Abstract
This study aimed to evaluate the concentration of metals such as aluminum (Al), cadmium (Cd), lead (Pb), barium (Ba), bismuth (Bi), cobalt (Co), nickel (Ni), chromium (Cr), iron (Fe), copper (Cu), zinc (Zn), and selenium (Se) in 360 samples of poultry meat and liver from six brands (A, B, C, D, E, and F) in Assiut, Egypt; compare these concentrations with Egyptian and world permissible limits; and determine their safety for human consumption according to health risk assessment. Chest, thigh muscles, and liver were collected randomly from Assiut city markets, and the concentration of heavy metals was measured in the central laboratory of the faculty of agriculture at Assiut University using inductively coupled plasma mass spectrometry (ICP-MS). All the analyzed samples were positive for the tested metals and were far below the allowed maximum permissible limits except for Pb and Fe, which exceeded the Egyptian Organization for Standardization (EOS) permissible limits with 33% and 67%, respectively, as well as Pb and Cd, which exceeded FAO/WHO permissible limits with 94% and 17%, respectively. Health risk assessment revealed the safety and minimum health risk for human consumption of metal residues in poultry tissues and liver using estimated daily intake (EDI), target hazard quotient (THQ), hazard index (HI), and target cancer risk (TR). Even though the THQ and HI values were significantly lower than 1.0 during our study, heavy metal monitoring in poultry products and byproducts is required for human security and safety.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal pollution in food and feed is increasing every day due to anthropogenic activities in industry and agriculture. Poultry are exposed to a massive array of metals through contaminated feed, drinking water, and litter, which can reduce the safety of their food products (Ahmed, 2003). Pollution occurs through different steps in industries that enter the food chain and contaminate our food (Gholizadeh et al., 2009; Khairy, 2009).
Poultry meat has high nutritional value thanks to its essential amino acids, vitamins, minerals, important trace elements, and antioxidants, which promote human health and growth, especially in developing countries (Hassanin et al., 2014; Taha, 2003). All over the world, the poultry industry is well developed due to its easy digestibility, acceptance, and relative cheapness compared with other meat products. Contamination of poultry feed with heavy metals has an effect not only on livestock health, production, and reproduction but also on the safety of its products (Peng et al., 2018).
Heavy metal residues are characterized by no taste or smell, persistence, bioaccumulation in tissues, biomagnification in the food chain, different toxic effects, and being hidden in meat and offal (Darwish et al., 2018; Demirezen & Uruç, 2006; Ragab et al., 2014). The accumulation of heavy metals varies by organ in the same animal or bird depending on the consumed dose, exposure interval, animal breed, and age (John & Jeanne, 1994). Estimation of heavy metals in poultry edibles must receive further serious attention (Iwegbue et al., 2008; Ramadan & Adam, 2007) due to their bioaccumulation in living tissues and further toxic effects on vital body systems (Ahmed et al., 2019; Devi & Yadav, 2018). Heavy metals in food are considered a risk, so health institutions and organizations set standardization limits and monitoring programs to adjust permissible and non-permissible limits of heavy metals in food (Andrée et al., 2010; EC, 2003, 2008). Metals are classified as essential metals, such as Fe, Cu, Zn, and Se, which play a role in the structure of vitamins and enzymes but can be harmful if consumed in excess (Filazi et al., 2003; Niedziółka et al., 2010), and heavy metal pollutants, which can cause toxic effects even at low levels, such as Al, Cd, and Pb (Lenntech, 2012).
Al is a neurotoxic metal, and there is a relationship between it and Alzheimer's disease (Alfrey et al., 1976). Food is the main source of Al intake due to its wide use in our lives. It has been classified as a non-carcinogenic metal in "Group 3" by the International Agency for Research on Cancer (IARC) (EPA, 2004; IARC, 2012) and the Joint FAO/WHO Expert Committee on Food Additives (JECFA) and the Scientific Committee for Food (SCF) set 7.00 mg/kg body weight per week as a tolerable weekly intake (PTWI) for Al (JECFA, 1989).
Cd is an endocrine disruptor metal that causes renal and pulmonary dysfunction, bone defects, and myocardial diseases in animals (Akan et al., 2010; Faten et al., 2014; McLaughlin et al., 1999). The IARC classified Cd as a human carcinogen in "Group 1" (EFSA, 2009). According to the EU, FAO/WHO, and Turkey, the maximum permissible limits of Cd are 0.05 and 0.5 mg/kg in poultry meat and liver, respectively (FAO/WHO, 2006; EC, 2008; TFC, 2008), and the EOS set 0.5 mg/kg as the guideline limit of Cd (EOS, 1993).
Pb can enter the body through inhalation or ingestion and cause disturbances in different body systems (Berny et al., 1994). It has been classified as a "Group 2B" potential human cancer by the IARC (IARC, 2012). According to the EU and FAO/WHO, the maximum residue limits for Pb in poultry meat and liver are 0.1 and 0.5 mg/kg, respectively (FAO/WHO, 2006; EC, 2008). The EOS also suggested 2 mg/kg as a guideline for Pb in food (EOS, 1993).
Co enters the food chain in the form of fertilizers, is absorbed by plants, and then reaches the animal body, where it threatens lung, heart, and thyroid functions (Bucher et al., 1990; Coates JR & Watson, 1971; Pier, 1975). According to IARC, it has been classified as "probably carcinogenic" to humans in "Group 2A" (IARC, 1999, 2006).
Ni disrupts respiratory, cardiovascular, and nervous system functions in the body (Macomber & Hausinger, 2011). It is lethal if it exceeds the permissible limit in edibles (Nriagu & Pacyna, 1988). According to IARC, it has been classified as a human carcinogen in "Group 1" (IARC, 1990, 2012). The Food and Nutrition Board (FNB, 2010) set the permissible limit of Ni at 4 mg/kg, but the EOS showed that the safe guideline for Ni in food is 10 mg/kg wet weight (EOS, 1993).
Cr (III) acts as a cofactor of insulin in the body, so it has a vital role in glucose, lipid, and protein metabolism. Cr (IV) is considered a carcinogen (Wilbur et al., 2012), and the IARC confirms that and classifies it as a human carcinogen in "Group 1" (IARC, 1990, 2012). The World Health Organization and the Food and Agriculture Organization of the United Nations set 1 mg/kg as a limit for Cr exposure (WHO/FAO, 2011). Finally, Ba, Bi, Cu, Zn, Fe, and Se have been classified by the IARC as "Group 3" non-carcinogenic metals (EPA, 2004; IARC, 2012).
In last year's, renal failure, liver cirrhosis, chronic anemia, and other health problems have increased in the Egyptian population due to exposure to metals (Salem et al., 2000), and about 25% of human diseases today are due to long-term exposure to environmental pollution (UNEP, 2008).
The purpose of this study was to determine the level of safety of heavy metals (Al, Cd, Pb, Ba, Bi, Co, and Ni) and essential metals (Cr, Fe, Cu, Zn, and Se) residues in poultry chest, thigh muscles, and liver from six brands in Assiut, Egypt, using EDI, THQ, HI, and TR health risk assessments.
