoopkumar, A., Puthur, S., Sindhu, R., Binod, P., Pandey, A., et al. (2018). Zinc oxide phytase nanocomposites as contributory tools to improved thermostability and shelflife. Bioresource Technology Reports, 3, 1–6.
Rebello, S., Anoopkumar, A., Sindhu, R., Binod, P., Pandey, A., Aneesh, E. M. (2020b). Comparative life-cycle analysis of synthetic detergents and biosurfactants—an overview. Refining Biomass Residues for Sustainable Energy and Bioproducts (pp. 511–521). Elsevier.
Rebello, S., Balakrishnan, D., Anoopkumar, A., Sindhu, R., Binod, P., Pandey, A., et al. (2019). Industrial Enzymes as Feed Supplements—Advantages to Nutrition and Global Environment. Green Bio-processes (pp. 293–304). Singapore: Springer.
Reisen, W. K., Fang, Y., & Martinez, V. M. (2006). Effects of temperature on the transmission of West Nile virus by Culex tarsalis (Diptera: Culicidae). Journal of Medical Entomology, 43(2), 309–317.
Rezza, G., Weaver, S. C. (2019). Chikungunya as a paradigm for emerging viral diseases: evaluating disease impact and hurdles to vaccine development. PLoS neglected tropical diseases, 13(1), e0006919.
Riehle, M. A., Moreira, C. K., Lampe, D., Lauzon, C., & Jacobs-Lorena, M. (2007). Using bacteria to express and display anti-Plasmodium molecules in the mosquito midgut. International Journal for Parasitology, 37(6), 595–603.
Rivero, A., Vezilier, J., Weill, M., Read, A. F., Gandon, S. (2010). Insecticide control of vector-borne diseases: when is insecticide resistance a problem? PLoS pathogens, 6(8), e1001000.
Roberts, D. R., Andre, R. G. (1994). Insecticide resistance issues in vector-borne disease control. The American journal of tropical medicine and hygiene, 50(6_Suppl), p. 21–34.
Robinson, M. C. (1955). An epidemic of virus disease in Southern Province, Tanganyika territory, in 1952–1953. Transactions of the Royal Society of Tropical Medicine and Hygiene, 49(1), 28–32.
Rowley, W. A., & Graham, C. L. (1968). The effect of temperature and relative humidity on the flight performance of female Aedes aegypti. Journal of Insect Physiology, 14(9), 1251–1257.
Rozendaal, J. A. (1997). Vector control: methods for use by individuals and communities. World Health Organization.
Russell, P. F. (1963). Practical Malariology: By Paul F. Oxford University Press.
Sadasivaiah, S., Tozan, Y., & Breman, J. G. (2007). Dichlorodiphenyltrichloroethane (DDT) for indoor residual spraying in Africa: how can it be used for malaria control? The American journal of tropical medicine and hygiene, 77(6_Suppl), 249–263.
Schiøler, K. L., Samuel, M., Wai, K. L. (2007). Vaccines for preventing Japanese encephalitis. Cochrane Database of Systematic Reviews, https://doi.org/10.1002/14651858.CD004263.pub2.
Scott, T. W., Clark, G. G., Lorenz, L. H., Amerasinghe, P. H., Reiter, P., & Edman, J. D. (1993). Detection of multiple blood feeding in Aedes aegypti (Diptera: Culicidae) during a single gonotrophic cycle using a histologic technique. Journal of Medical Entomology, 30(1), 94–99.
Sharma, G. (1984) 'Review of malaria and its control in India' Proceeding of the Indo-UK workshop on malaria education. VP Sharma (MRC ICMR Delhi). p. 13–40.
Sharrel Rebello, A. N. A., Embalil Mathachan Aneesh, Raveendran Sindhu, Parameswaran Binod, AshokPandey (2019). Sustainability and life cycle assessments of lignocellulosic and algal pretreatments. Bioresource Technology Reports, In Press, Journal Pre-proof, Available online 26 December 2019.
Shretta, R., Liu, J., Cotter, C., Cohen, J., Dolenz, C., Makomva, K., et al. (2017). Malaria elimination and eradication. Major Infectious Diseases. 3rd edition: The International Bank for Reconstruction and Development/The World Bank.
Sikka, V., Chattu, V. K., Popli, R. K., Galwankar, S. C., Kelkar, D., Sawicki, S. G., et al. (2016). The emergence of Zika virus as a global health security threat: A review and a consensus statement of the INDUSEM Joint Working Group (JWG). Journal of Global Infectious Diseases, 8(1), 3.
Singh, K., Rahman, S., & Joshi, G. (1989). Village scale trial of deltamethrin against mosquitoes. The Journal of Communicable Diseases, 21(4), 339–353.
Slater, H., Michael, E. (2012). Predicting the current and future potential distributions of lymphatic filariasis in Africa using maximum entropy ecological niche modelling. PloS one, 7(2), e32202.
