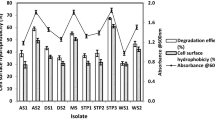
Abstract
In this study, the biosurfactants (Bio-SFs) producing bacteria are screened from the selected alkaline lake of Ethiopia, and the potential bacterial strain and their produced Bio-SFs are further characterized. In an initial screening, 25 bacterial isolates were isolated, and among those, the bacterial isolate assigned as CS1 was identified as the most potent producer of Bio-SFs using a subsequent characterization process. The CS1 strain was identified as Serratia sp. via biochemical and molecular methods. An emulsion index (E24) of 69.06 ± 0.11% was obtained for CS1 after 5 days of incubation time at 30 °C. The CS1-extracted Bio-SFs were characterized by Fourier transform infrared (FTIR), and it indicated that the type of biosurfactant produced was a glycolipid. The stability of the crude Bio-SFs was characterized, and the optimal conditions were found to be 80 °C, pH 8, and 3% NaCl, respectively. The extracted Bio-SFs were compatible with tested commercial detergents, and its efficiency increased from 12.2 ± 0.1% to 67.1 ± 0.17% and 70.43 ± 0.11% when combined with commercially available detergent brands in Ethiopia such as Taza and Largo, respectively. This study suggests that the isolated S. marcescens CS1 strain has the potential to produce Bio-SFs that are viable competence to replace the use of synthetic chemicals in the production of commercial detergents.






Similar content being viewed by others
Availability of data and material
The research data used to support the findings of this study are available from the corresponding author upon request.
Code availability
Not applicable.
References
Abdulrasheed, M., Zakaria, N. N., Roslee, A. F. A., Shukor, M. Y., Zulkharnain, A., et al. (2020). Biodegradation of diesel oil by cold-adapted bacterial strains of Arthrobacter spp. from Antarctica. Antarctic Science, 32(5), 341–353. https://doi.org/10.1017/S0954102020000206
Adetunji, A. I., & Olaniran, A. O. (2021). Production and potential biotechnological applications of microbial surfactants: An overview. Saudi Journal of Biological Sciences, 28(1), 669–679. https://doi.org/10.1016/j.sjbs.2020.10.058
Ahuja, V., Macho, M., Thabet, J., Banerjee, A., Ewe, D., Saha, S., & Saurav, K. (2022). Optimization and characterization of biosurfactant from Streptomyces. In: Dharumadurai D. (eds) Methods in Actinobacteriology. Springer Protocols Handbooks. Humana, New York, NY. pp 647–659. https://doi.org/10.1007/978-1-0716-1728-1_96
Al-Ghanayem, A. A., Joseph, B., Alhussaini, M. S., & Ramteke, P. W. (2022). Current applications and future trends of extremozymes in detergent industries. In Microbial Extremozymes (pp. 223–230). Academic Press. https://doi.org/10.1016/B978-0-12-822945-3.00020-8
Ambaye, T. G., Vaccari, M., Prasad, S., & Rtimi, S. (2021). Preparation, characterization and application of biosurfactant in various industries: A critical review on progress, challenges and perspectives. Environmental Technology & Innovation, 24, 102090. https://doi.org/10.1016/j.eti.2021.102090
Anyanwu, C. U., Obi, S. K. C., & Okolo, B. N. (2011). Lipopeptide biosurfactant production by Serratia marcescens NSK-1 strain isolated from petroleum-contaminated soil. Journal of Applied Sciences Research, 79–87. http://www.aensionline.com/jasr/jasr/2011/79-87.pdf
Araujo, H. W., Andrade, R. F., Montero-Rodríguez, D., Rubio-Ribeaux, D., Da Silva, C. A. A., & Campos-Takaki, G. M. (2019). Sustainable biosurfactant produced by Serratia marcescens UCP 1549 and its suitability for agricultural and marine bioremediation applications. Microbial Cell Factories, 18(1), 1–13. https://doi.org/10.1186/s12934-018-1046-0