Materials and Methods
Sample preparation and analysis
Three hundred and sixty chest, thigh, and liver samples were randomly collected from Assiut city markets from six brands, 20 samples per brand. Samples were labeled, stored in a polyethylene bag, and frozen at -20 °C until the time of analysis. All laboratory containers were washed with a 10% solution of nitric acid prior to use. About one gram of each sample was individually soaked in 5 ml of high-grade nitric acid (68%; Merck, Germany) over night at room temperature as a digestive solution. The solution was heated at 150 °C for 5 h until the brown vapors disappeared and the sample solution became colorless. The residual solutions (~ 1.5–2 ml) were allowed to cool at room temperature, washed with 25 ml of distilled water, filtrated with ashless No. 42 filter paper, and stored in the freezer until analysis for heavy metals.
The concentration of each metal was determined by inductively coupled plasma mass spectrometry (ICP-MS, iCAP 6000 series) in the central laboratory of the faculty of agriculture at Assiut University against standard solutions of each metal, which were purchased from Sigma, USA.
For assurance quality control of analysis, procedural blanks and duplicate of the samples were done in each batch of digested samples. In addition, a recovery of the total analytical procedure was processed for metals in poultry edibles by spiking the analyzed samples with different standard concentrations. In order to ensure the reliability of instruments, a blank and known standard were run after every 10 samples. Accepted recoveries % were 97.3, 99.6, 93.6, 105.3, 103.16, 105.6, 92.1, 104.9, 104.5, 99.5, 97.6, and 100.46 for Al, Cd, Pb, Ba, Bi, Co, Ni, Cr, Fe, Cu, Zn, and Se, respectively. Metal concentrations were interpreted in µg/g (ppm).
Statistical analysis
Using the SPSS program for Windows, version 16.0, for expressing heavy metal concentrations as the mean ± SE. The results were analyzed statistically using one-way analysis of variance (ANOVA) with Tukey and Dunnett multiple comparison tests.
Health risk assessment
Health risks for consumption of metal residues in poultry tissues and liver were assessed based on calculations of EDI, THQ, HI, and TR.
The EDI was calculated according to the United States Environment Protection Agency (USEPA) (2010) as the following equation:
where C is the concentration of the metal in each sample (µg/g). FIR is the rate of ingestion of poultry meat (muscles) in Egypt, which was estimated to be 39.53 g/day (FAOSTAT, 2013) and 0.1 g/day for liver tissue (WHO, 2003). BW is the body weight of the Egyptian adults, which was set at 70 kg.
According to the USEPA (1989), the non-cancer risk of heavy metals was calculated as follows:
where THQ is the target hazard quotient; ED is exposure duration, which equals 30 years for non-cancer risk and 70 years for cancer risk as suggested by the USEPA; C represents the metal concentration in poultry meat (µg/g); FIR represents the food ingested rate (g/day); EF represents the exposure frequency (365 days/year); and RfD represents the metal reference dose (mg/kg/day), which represents the daily oral exposure dose of metal with the human population over a lifetime without an appreciable risk of deleterious effects (Akoto et al., 2014). RfD values for Al, Cd, Pb, Ba, Bi, Co, Ni, Cr, Fe, Cu, Zn, and Se are 0.0004, 0.001, 0.07, 0.00029, 0.0003, 0.02, 0.003, 0.7, 0.005, 0.3, and 0.005 (µg/g BW/day), respectively (USEPA, 2000, 2006). BW is the body weight of the Egyptian adults, and AT is the average exposure time (365 days × exposure years, which are set at 70 years). If the ratio is equal to or greater than 1, the Egyptian population will be at risk from these poultry edibles (Chien et al., 2002).
Hazard indexes aid in assessing the risk of combined metal exposure using the following equation:
In our study, HI = HQ Al + HQ Cd + HQ Pb + HQ Ba + HQ Bi + HQ Co + HQ Ni + HQ Cr + HQ Fe + HQ Cu + HQ Zn + HQ Se (USEPA, 1989). where ∑HQ means the sum of the hazard quotients of metals in the study. When HI exceeded 1, there was concern for a potential health effect (Huang et al., 2008).
Target cancer risk was used to indicate the carcinogenic risk of heavy metal residues in poultry edibles. It was estimated, according to USEPA (2018), as the following equation:
where TR is the target cancer risk; ED is exposure duration, which equals 70 years for the cancer risk suggested by the USEPA; EF is exposure frequency (365 days/year); FIR is the food ingested rate (g/day); CM is the concentration of metal in poultry meat (µg/g); CPSo is the carcinogenic potency slope, oral (mg/kg body weight/day). The CPSo values are 0.38, 0.0085, 0.7, 1.7, and 0.5 for Cd, Pb, Co, Ni, and Cr, respectively (USEPA, 2018). The average body weight (ABW) is 70 kg for Egyptian adults, and ATc is the average time for carcinogens is 365 days per year for 70 years.
Results and Discussion
Metal concentrations in poultry muscles and liver
The concentration mean, standard error, and range of heavy metals (Al, Cd, Pb, Ba, Bi, Co, and Ni) and essential metals (Cr, Fe, Cu, Zn, and Se) were analyzed in the muscles and liver of six brands of poultry sold in Assiut province markets. Results were discussed and compared with available national and international maximum permissible limits.
Among the analyzed heavy metals in poultry edibles, Al was the most highly concentrated metal; its range was 8.610–21.985 µg/g in chest muscles, 5.729–18.533 µg/g in thigh muscles, and 5.873–14.005 µg/g in the liver of six brands (Table 1). The obtained results for Al were in agreement with the study conducted by Mahmoud and Abdel-Mohsein (2015), where Al was the highest analyzed metal with concentrations of 8.44 and 16.44 µg/g in liver and 7.74 and 10.1 µg/g in muscles of Assiut and Qena broiler farms, respectively, and Wang et al. (2020), where the Al mean was 5.199 µg/g in broilers, but disagreed with the study done by Korish and Attia (2020), where undetectable Al levels were found in different poultry meat products and liver.
Human and animal exposure to Al is uncontrolled due to its widespread use in our lives (Bohrer et al., 2008). Its daily applications include beverage cans, pots and pans, foil, and water filtration. Contaminated food and water are the main sources of Al for humans and animals (Mahmoud & Abdel-Mohsein, 2015). Its residue poses a threat to humans, causing different deleterious effects on the nervous, skeletal, and hematopoietic systems (Domingo, 1995). Al+3 can replace Mg+2 and Fe+3 in the human body and result in cellular growth and communication disturbances as well as neurotoxicity and endocrine disruption (Barabasz et al., 2002; Vardar & Ünal, 2007).
Till now, there has been no Egyptian maximum permissible limit for Al in poultry edibles, but the recommended dietary allowance (RDA) is 60 mg/day for adults (WHO, 1989). In our study, the concentration of Al in poultry muscles and liver was lower than the RDA of the WHO.
