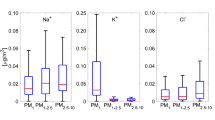
Abstract
The equilibrium between nitric acid gas (HNO3(g)) and ammonium nitrate aerosol (NH4NO3(p)) in ambient air was studied based on the monitoring data obtained using a five-stage filter-pack system, in which the fine aerosol and the coarse aerosol were separately collected; this made it possible to evaluate the actual situation of the equilibrium more accurately. The partition between HNO3(g) and coarse particulate nitrate (c-NO3-(p)), as well as that between HNO3(g) and fine particulate nitrate (f-NO3-(p)), could be evaluated individually thanks to the classification separation of the aerosol by size. The c-particle proportion c-NO3-(p)/(c-NO3-(p) + HNO3(g)) between HNO3(g) and c-NO3-(p) had a weak negative correlation (r = -0.46, p<0.001) with air temperature; in contrast, the f-particle proportion f-NO3-(p)/(f-NO3-(p) + HNO3(g)) between HNO3(g) and f-NO3-(p) had a moderate negative correlation (r = -0.80, p<0.001) with air temperature in total; furthermore, the f-particle proportion had an interesting and discriminative dependence on air temperature which could be divided into two regions by an air temperature around 15°C. The condition of high air temperature accompanied by high relative humidity frequently resulted in the deliquescent state of NH4NO3(p), providing the disconnect from the theoretical prediction for the products of [NH3(g)] and [HNO3(g)] ([NH3(g)][HNO3(g)]) by Seinfeld and Pandis (1998).









Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Aikawa, M., & Hiraki, T. (2008). Methodology of analysis associating survey results by the filter-pack method with those of precipitation- a cid-base balance on acid-related and alkali-related chemical species in urban ambient air and its influence on the acidification of precipitation. Journal of Atmospheric Chemistry, 61(1), 21–29. https://doi.org/10.1007/s10874-009-9122-9
Aikawa, M., Hiraki, T., Mukai, H., & Murano, K. (2008). Characteristic variation of concentration and chemical form in sulfur, nitrate, ammonium, and chloride species observed at urban and rural sites of Japan. Water, Air, and Soil Pollution, 190(1–4), 287–297. https://doi.org/10.1007/s11270-007-9600-0
Aikawa, M., Hiraki, T., & Tamaki, M. (2005). Characteristics in concentration of chemical species in ambient air based on three-year monitoring by filter pack method. Water, Air, and Soil Pollution, 161(1–4), 335–352. https://doi.org/10.1007/s11270-005-4774-9
Aikawa, M., Ohara, T., Hiraki, T., Oishi, O., Tsuji, A., Yamagami, M., Murano, K., & Mukai, H. (2010). Significant geographic gradients in particulate sulfate over Japan determined from multiple site measurements and a chemical transport model: Impacts of transboundary pollution from the Asian continent. Atmospheric Environment, 44, 381–391. https://doi.org/10.1016/j.atmosenv.2009.10.025
Bari, A., Ferraro, V., Wilson, L. R., Luttinger, D., & Husain, L. (2003). Measurements of gaseous HONO, HNO3, SO2, HCl, NH3, particulate sulfate and PM2.5 in New York, NY. Atmospheric Environment, 37(20), 2825–2835. https://doi.org/10.1016/S1352-2310(03)00199-7
Do, H. D., Lim, Y. B., & Kim, Y. P. (2022). Water uptake and the gas-particle partitioning of nitrate aerosols. Atmospheric Chemistry and Physics Discussions, 1, 23. https://doi.org/10.5194/acp-2022-364
EANET, 2018, DataReport, https://monitoring.eanet.asia/document/public/index (last access: Dec. 6th, 2021)
Fujiwara, H., Sadanaga, Y., Urata, J., Masui, Y., Bandow, H., Ikeda, K., Hanaoka, S., Watanabe, I., Arakaki, T., Kato, S., Kajii, Y., & Zhang, D. (2014). Aerial observation of nitrogen compounds over the East China Sea in 2009 and 2010. Atmospheric Environment, 97, 462–470. https://doi.org/10.1016/j.atmosenv.2014.01.064
Hicks, B. B., Saylor, R. D., & Baker, B. D. (2016). Dry deposition of particles to canopies—A lookback and the road forward. Journal of Geophysical Research: Atmospheres. https://doi.org/10.1002/2015JD024742
Katata, G., Matsuda, K., Sorimachi, A., Kajino, M., & Takagi, K. (2020). Effects of aerosol dynamics and gas–particle conversion on dry deposition of inorganic reactive nitrogen in a temperate forest. Atmospheric Chemistry and Physics, 20, 4933–4949. https://doi.org/10.5194/acp-20-4933-2020
Lee, H. S., Kang, C.-M., Kang, B.-W., & Kim, H.-K. (1999). Seasonal variations of acidic air pollutants in Seoul, South Korea. Atmospheric Environment, 33(19), 3143–3152. https://doi.org/10.1016/S1352-2310(98)00382-3
Matsumoto, M., & Okita, T. (1998). Long term measurements of atmospheric gaseous and aerosol species using an annular denuder system in Nara, Japan. Atmospheric Environment, 32(8), 1419–1425. https://doi.org/10.1016/S1352-2310(97)00270-7