Azizi-Shotorkhoft, A., Mohammadabadi, T., Motamedi, H., Chaji, M., & Fazaeli, H. (2016). Isolation and identification of termite gut symbiotic bacteria with lignocellulose-degrading potential, and their effects on the nutritive value for ruminants of some by-products. Animal Feed Science and Technology, 221, 234–242.https://doi.org/10.1016/j.anifeedsci.2016.04.016
Balakrishnan, S., Arunagirinathan, N., Rameshkumar, M. R., Indu, P., Vijaykanth, N., Almaary, K. S., & Chen, T. W. (2022). Molecular characterization of biosurfactant producing marine bacterium isolated from hydrocarbon-contaminated soil using 16S rRNA gene sequencing. Journal of King Saud University-Science, 34(3), 101871. https://doi.org/10.1016/j.jksus.2022.101871
De Giani, A., Zampolli, J., & Di Gennaro, P. (2021). Recent trends on biosurfactants with antimicrobial activity produced by bacteria associated with human health: Different perspectives on their properties, challenges, and potential applications. Frontiers in Microbiology, 12, 678. https://doi.org/10.3389/fmicb.2021.655150
Delgado-García, M., Contreras-Ramos, S. M., Rodríguez, J. A., Mateos-Díaz, J. C., Aguilar, C. N., & Camacho-Ruíz, R. M. (2018). Isolation of halophilic bacteria associated with saline and alkaline-sodic soils by culture dependent approach. Heliyon, 4(11), e00954. https://doi.org/10.1016/j.heliyon.2018.e00954
Derguine-Mecheri, L., Kebbouche-Gana, S., & Djenane, D. (2021). Biosurfactant production from newly isolated Rhodotorula sp. YBR and its great potential in enhanced removal of hydrocarbons from contaminated soils. World Journal of Microbiology and Biotechnology, 37(1), 1–18. https://doi.org/10.1007/s11274-020-02983-3
Doshi, D. V., Maniyar, J. P., Bhuyan, S. S., & Mujumdar, S. S. (2010). Studies on bioemulsifier production by Actinopolyspora sp. A18 isolated from garden soil. Indian Journal of Biotechnology, 9, 391–397. http://nopr.niscair.res.in/handle/123456789/10437
Dusane, D. H., Pawar, V. S., Nancharaiah, Y. V., Venugopalan, V. P., Kumar, A. R., & Zinjarde, S. S. (2011). Anti-biofilm potential of a glycolipid surfactant produced by a tropical marine strain of Serratia marcescens. Biofouling, 27(6), 645–654. https://doi.org/10.1080/08927014.2011.594883
Ferraz, C., De Araújo, Á. A., & Pastore, G. M. (2002). The influence of vegetable oils on biosurfactant production by Serratia marcescens. Applied Biochemistry and Biotechnology, 98(1), 841–847. https://doi.org/10.1385/ABAB:98-100:1-9:841
Gandhimathi, R., Kiran, G. S., Hema, T. A., Selvin, J., Raviji, T. R., & Shanmughapriya, S. (2009). Production and characterization of lipopeptide biosurfactant by a sponge-associated marine actinomycetes Nocardiopsis alba MSA10. Bioprocess and Biosystems Engineering, 32(6), 825–835. https://doi.org/10.1007/s00449-009-0309-x
Gaur, V. K., Sharma, P., Sirohi, R., Varjani, S., Taherzadeh, M. J., Chang, J. S., & Kim, S. H. (2022). Production of biosurfactants from agro-industrial waste and waste cooking oil in a circular bioeconomy: An overview. Bioresource Technology, 343, 126059. https://doi.org/10.1016/j.biortech.2021.126059
Gayathiri, E., Prakash, P., Karmegam, N., Varjani, S., Awasthi, M. K., & Ravindran, B. (2022). Biosurfactants: Potential and eco-friendly material for sustainable agriculture and environmental safety—A review. Agronomy, 12(3), 662. https://doi.org/10.3390/agronomy12030662
Goutx, M., Mutaftshiev, S., & Bertrand, J. C. (1987). Lipid and exopolysaccharide production during hydrocarbon growth of a marine bacterium from the sea surface. Marine Ecology Progress Series. Oldendorf, 40(3), 259–265.