The range of Cd concentration (µg/g) was 0.014–0.054 in chest muscles, 0.015–0.088 in thigh muscles, and 0.027–0.104 in the liver of six brands (Table 1). Cd concentration results agree with studies done by Oforka et al. (2012), Nigeria; Cd means in chicken liver and muscles were 0.0457 and 0.0162 µg/g, respectively; Abbas (2017), Cd means (µg/g) in broiler chickens were 0.075, 0.056, and 0.054 in liver, thigh, and breast, respectively; and Reda et al. (2021), Ismailia province, Egypt. Results were lower than the study conducted by Okoye et al. (2011), Enugu State; Cd concentrations were 5.57 and 10.30 µg/g in chicken liver and muscles, respectively; Badis et al. (2014), Cd concentations were 1.39 and 1.49 µg/g in North and South areas of Algeria, respectively; Mahmoud and Abdel-Mohsein (2015), Cd concentations were 1.41, 0.24 µg/g in liver samples and 0.88 and 2.44 µg/g in muscles samples of broiler farms from Assiut and Qena, respectively, and Mottalib et al. (2018), Bangladesh; Cd concentrations mean were 0.243 and 1.092 µg/g in broiler breast and liver, respectively, and higher than the study done by Wang et al. (2020), Jilin Province, China; broiler Cd concentration was 0.003 µg/g.
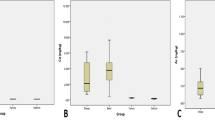
The presence of Cd in the earth's crust is thought to be its primary route to reach the food chain, and then animals and humans (Rashid et al., 2018). In addition, there are different sources of Cd in our lives, such as fertilizers, sewage, soil, lakes, and groundwater (Chowdhury et al., 2011; Hu et al., 2018). Inhaled or ingested Cd causes respiratory, renal, hepatic, cardiovascular, and skeletal system dysfunction (Mansour, 2014; Rehman et al., 2013), as well as carcinogenesis and mutagenesis (Jaishankar et al., 2014; Jan et al., 2015). Cd levels were below FAO/WHO permissible limits in all samples except the chest muscles of brand 1 and the thigh muscles of brands 1 and 2 (Fig. 1) with 17% (40 muscles and 20 liver); however, all Cd levels during our study were below the Egyptian permissible limits according to EOS.
The range of Pb concentration (µg/g) during the study was 2.560–5.552 in chest muscles, 0.334–1.082 µg/g in thigh muscles, and 0.146–0.952 µg/g in the liver of six brands. Pb residues were significantly higher in chest muscles than thigh muscles and liver (Table 1). Results were in agreement with the study conducted by Mahmoud and Abdel-Mohsein (2015); the Pb mean was 2.75 µg/g in broiler farms and lower than the study done by Okoye et al. (2011), Nigeria; Pb concentrations were 26.29 and 45.05 µg/g in the liver and muscles of different chicken types, respectively; and Badis et al. (2014), Algeria; Pb concentrations were 8.80 and 8.18 µg/g in the North and South areas, respectively. Results in the current study showed that poultry muscle Pb concentration was higher than the liver level, which agrees with the study done by Mahmoud and Abdel-Mohsein (2015) but disagrees with studies conducted by Oforka et al. (2012) and Reda et al. (2021), who showed that internal organs of poultry accumulated more Pb than muscles.
Pb is considered one of the most toxic heavy metals and has no benefits for animals or humans. The wide spread of industrial products such as batteries, limestone, leaded gasoline in vehicles, and perfumes, in addition to agricultural and sewage discharges, causes environmental pollution with Pb (EC, 2001; Hussain et al., 2012; Ismail & Abolghait, 2013; Ogwok et al., 2014). Pb can accumulate in the liver and bones as well as affect the nervous, hematopoietic, reproductive, cardiovascular, and adrenal systems (Canfield et al., 2003; Codling et al., 2008; EC, 2001; Khan et al., 2016). In comparison of our Pb results with the previously mentioned FAO/WHO permissible limits, 94% of samples (240 muscles and 100 livers) exceeded permissible limits. All analyzed samples exceeded permissible limits except the liver of brand 1 (Fig. 2). According to EOS, only chest muscles from six brands exceeded permissible limits and represented 33%.
The range of Ba concentrations (µg/g) was 1.971–6.226 in chest muscles, 3.216–6.779 in thigh muscles, and 1.038–4.416 in the liver of different brands (Table 1). It is found in all foods in concentrations ranging from 0.21 to 11 mg/kg (Howe et al., 2005; WHO, 2004; Ysart et al., 1999). Our results were higher than those of studies conducted by Ysart et al. (1999), UK; Rose et al. (2010), UK; Ba concentration was 0.03 µg/g; and González-Weller et al. (2013), whose Ba mean was 0.113 and 0.169 µg/g in chicken breast and liver, respectively.
In addition, Ba is abundant in the earth's crust; its compounds are used in wide applications in our lives, such as fluorescent lamps, paints, bricks, tiles, glass, rubber, oil, and gas industries, as well as barium nitrate and chlorate, which give fireworks a green color. The widespread use of Ba in industrial human activities raises its natural level in the environment.
The range of Bi concentrations (µg/g) was 0.587–3.526 in chest muscles, 0.0043–0.0133 in thigh muscles, and 0.0010–0.0110 in the liver of six brands. Results also showed that Bi residues were significantly higher in chest muscles than thigh muscles and liver (Table 1). Our results disagree with the study done by González-Weller et al. (2013); liver Bi level (53.28 µg/g) was higher than muscle (0.318 µg/g).
Bi is used in the manufacture of shots, shotguns, fishing sinkers, fibers, rubbers, and pharmaceuticals. It is considered one of the less toxic heavy metals and poses a minimal threat to the environment; its toxicity may occur due to high doses applied to burns. There is no evidence that Bi or Ba are carcinogenic, and no permissible limit has been established for either element yet.
The range of Co concentrations (µg/g) was 0.039–0.083 in the chest, 0.014–0.039 in the thigh muscles, and 0.028–0.053 in the liver of six brands. Results also showed that Co residues were significantly higher in chest muscles than thigh muscles and liver (Table 1). Our results agree with the study conducted by Hozan and Hemin (2013): the Co range was 0.00–0.04 µg/g in chicken luncheon that was sold in Sulaymaniah markets; Mottalib et al. (2018), the Co mean was 0.061 and 0.07 µg/g in broiler breast and liver, respectively; and Ersoy et al. (2015), the Co level was determined in the thigh, chest, and liver of poultry around the cement factory in a residential area. On the other hand, the Co range was lower than in the study done by Abdel-Salam et al. (2013), where the Co concentration was 0.2 µg/g.
Co is released into the environment as a result of both natural and anthropogenic activities. It plays a vital role as a constituent of vitamin B12, but excessive intake can cause adverse health effects on the endocrine, nervous, and cardiovascular systems (Zahrana & Hendy, 2015). In the previous literature, we have not found Egyptian or international permissible limits for Co in poultry edibles.
The range of Ni concentrations (µg/g) was 0.154–0.228 in chest muscles, 0.143–0.255 in thigh muscles, and 0.112–0.217 in the liver of six brands (Table 1). Our results agree with the study conducted by Mottalib et al. (2018), whose Ni mean in broiler breast and liver was 0.298 and 0.398 µg/g, respectively, but are lower than the study done by Oforka et al. (2012), whose Ni mean was 0.062 and 0.108 µg/g in muscles and liver, respectively, and Mahmoud and Abdel-Mohsein (2015), whose Ni concentration was 4.1 µg/g.