Solano-Villarreal, E., Valdivia, W., Pearcy, M., Linard, C., Pasapera-Gonzales, J., Moreno-Gutierrez, D., et al. (2019). Malaria risk assessment and mapping using satellite imagery and boosted regression trees in the Peruvian Amazon. Scientific Reports, 9(1), 1–12.
Southwood, T. R. (1977). Habitat, the templet for ecological strategies?. The Journal of Animal Ecology, 46(2), 337–365.
Strode, C., Donegan, S., Garner, P., Enayati, A. A., Hemingway, J. (2014). The impact of pyrethroid resistance on the efficacy of insecticide-treated bed nets against African anopheline mosquitoes: systematic review and meta-analysis. PLoS medicine, 11(3), e1001619.
Suk, J. E. (2016). Climate change, malaria, and public health: accounting for socioeconomic contexts in past debates and future research. Wiley Interdisciplinary Reviews: Climate Change, 7(4), 551–568.
Thu, H. M., Aye, K. M., & Thein, S. (1998). The effect of temperature and humidity on dengue virus propagation in Aedes aegypti mosquitos. Southeast Asian Journal of Tropical Medicine and Public Health, 29(2), 280–284.
Trampuz, A., Jereb, M., Muzlovic, I., & Prabhu, R. M. (2003). Clinical review: severe malaria. Critical Care, 7(4), 1–9.
Tu, Y. (2011). The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nature Medicine, 17(10), 1217.
Van Bortel, W., Dorleans, F., Rosine, J., Blateau, A., Rousset, D., Matheus, S., et al. (2014). Chikungunya outbreak in the Caribbean region, December 2013 to March 2014, and the significance for Europe. Eurosurveillance, 19(13), 20759.
Vijayan, V. (2010). Laboratory selection of carbofuran tolerant line of Culex quinquefasciatus Say, the filarial vector at Mysore. Journal of Communicable Diseases, 42(3), 201–207.
Watson, M. (1921). The prevention of malaria in the Federated Malay States: a record of twenty years' progress. EP Dutton & Company.
Weltman, J. (2016). Medical Microbiology & Diagnosis An Immuno-Bioinformatic Analysis of Zika virus (ZIKV) envelope E Protein. Journal of Medical Microbiology Diagn, 5(2), 1–2.
White, R. S. (1945). House spraying with DDT and with pyrethrum extract compared: first results. Journal of the Malaria Institute of India, 6(1), 83–93.
WHO (1982). Manual on environmental management for mosquito control, with special emphasis on malaria vectors.
WHO. (2010). World health statistics 2010. World Health Organization.
WHO. (2014). Quantitative risk assessment of the effects of climate change on selected causes of death, 2030s and 2050s. World Health Organization.
WHO. (2017). Global vector control response 2017–2030. WHO.
WHO (2019a). Dengue and severe dengue. https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue. Last accessed on 25–10–2020.
WHO (2019b). Dengue and severe dengue. Available at https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue. Last Acessed on 25 December 2019.
Wichit, S., Hamel, R., Yainoy, S., Gumpangseth, N., Panich, S., Phuadraksa, T., et al. (2019). Interferon-inducible protein (IFI) 16 regulates Chikungunya and Zika virus infection in human skin fibroblasts. EXCLI Journal, 18, 467.
Wilke, A. B. B., & Marrelli, M. T. (2015). Paratransgenesis: a promising new strategy for mosquito vector control. Parasites & Vectors, 8(1), 342.
Yacoub, S., Mongkolsapaya, J., Screaton, G. (2016). Recent advances in understanding dengue. F1000Research, 5, 78.
Zanluca, C., & Dos Santos, C. N. D. (2016). Zika virus–an overview. Microbes and Infection, 18(5), 295–301.
Zeller, H., & Schuffenecker, I. (2004). West Nile virus: an overview of its spread in Europe and the mediterranean basin in contrast to its spread in the Americas. European Journal of Clinical Microbiology and Infectious Diseases, 23(3), 147–156.
Acknowledgements
The authors would like to thank Principal, St. Joseph’s College, Irinjalakuda, for the laboratory facilities provided. The author Embalil Mathachan Aneesh thanks University Grants Commission, Government of India (UGC Research Award—F30-6/20-16(SA-II), and Department of Biotechnology, Ministry of Science & Technology, Government of India (BT/PR39753/FCB/125/95/2020), for their fundings.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. A.N. Anoopkumar and Embalil Mathachan Aneesh declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Anoopkumar, A., Aneesh, E.M. A critical assessment of mosquito control and the influence of climate change on mosquito-borne disease epidemics. Environ Dev Sustain 24, 8900–8929 (2022). https://doi.org/10.1007/s10668-021-01792-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10668-021-01792-4