Ministry of the Environment of Japan (2019). Report on acid deposition and air pollution in Japan (Fiscal year 2013–2017). http://www.env.go.jp/air/acidrain/monitoring/rep4/1_2syou.pdf (last access: July 4th, 2021) (in Japanese)
Nah, T., Guo, H., Sullivan, A. P., Chen, Y., Tanner, D. J., Nenes, A., Russell, A., Ng, N. L., Huey, L. G., & Weber, R. J. (2018). Characterization of aerosol composition, aerosol acidity, and organic acid partitioning at an agriculturally intensive rural southeastern US site. Atmospheric Chemistry and Physics, 18(15), 11471–11491. https://doi.org/10.5194/acp-18-11471-2018
Network Center of EANET. (2013). Technical Manual for Air Concentration Monitoring in East Asia, https://www.eanet.asia/wp-content/uploads/2019/04/techacm.pdf (last access; Aug. 13th, 2022)
Nguyen, K. L., & Aikawa, M. (2022a). Record of pre-neutralized H2SO4 implied in the results of four-stage and five-stage paralleled filter pack observations on the western edge of Japan. Water, Air and Soil Pollution, 233, 319. https://doi.org/10.1007/s11270-022-05823-2
Nguyen, K. L., & Aikawa, M. (2022b). A novel developed step-by-step assessment methodology for the correction of sampling artifact and chlorine loss on HNO3 in ambient air. Water, Air and Soil Pollution, 233, 110. https://doi.org/10.1007/s11270-022-05570-4
Osada, K., Ura, S., Kagawa, M., Mikami, M., Tanaka, T. Y., Matoba, S., Aoki, K., Shinoda, M., Kurosaki, Y., Hayashi, M., Shimizu, A., & Uematsu, M. (2014). Wet and dry deposition of mineral dust particles in Japan: factors related to temporal variation and spatial distribution. Atmospheric Chemistry and Physics, 14, 1107–1121 http://www.atmos-chem-phys.net/14/1107/2014/doi:10.5194/acp-14-1107-2014
Peng, Y., Suzuki, M., Nguyen, K. L., Zhang, X., & Aikawa, M. (2021). Presence and source attribution of airborne anthropogenic/non-sea-salt inorganic chloride determined by filter-pack method at eastern edge in East Asia. Water, Air and Soil Pollution, 232, 238. https://doi.org/10.1007/s11270-021-05186-0
Robarge, W. P., Walker, J. T., McCulloch, R. B., & Murray, G. (2002). Atmospheric concentrations of ammonia and ammonium at an agricultural site in the southeast United States. Atmospheric Environment, 36(10), 1661–1674. https://doi.org/10.1016/S1352-2310(02)00171-1
Seinfeld, J. H., & Pandis, S. N. (1998). Atmospheric Chemistry and Physics from Air Pollution to Climate Change (pp. 531–537). John Wiley & Sons, Inc..
Seinfeld, J. H., & Pandis, S. N. (2016). Atmospheric Chemistry and Physics from Air Pollution to Climate Change (Third ed., p. 19). John Wiley & Sons, Inc..
Shi, X., Nenes, A., Xiao, Z., Song, S., Yu, H., Shi, G., Zhao, Q., Chen, K., Feng, Y., & Russell, A. G. (2019). High-resolution data sets unravel the effects of sources and meteorological conditions on nitrate and its gas-particle partitioning. Environmental Science & Technology, 53(6), 3048–3057.
Stelson, A. W., & Seinfeld, J. H. (2007). Relative humidity and temperature dependence of the ammonium nitrate dissociation constant. Atmospheric Environment, 41, S126–S135. https://doi.org/10.1016/j.atmosenv2007.10.063
Tamaki, M., Hiraki, T., & Aikawa, M. (2000). Progress in Acid Deposition Monitoring Technology in Japan. Global Environmental Research, 4(1), 25–38.
Yamaga, S., Ban, S., Xu, M., Sakurai, T., Itahashi, S., & Matsuda, K. (2021). Trends of sulfur and nitrogen deposition from 2003 to 2017 in Japanese remote areas. Environmental Pollution, 289(2021), 117842. https://doi.org/10.1016/j.envpol.2021.117842
Zhang, X., Eto, Y., Wang, J., & Aikawa, M. (2021a). Risk assessment and management of PM2.5-bound heavy metals in the urban area of Kitakyushu, Japan. Science of the Total Environment, 763, 143001. https://doi.org/10.1016/j.scitotenv.2021.148748
Zhang, X., Murakami, T., Wang, J., & Aikawa, M. (2021b). Sources, species and secondary formation of atmospheric aerosols and gaseous precursors in the suburb of Kitakyushu, Japan. Science of the Total Environment, 763(2021), 143001. https://doi.org/10.1016/j.scitotenv.2020.143001
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
Sho Oniwa : Sample collection, Chemical analysis, Statistical analysis, Visualization, Writing of draft manuscript
Momoko Abe : Sample collection, Chemical analysis
Masahide Aikawa : Design, Conceptualization, Organization and Supervision of the study, Writing of the final manuscript
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementray information
ESM 1
(DOCX 13 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oniwa, S., Abe, M. & Aikawa, M. Significant parameter for controlling the partition of ambient nitrate species between HNO3(g) and NH4NO3(p). Environ Monit Assess 195, 1134 (2023). https://doi.org/10.1007/s10661-023-11751-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-023-11751-3