Ni is naturally present in the earth's crust, and the main sources of it in our environment are emissions from refineries, mining, and the combustion of coal, oil, and municipal wastes (USEPA, 2000). Ni is not an accumulated element in the food chain, but it can cause respiratory dysfunction, oxidative stress, allergic skin reactions, hepatotoxicity, immunotoxicity, and cancer (ATSDR, 2004; Das & Büchner, 2007; Lee, 2006). According to the previously mentioned Food and Nutrition Board, WHO/FAO, and EOS safe guidelines for Ni, its concentrations in all samples of different brands examined in our study were far below those limits and considered safe for human consumption.
Fe and Zn were the two highest trace elements in different poultry edible of the study, according to essential trace metal results. The liver accumulated more Fe, Cu, Zn, and Se than the thigh and chest muscles, depending on the brand.
The range of Cr concentrations (µg/g) was 0.266–2.215 in chest muscles, 0.170–0.460 in thigh muscles, and 0.161–1.422 in the liver of six brands (Table 2). Results agree with the study conducted by Iwegbue et al. (2008); the Cr range was 0.01–3.43 µg/g. On the other hand, lower than the study done by Mottalib et al. (2018), the Cr mean was 3.976 and 1.683 µg/ in broiler breast and liver, respectively. Cr is used in dyes, paints, the tanning of leather, the firing of bricks, and metallurgy to impart corrosion resistance and a shiny finish. Due to its wide applications in agriculture, such as fertilizers and wastewater irrigation, people are exposed to it through breathing, eating, drinking, and skin contact (Korish & Attia, 2020). Forms of Cr can be detected by their degree of toxicity; Cr (III) is an essential nutrient for humans and a non-carcinogenic compound, but when they exceed a certain value, negative health effects can occur. Cr (IV) is dangerous for human health and can cause skin rashes, respiratory and heart problems, kidney and liver damage, and cancer (Dębski et al., 2004; Jaishankar et al., 2014; Sahin et al., 2002; Toghyani et al., 2012). The international permissible limit for Cr in edible poultry has been proposed at 1.0 ppm (Youssef A. Attia et al., 2019; National Standard of the People’s Republic of China, 2005; Roychowdhury et al., 2003). According to the previous limit, 11% (20 chest muscle and 20 liver) of the samples in the current study exceeded the permissible limit.
The range of Fe concentrations (µg/g) was 7.631–31.604 in chest muscles, 4.583–18.360 in thigh muscles, and 24.151–48.142 in the liver of brands (Table 2). Fe results were lower than Badis et al. (2014), Algeria; Fe concentration was 246.83 and 186.33 µg/g in Nourth and South areas, respectively; Korish and Attia (2020); Fe mean was 87.8, 63.1, and 288.2 µg/g in frozen, fresh broiler meat, and liver, respectively, and Reda et al. (2021), Ismailia Province. Thanks to its combination of low cost and high strength, Fe is an indispensable element worldwide. Its applications range from food containers to cars. For humans, it facilitates protein, lipid, and carbohydrate metabolism in the body, but its deficiency causes anemia, a high susceptibility to myocardial infarction, gut infections, and bleeding (Elsharawy, 2015; Jaishankar et al., 2014). High Fe concentrations also cause cardiac arrest and respiratory failure (Korish & Attia, 2020). The Egyptian Organization for Standardization and Quality Control suggested 15.0 μg/g as a safe line for Fe in poultry meat and 20.0 μg/g in offal (EOS, 2010). According to the previous limit, 67% (120 chest and thigh muscles as well as 120 liver) of samples exceeded the mentioned permissible limit (Fig. 3).
The range of Cu concentrations (µg/g) was 0.720–0.989 in chest muscles, 0.606–1.417 in thigh muscles, and 3.071–4.108 in the liver of six brands in the study (Table 2). This study's results were compared with those reported by Okoye et al. (2011), whose Cu mean was 26.29 and 45.05 µg/g in different chicken liver and muscles, respectively; Alturiqi and Albedair (2012)'s Cu range was (2.31–7.79 µg/g). Elsharawy (2015), Cu range was (0.15–1.16 µg/g); Mottalib et al. (2018), Cu mean was 2.422 and 4.092 µg/g in broiler breast and liver, respectively; and Korish and Attia (2020), Cu mean was 0.036, 0.056, and 19.24 µg/g in frozen, fresh broiler meat and liver, respectively. Cu is very common and abundant in our environment due to its high natural presence and its industrial and agricultural applications. It is an essential element for various enzymes in our body, but if levels exceed limits, they cause liver and kidney disorders (Elsharawy, 2015; National Research Council, 1994; Ogwok et al., 2014). Cu does not break down in the environment, so it can accumulate in plants, animals, and soil. Consumption of highly Cu-leveled animal products acts as a threat to public health (Alturiqi & Albedair, 2012; Elsharawy, 2015; Hu et al., 2018; Jaishankar et al., 2014). Its toxic effects extend from the activity of microorganisms and earthworms to human health. The Egyptian Organization for Standardization and Quality Control set 15 μg/g as a safe permissible limit for Cu residues in meat and offal (EOS, 2010). According to the previous limit, all the analyzed samples in the current study were within the permissible limits.
The range of Zn concentrations (µg/g) was 7.963–15.295 in chest muscles, 15.171–22.792 in thigh muscles, and 22.952–31.980 in the liver of brands in the study (Table 2). Zn results agree with studies conducted by Chowdhury et al. (2011), Badis et al. (2014), Algeria, and Hu et al. (2018), China. On the other hand, they were higher than the study done by El Bayomi et al. (2018), whose Zn mean was 2.46 and 4.76 µg/g in broiler muscle and live, respectively. Our results also revealed that Fe, Cu, and Zn concentrations were higher in the liver than in the muscles of poultry, which agrees with results reported by El Bayomi et al. (2018), Hu et al. (2018), and Korish and Attia (2020). The international permissible limit for Zn in edible poultry is set at 20 μg/g (Attia et al., 2019; National Standard of the People’s Republic of China, 2005; Roychowdhury et al., 2003). According to the previous permissible limit, 44% (40 thigh muscles and 120 liver) of our study samples exceeded the mentioned limit.
The range of Se concentrations (µg/g) was 0.239–0.335 in chest muscles, 0.192–0.383 in thigh muscles, and 0.601–0.816 in the liver of brands in the study (Table 2). Our results showed that Se concentrations were higher in the liver than in the muscles of poultry, which agrees with Hu et al. (2018), China. Se is one of the essential trace elements and has a role in metabolism, reproduction, immunological function, and antioxidant balance (Attia et al., 2010; Hosnedlova et al., 2018; Saleh & Ebeid, 2019). The international permissible limit for Se in edible poultry has been proposed at 0.5 μg/g ( Attia et al., 2019; National Standard of the People’s Republic of China, 2005; Roychowdhury et al., 2003), and our results showed that 33% (120 liver) of the samples exceeded the previous limit.
Health risk assessment
EDI of metals in poultry muscles and liver
The daily intake of metals was estimated and compared with the RDA and tolerable daily intake (TDI) levels, which are considered good monitoring parameters for human exposure to metals. According to Table 3, the EDI of poultry muscles and liver in the current study was lower than the RDI and TDI (FNB, 2004; FSA, 2009; WHO, 2003), which means that human consumption of poultry edibles with such metal residues had a minimum health risk. The EDI results in the current study agree with those reported by El Bayomi et al. (2018).
THQ and HI of metals in poultry muscles and liver
The THQ and HI were used to assess the non-carcinogenic risks of eating poultry muscles and liver. THQ and HI results were far below 1.0 (Tables 4 and 5), and there was no obvious risk for Egyptian consumers from the current study of poultry muscles and liver, which agrees with the previous studies conducted by Bortey-Sam et al. (2015), Darwish et al. (2018), El Bayomi et al. (2018), and Ogbomida et al. (2018).
TR of metals in poultry muscles and liver
TR is a tool used to assess the cancer risk of analyzed metals. The US Environmental Protection Agency accepted for regulatory purposes a cancer risk in the range of 1 × 10–6 to 1 × 10–4 (USEPA, 2004), and South Africa considered that the individual cancer risk limit was 5 × 10–6 (Government of South Africa, 2006). TR results revealed that none of the analyzed metals in the current study pose a carcinogenic risk (Table 6).
Conclusion
Although all the analyzed poultry meat and liver were positive for metals (Al, Cd, Pb, Ba, Bi, Co, Ni, Cr, Fe, Cu, Zn, and Se), concentrations were far below the maximum permissible limits except for Pb, Cd, and Fe, which exceeded the EOS and/or FAO/WHO permissible limits. Health risk assessment conducted during the study using EDI, THQ, HI, and TR revealed the safety and minimum health risk for human consumption of metal residues in poultry tissues and liver. Even though THQ and HI values were significantly lower than 1.0 during our study, metal monitoring in poultry products and byproducts is required for human security and safety, especially in Egypt due to a shortage of different sources of red proteins and high poultry meat consumption.
Data availability
Is applicable.
References
Abbas, K. H. (2017). Detection of some heavy metals in poultry meats from some sources of meat and poultry rations. Al-Qadisiyah Journal of Veterinary Medicine Sciences, 16(2), 99–104.
Abdel-Salam, N. M., Ahmed, S., Basir, A., Rais, A. K., Bibi, A., Ullah, R., et al. (2013). Distribution of heavy metals in the liver, kidney, heart, pancreas and meat of cow, buffalo, goat, sheep and chicken from Kohat market Pakistan. Life Sci J, 10(7s), 937–940.
Ahmed, S., Khurshid, S., Qureshi, F., Hussain, A., & Bhattacharya, A. (2019). Heavy metals and geo-accumulation index development for groundwater of Mathura city, Uttar Pradesh. Desalination and Water Treatment, 138, 291–300.
Ahmed, W. M. S. (2003). Studies on heavy metals pollution in poultry farms relation to production performance. agris.fao.org
Akan, J. C., Abdulrahman, F. I., Sodipo, O. A., & Chiroma, Y. A. (2010). Distribution of heavy metals in the liver, kidney and meat of beef, mutton, caprine and chicken from Kasuwan Shanu market in Maiduguri Metropolis, Borno State, Nigeria. Research Journal of Applied Sciences, Engineering and Technology, 2(8), 743–748.
Akoto, O., Bismark, E. F., Darko, G., & Adei, E. (2014). Concentrations and health risk assessments of heavy metals in fish from the Fosu Lagoon. International Journal of Environmental Research, 8(2), 403–410.
Alfrey, A. C., LeGendre, G. R., & Kaehny, W. D. (1976). The dialysis encephalopathy syndrome: Possible aluminum intoxication. New England Journal of Medicine, 294(4), 184–188.
Alturiqi, A. S., & Albedair, L. A. (2012). Evaluation of some heavy metals in certain fish, meat and meat products in Saudi Arabian markets. The Egyptian Journal of Aquatic Research, 38(1), 45–49.
Andrée, S., Jira, W., Schwind, K.-H., Wagner, H., & Schwägele, F. (2010). Chemical safety of meat and meat products. Meat Science, 86(1), 38–48.
ATSDR. (2004). Agency for toxic substances and disease registry division of toxicology. Clifton Road, NE, Atlanta, GA,USA. Available at: http://www.atsdr.cdc.gov/toxprofiles
Attia, Y. A., Qota, E. M., Bovera, F., Tag El-Din, A. E., & Mansour, S. A. (2010). Effect of amount and source of manganese and/or phytase supplementation on productive and reproductive performance and some physiological traits of dual purpose cross-bred hens in the tropics. British Poultry Science, 51(2), 235–245.
Attia, Y. A., Addeo, N. F., Al-Hamid, A. A. H. E. A., & Bovera, F. (2019). Effects of phytase supplementation to diets with or without zinc addition on growth performance and zinc utilization of white pekin ducks. Animals, 9(5), 1–12. https://doi.org/10.3390/ani9050280
Badis, B., Rachid, Z., & Esma, B. (2014). Levels of selected heavy metals in fresh meat from cattle, sheep, chicken and camel produced in Algeria. Annual Research & Review in Biology, 4(8), 1260–1267. https://doi.org/10.9734/ARRB/2014/7430
Barabasz, W., Albinska, D., Jaskowska, M., & Lipiec, J. (2002). Ecotoxicology of aluminium. Polish Journal of Environmental Studies, 11(3), 199–204.
Berny, P. J., Cote, L. M., & Buck, W. B. (1994). Relationship between soil lead, dust lead, and blood lead concentrations in pets and their owners: Evaluation of soil lead threshold values. Environmental Research, 67(1), 84–97.
Bohrer, D., Dessuy, M. B., Kaizer, R., Do Nascimento, P. C., Schetinger, M. R. C., Morsch, V. M., et al. (2008). Tissue digestion for aluminum determination in experimental animal studies. Analytical Biochemistry, 377(2), 120–127.
Bortey-Sam, N., Nakayama, S. M. M., Ikenaka, Y., Akoto, O., Baidoo, E., Yohannes, Y. B., et al. (2015). Human health risks from metals and metalloid via consumption of food animals near gold mines in Tarkwa, Ghana: Estimation of the daily intakes and target hazard quotients (THQs). Ecotoxicology and Environmental Safety, 111, 160–167.
Bucher, J. R., Elwell, M. R., Thompson, M. B., Chou, B. J., Renne, R., & Ragan, H. A. (1990). Inhalation toxicity studies of cobalt sulfate in F344/N rats and B6C3F1 mice. Toxicological Sciences, 15(2), 357–372.
Canfield, R. L., Henderson, C. R., Jr., Cory-Slechta, D. A., Cox, C., Jusko, T. A., & Lanphear, B. P. (2003). Intellectual impairment in children with blood lead concentrations below 10 μg per deciliter. New England Journal of Medicine, 348(16), 1517–1526.
Chien, L.-C., Hung, T.-C., Choang, K.-Y., Yeh, C.-Y., Meng, P.-J., Shieh, M.-J., & Han, B.-C. (2002). Daily intake of TBT, Cu, Zn, Cd and As for fishermen in Taiwan. Science of the Total Environment, 285(1–3), 177–185.
Chowdhury, M. Z. A., Siddique, Z. A., Hossain, S. M. A., Kazi, A. I., Ahsan, A. A., Ahmed, S., & Zaman, M. M. (2011). Determination of essential and toxic metals in meats, meat products and eggs by spectrophotometric method. Journal of the Bangladesh Chemical Society, 24(2), 165–172.
Coates JR, E. O., & Watson, J. H (1971). Diffuse interstitial lung disease in tungsten carbide workers. Annals of internal medicine, 75(5), 709–716.
Codling, E. E., Chaney, R. L., & Mulchi, C. L. (2008). Effects of broiler litter management practices on phosphorus, copper, zinc, manganese, and arsenic concentrations in Maryland coastal plain soils. Communications in Soil Science and Plant Analysis, 39(7–8), 1193–1205.
Darwish, W. S., Atia, A. S., Khedr, M. H. E., & Eldin, W. F. S. (2018). Metal contamination in quail meat: Residues, sources, molecular biomarkers, and human health risk assessment. Environmental Science and Pollution Research, 25(20), 20106–20115.
Das, K. K., & Büchner, V. (2007). Effect of nickel exposure on peripheral tissues: Role of oxidative stress in toxicity and possible protection by ascorbic acid. Reviews on Environmental Health, 22(2), 157–173.
Dębski, B., Zalewski, W., Gralak, M. A., & Kosla, T. (2004). Chromium-yeast supplementation of chicken broilers in an industrial farming system. Journal of Trace Elements in Medicine and Biology, 18(1), 47–51.
Demirezen, D., & Uruç, K. (2006). Comparative study of trace elements in certain fish, meat and meat products. Meat Science, 74(2), 255–260.
Devi, N. L., & Yadav, I. C. (2018). Chemometric evaluation of heavy metal pollutions in Patna region of the Ganges alluvial plain, India: Implication for source apportionment and health risk assessment. Environmental Geochemistry and Health, 40(6), 2343–2358.
Domingo, J. L. (1995). Reproductive and developmental toxicity of aluminum: A review. Neurotoxicology and Teratology, 17(4), 515–521.
EC. (2001). European commission regulation no. 466 of 8 March 2001; setting maximum levels for certain contaminants in foodstuff. Official Journal of the European Communities: Brussels
EC. (2003). European commission. Opinion of the scientific committee on animal nutrition on the use of zinc in feedstuffs. European commission, health and consumer protection directorate, brussels, Belgium. Scientific Committee on Food.
EC. (2008). European Commission regulation 629/2008/EC of 2 July 2008 setting maximum levels for certain contaminants in foodstuffs. Official Journal of the European Union. L173, 63.7.3008. https://www.researchgate.net/publication/273130016
EFSA. (2009). Cadmium in food. Scientific opinion of the panel on contaminants in the food chain. European Food Safety Authority J, 980, 1–139.
El Bayomi, R. M., Darwish, W. S., Elshahat, S. S. M., & Hafez, A. E. (2018). Human health risk assessment of heavy metals and trace elements residues in poultry meat retailed in Sharkia Governorate. Egypt. Slovenian Veterinary Research, 55(Suppl 20), 211–219.
Elsharawy, N. T. M. (2015). Some heavy metals residues in chicken meat and their edible offal in New Valley. In 2nd conference of food safety. Suez Canal University, Faculty of Veterinary Medicine Ismailia. Vol. 1, pp. 50–57.
EOS. (1993). Egyptian organization for standardization. Maximum levels for heavy metal concentrations in food. ES 2360-1993, UDC:546.19:815, Egypt. https://doi.org/10.14737/journal.aavs
EOS. (2010). Egyptian organization for standardization and quality control. Maximum level for certain contaminants in food stuffs. Available online: https://www.slideshare.net/flash_hero/es-7136. Accessed 26 Mar 2019.
EPA. (2004). Human health evaluation manual (Part a), vol I. Washington, DC, USA: EPA; Risk assessment guidance for superfund. https://www.epa.gov/sites/default/files
Ersoy, İE., Uzatıcı, A., & Bilgücü, E. (2015). Possible heavy metal residues in poultry and their products that are bred around cement industry. Journal of Animal Behaviour and Biometeorology, 3(2), 63–68.
FAO/WHO. (2006). Report of the 32nd session of the codex committee of the food additives and contaminants. Beijing, Peoples Republic China. https://agris.fao.org
FAOSTAT. (2013). Food balance sheets. Available online from: http://www.fao.org/faostat/en/#data/FBS
Faten, S., Hassan, M., Amira, M., & Enas, A. (2014). Heavy metals residues in some chicken meat product. Benha Veterinary Medical Journal, 27(2), 256–263.
Filazi, A., Baskaya, R., Kum, C., & Hismiogullari, S. E. (2003). Metal concentrations in tissues of the Black Sea fish Mugil auratus from Sinop-Icliman. Turkey. Human & Experimental Toxicology, 22(2), 85–87.
FNB. (2004). Dietary Reference Intakes (DRIs). Institute of Medicine, The National Academies press Washington, D.C.
FNB. (2010). Determination of Cadmium, Copper, Nickel, Lead in Some Tea Samples in India. International Journal Of Pharmaceutical And Bio-Medical Science, 3(2), 943–946.
FSA. (2009). Measurement of the concentrations of metals and other elements from the 2006 UK total diet study. London, UK, Report no. 01/09.
Gholizadeh, A., Ardalan, M., Tehrani, M. M., Hosseini, H. M., & Karimian, N. (2009). Solubility test in some phosphate rocks and their potential for direct application in soil. World Applied Sciences Journal, 6(2), 182–190.
González-Weller, D., Rubio, C., Gutiérrez, Á. J., González, G. L., Mesa, J. M. C., Gironés, C. R., et al. (2013). Dietary intake of barium, bismuth, chromium, lithium, and strontium in a Spanish population (Canary Islands, Spain). Food and Chemical Toxicology, 62, 856–868.
Government of South Africa. (2006). Gazette: No. 8454, Vol 490, No. 28755. Pretoria, South Africa: Government of South Africa. https://www.greengazette.co.za/documents/
Hassanin, F., Mahmoud, A., & Mohamed, E. (2014). Heavy metals residues in some chicken meat products. Benha Veterinary Medical Jounal, 27, 256–263.
Hosnedlova, B., Kepinska, M., Skalickova, S., Fernandez, C., Ruttkay-Nedecky, B., Peng, Q., et al. (2018). Nano-selenium and its nanomedicine applications: A critical review. International Journal of Nanomedicine, 13, 2107.
Howe, A., Fung, L. H., Lalor, G., Rattray, R., & Vutchkov, M. (2005). Elemental composition of Jamaican foods 1: A survey of five food crop categories. Environmental Geochemistry and Health, 27(1), 19–30.
Hozan, J. H., & Hemin, N. M. (2013). Determination of heavy metals in exposed corned beef and chicken luncheon that sold in Sulaymaniah markets. African Journal of Food Science, 7(7), 178–182.
Hu, Y., Zhang, W., Chen, G., Cheng, H., & Tao, S. (2018). Public health risk of trace metals in fresh chicken meat products on the food markets of a major production region in southern China. Environmental Pollution, 234, 667–676. https://doi.org/10.1016/j.envpol.2017.12.006
Huang, C.-F., Hsu, C.-J., Liu, S.-H., & Lin-Shiau, S.-Y. (2008). Neurotoxicological mechanism of methylmercury induced by low-dose and long-term exposure in mice: Oxidative stress and down-regulated Na+/K+-ATPase involved. Toxicology Letters, 176(3), 188–197.
Hussain, R. T., Ebraheem, M. K., & Moker, H. M. (2012). Assessment of heavy metals (Cd, Pb and Zn) contents in livers of chicken available in the local markets of Basrah city. Iraq. Bas. J. Vet. Res., 11, 43–51.
IARC. (1990). Chromium, nickel and welding. IARC monographs on the evaluation of carcinogenic risks to humans. Int Agency Res Cancer Lyon, 49, 49–256.
IARC. (1999). Surgical implants and other foreign bodies. Monographs on the evaluation of carcinogenic risks to humans, vol. 74. Lyon, France: World Health Organization, International Agency for Research on Cancer. https://monographs.iarc
IARC. (2006). Cobalt in hard metals and cobalt sulfate, gallium arsenide, indium phosphide and vanadium pentoxide. IARC monographs on the evaluation of carcinogenic risks to humans, vol. 86. Lyon, France: World Health Organization. https://publications.iarc.fr/Book-And-Report-Series/
IARC. (2012). IARC monographs on the evaluation of carcinogenic risks to humans. A review of human carcinogens. IARC Scientific Publications, 100C, 169–218 http://monographs.iarc.fr/ENG
Ismail, S. A., & Abolghait, S. K. (2013). Estimation of Lead and Cadmium residual levels in chicken giblets at retail markets in Ismailia city, Egypt. International Journal of Veterinary Science and Medicine, 1(2), 109–112.
Iwegbue, C. M. A., Nwajei, G. E., & Iyoha, E. H. (2008). Heavy metal residues of chicken meat and gizzard and turkey meat consumed in southern Nigeria. Bulgarian Journal of Veterinary Medicine, 11(4), 275–280.
Jaishankar, M., Tseten, T., Anbalagan, N., Mathew, B. B., & Beeregowda, K. N. (2014). Toxicity, mechanism and health effects of some heavy metals. Interdisciplinary Toxicology, 7(2), 60.
Jan, A. T., Azam, M., Siddiqui, K., Ali, A., Choi, I., & Haq, Q. M. R. (2015). Heavy metals and human health: Mechanistic insight into toxicity and counter defense system of antioxidants. International Journal of Molecular Sciences, 16(12), 29592–29630.
JECFA. (1989). Joint FAO/WHO Expert Committee on Food Additives (1989) Toxicological evaluation of certain food additives and contaminants. WHO Food Additives Series, 24, 113–154.
John, H. H., & Jeanne, I. R. (1994). Food additives, contaminants and natural toxins. Modern Nutrition in Health and Disease, 8, 1597–1598.
Khairy, H. M. (2009). Toxicity and accumulation of copper in Nannochloropsis oculata (Eustigmatophyceae, Heterokonta). World Applied Sciences Journal, 6(3), 378–384.
Khan, Z., Sultan, A., Khan, R., Khan, S., Imranullah, F. K., & Farid, K. (2016). Concentrations of heavy metals and minerals in poultry eggs and meat produced in Khyber Pakhtunkhwa, Pakistan. Meat Sci Vet Public Health [internet], 1(1), 4–10.
Korish, M. A., & Attia, Y. A. (2020). Evaluation of heavy metal content in feed, litter, meat, meat products, liver, and table eggs of chickens. Animals, 10(4), 727.
Lee, S.-H. (2006). Differential gene expression in nickel (II)-treated normal rat kidney cells. Research Communications in Molecular Pathology and Pharmacology, 119(1–6), 77–87.
Lenntech, B. V. (2012). Heavy metals. Available at website: www.lenntech.com/periodic/periodic-chart.htm. Available at: 10.21608/EAJBSZ. 2021. 209055.
Macomber, L., & Hausinger, R. P. (2011). Mechanisms of nickel toxicity in microorganisms. Metallomics, 3(11), 1153–1162.
Mahmoud, M. A. M., & Abdel-Mohsein, H. S. (2015). Health risk assessment of heavy metals for Egyptian population via consumption of poultry edibles. Adv. Anim. Vet. Sci, 3(1), 58–70.
Mansour, S. A. (2014). Monitoring and health risk assessment of heavy metal contamination in food. Practical Food Safety: Contemporary Issues and Future Directions, 13, 235–255.
McLaughlin, M. J., Parker, D. R., & Clarke, J. M. (1999). Metals and micronutrients–food safety issues. Field Crops Research, 60(1–2), 143–163.
Mottalib, M. A., Zilani, G., Suman, T. I., Ahmed, T., & Islam, S. (2018). Assessment of Trace Metals in Consumer Chickens in Bangladesh. Journal of health and Pollution, 8(20). https://doi.org/10.5696/2156-9614-8.20.181208
National Research Council. (1994). Nutrient requirements of poultry, 9th ed.; 8. Toxicity of certain inorganic elements (pp. 58–60). Washington, DC, USA: National Academy Press. Available at https://www.mdpi.com/2076-2615/10/4/727
National Standard of the People’s Republic of China. (2005). Maximum levels of contaminants in foods issued on January 25, 2005 implemented on October 1, 2005 issued by the ministry of hygienic and the standardization administration of China. Beijing, China. Available at: https://www.mdpi.com/2076-2615/10/4/727
Niedziółka, R., Pieniak-Lendzion, K., Horoszewicz, E., & Remiszewska, G. (2010). Content of metals in the muscle tissue and internal organs of male goats of the white improved breed. Fresenius Environmental Bulletin, 19(4), 616–619.
Nriagu, J. O., & Pacyna, J. M. (1988). Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature, 333(6169), 134–139.
Oforka, N. C., Osuji, L. C., & Onwuachu, U. I. (2012). Estimation of dietary intake of cadmium, lead, manganese, zinc and nickel due to consumption of chicken meat by inhabitants of Port-Harcourt Metropolis, Nigeria. Archives of Applied Science Research, 4(1), 675–684.
Ogbomida, E. T., Nakayama, S. M. M., Bortey-Sam, N., Oroszlany, B., Tongo, I., Enuneku, A. A., et al. (2018). Accumulation patterns and risk assessment of metals and metalloid in muscle and offal of free-range chickens, cattle and goat in Benin City, Nigeria. Ecotoxicology and Environmental Safety, 151, 98–108.
Ogwok, P., Bamuwamye, M., Apili, G., & Musalima, J. H. (2014). Health risk posed by lead, copper and iron via consumption of organ meats in Kampala City (Uganda). Journal of Environment Pollution and Human Health, 2(3), 69–73.
Okoye, C. O. B., Aneke, A. U., Ibeto, C. N., & Ihedioha, I. J. N. (2011). Heavy metals analysis of local and exotic poultry meat. International J. of Applied Environmntal Sciences, 6(1), 49–55.
Peng, W.-X., Marchal, J. L. M., & Van der Poel, A. F. B. (2018). Strategies to prevent and reduce mycotoxins for compound feed manufacturing. Animal Feed Science and Technology, 237, 129–153.
Pier, S. M. (1975). The role of heavy metals in human health. Texas Reports on Biology and Medicine, 33(1), 85–106.
Ragab, A. R., Farouk, O., Afify, M. M., Attia, A. M., El Samanoudy, A., & Taalab, Y. M. (2014). The role of oxidative stress in carcinogenesis induced by metals in breast cancer Egyptian females sample at Dakahlia Governorate. Journal of Environmental & Analytical Toxicology, 4(2), 207.
Ramadan, M. A. E., & Adam, S. M. (2007). The effect of chicken manure and mineral fertilizers on distribution of heavy metals in soil and tomato organs. Australian Journal of Basic and Applied Sciences, 1(3), 226–231.
Rashid, M. A., Sarker, M. S. K., Khatun, H., Sarker, N. R., Ali, M. Y., & Islam, M. N. (2018). Detection of heavy metals in poultry feed, meat and eggs. Asian-Australasian Journal of Food Safety and Security, 2(1), 1–5.
Reda, Y., Mahmoud, S. M., Abdou, R. H., & Elhady, K. A. (2021). Evaluation of Heavy Metal Residues in Poultry Farms in Ismailia Province. Egyptian Academic Journal of Biological Sciences, B. Zoology, 13(2), 271–284.
Rehman, H. U., Rehman, A., & Ullah, F. (2013). Comparative study of heavy metals in different parts of domestic and broiler chickens. International Journal of Pharmaceutical Sciences Review and Research, 23, 151–154.
Rose, M., Baxter, M., Brereton, N., & Baskaran, C. (2010). Dietary exposure to metals and other elements in the 2006 UK Total Diet Study and some trends over the last 30 years. Food Additives and Contaminants, 27(10), 1380–1404.
Roychowdhury, T., Tokunaga, H., & Ando, M. (2003). Survey of arsenic and other heavy metals in food composites and drinking water and estimation of dietary intake by the villagers from an arsenic-affected area of West Bengal, India. Science of the Total Environment, 308(1–3), 15–35.
Sahin, K., Sahin, N., Onderci, M., Gursu, F., & Cikim, G. (2002). Optimal dietary concentration of chromium for alleviating the effect of heat stress on growth, carcass qualities, and some serum metabolites of broiler chickens. Biological Trace Element Research, 89(1), 53–64.
Saleh, A. A., & Ebeid, T. A. (2019). Feeding sodium selenite and nano-selenium stimulates growth and oxidation resistance in broilers. South African Journal of Animal Science, 49(1), 176–183.
Salem, H. M., Eweida, E. A., & Farag, A. (2000). Heavy metals in drinking water and their environmental impact on human health. In Int conference on the environ hazards mitigation (pp. 542–556), Cairo Univ Egypt.
Taha, F. A. (2003). Poultry Sector in Middle-income Countries and Its Feed Requirements: The Case of Egypt. United States Department of Agriculture, Economic Research Service.
TFC. (2008). Regulation of setting maximum levels for certain contaminants in foodstuffs)_2008/26. Turkish Official Gazette, May 17th, 2008, Iss: 26879. https://www.researchgate.net/publication/273130016
Toghyani, M., Toghyani, M., Shivazad, M., Gheisari, A., & Bahadoran, R. (2012). Chromium supplementation can alleviate the negative effects of heat stress on growth performance, carcass traits, and meat lipid oxidation of broiler chicks without any adverse impacts on blood constituents. Biological Trace Element Research, 146(2), 171–180.
UNEP. (2008). United nations environment programme, environmental pollution and impacts on public health: Implication of the Dandora municipal dumping site. Nairobi, Kenya. https://doi.org/10.14737/journal.aavs
USEPA. (1989). Risk assessment guidance for superfund. In Human health evaluation manual part a, interim final, vol 1. Washington (DC): United States Environmental Protection Agency. EPA/540/1-89/002. https://doi.org/10.14737/journal.aavs
USEPA. (2000). Guidance for assessing chemical contaminant data for use in fish advisories: EPA 823-B-00-007, 3rd ed., vol. 1. Washington, DC., USA: Office of Science and Technology and Office of water, USEPA. https://doi.org/10.14737/journal.aavs
USEPA. (2004). Risk assessment guidance for superfund volume I: Human health evaluation manual (part E, supplemental guidance for dermal risk assessment). Washington, DC, USA: USEPA. https://www.epa.gov/risk/risk
USEPA. (2006). United States, environmental protection agency, integrated risk information system. Available at: http://www.epa.gov/iris/substS
USEPA. (2010). Integrated risk information system (IRIS). Cadmium (CASRN- 7440-43-9. http://www.epa.gov/iris/subst/0141.html
USEPA. (2018). Regional screening levels (RSLs) - generic tables. Washington, D.C. Available from: https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables. Accessed 2018 Oct 4.
Vardar, F., & Ünal, M. (2007). Aluminum toxicity and resistance in higher plants. Advanced molecular biology, 1, 1–12.
Wang, B., Liu, Y., Wang, H., Cui, L., Zhang, Z., Guo, J., et al. (2020). Contamination and health risk assessment of lead, arsenic, cadmium, and aluminum from a total diet study of Jilin Province, China. Food Science & Nutrition, 8(10), 5631–5640.
WHO. (1989). World Health Organization, Evaluation of certain food additives and contaminants. Thirty-third Report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, 776, 26–27.
WHO. (2004). Barium in Drinking-water. Background document for development of WHO Guidelines for Drinking-water Quality.
WHO. (2003). Food safety issues GEMS/food regional diets. Regional per capita consumption of raw and semi-processed agricultural commodities prepared by Global Environment Monitoring System/Food Contamination Monitoring and Assessment Programme (GEMS/Food). Food Safety issue. https://pesquisa.bvsalud.org/portal/resource/pt/who-42833
WHO/FAO. (2011). Working document for information and use in discussion related to contaminants and toxins in the GSCTFF. Joint food standards programme codex committee on contaminants in foods: Fifth session; The Hague, The Netherlands.
Wilbur, S., Abadin, H., Fay, M., Yu, D., Tencza, B., & Ingerman, L. (2012). Toxicological profile for chromium. Atlanta (GA): US Department of Health and Human Services. Public Health Service, Agency for Toxic Substances and Disease Registry, 24049864.
Ysart, G., Miller, P., Crews, H., Robb, P., Baxter, M., L’Argy, C. D., et al. (1999). Dietary exposure estimates of 30 elements from the UK Total Diet Study. Food Additives & Contaminants, 16(9), 391–403.
Zahrana, D. A., & Hendy, B. (2015). Heavy metals and trace elements composition in certain meat and meat products sold in Egyptian markets. International Journal of Sciences: Basic and Applied Research. IJSBAR, 20(1), 282–293.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). “No funding was obtained for this study.”
Author information
Authors and Affiliations
Contributions
(1) Dr. Heba Kamaly contributed substantially to the practical section, the composition of the paper, data analysis, and statistical analysis. (2) Dr. Ahmed Sharkawy has contributed to the practical section, the composition, and the revision of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Is applicable
Ethical responsibilities of authors
All authors have read, understood, and complied, as applicable, with the ethical responsibilities of authors as found in the Instructions for Authors and are aware that, with minor exceptions, no changes can be made to authorship once the paper is submitted.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kamaly, H.F., Sharkawy, A.A. Health risk assessment of metals in chicken meat and liver in Egypt. Environ Monit Assess 195, 802 (2023). https://doi.org/10.1007/s10661-023-11365-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-023-11365-9