Guadie, A., Gessesse, A., & Xia, S. (2018). Halomonas sp. strain A55, a novel dye decolorizing bacterium from dye-uncontaminated Rift Valley Soda lake. Chemosphere, 206, 59–69. https://doi.org/10.1016/j.chemosphere.2018.04.134
Jain, R. M., Mody, K., Joshi, N., Mishra, A., & Jha, B. (2013). Effect of unconventional carbon sources on biosurfactant production and its application in bioremediation. International Journal of Biological Macromolecules, 62, 52–58. https://doi.org/10.1016/j.ijbiomac.2013.08.030
Jimoh, A. A., & Lin, J. (2019a). Enhancement of Paenibacillus sp. D9 lipopeptide biosurfactant production through the optimization of medium composition and its application for biodegradation of hydrophobic pollutants. Applied Biochemistry and Biotechnology, 187(3), 724–743. https://doi.org/10.1007/s12010-018-2847-7
Jimoh, A. A., & Lin, J. (2019b). Biosurfactant: A new frontier for greener technology and environmental sustainability. Ecotoxicology and Environmental Safety, 184, 109607. https://doi.org/10.1016/j.ecoenv.2019.109607
Jimoh, A. A., & Lin, J. (2020). Biotechnological applications of Paenibacillus sp. D9 lipopeptide biosurfactant produced in low-cost substrates. Applied Biochemistry and Biotechnology, 1–21. https://doi.org/10.1007/s12010-020-03246-5
Khaje Bafghi, M., & Fazaelipoor, M. H. (2012). Application of rhamnolipid in the formulation of a detergent. Journal of Surfactants and Detergents, 15(6), 679–684. https://doi.org/10.1007/s11743-012-1386-4
Khopade, A., Biao, R., Liu, X., Mahadik, K., Zhang, L., & Kokare, C. (2012). Production and stability studies of the biosurfactant isolated from marine Nocardiopsis sp. B4. Desalination, 285, 198–204. https://doi.org/10.1016/j.desal.2011.10.002
Kumar, A., Singh, S. K., Kant, C., Verma, H., Kumar, D., Singh, P. P., et al. (2021). Microbial biosurfactant: A new frontier for sustainable agriculture and pharmaceutical industries. Antioxidants, 10(9), 1472. https://doi.org/10.3390/antiox10091472
Lamilla, C., Schalchli, H., Briceño, G., Leiva, B., Donoso-Piñol, P., et al. (2021). A pesticide biopurification system: A source of biosurfactant-producing bacteria with environmental biotechnology applications. Agronomy, 11(4), 624. https://doi.org/10.3390/agronomy11040624
Mahalingam, P. U., & Sampath, N. (2014). Isolation, characterization and identification of bacterial biosurfactant. European Journal of Experimental Biology, 4(6), 59–64.
Markande, A. R., Patel, D., & Varjani, S. (2021). A review on biosurfactants: Properties, applications and current developments. Bioresource Technology, 330, 124963. https://doi.org/10.1016/j.biortech.2021.124963
Maneerat, S., & Phetrong, K. (2007). Isolation of biosurfactant-producing marine bacteria and characteristics of selected biosurfactant. Songklanakarin Journal of Science and Technology, 29(3), 781–791.
Miceli, T. R., Corr, T. D., Barroso, M. M., Dogra, N., & Gross, A. R. (2022). Sophorolipids: Anti-cancer activities and mechanisms. Bioorganic & Medicinal Chemistry. https://doi.org/10.1016/j.bmc.2022.116787
Mnif, I., Ellouz-Chaabouni, S., & Ghribi, D. (2018). Glycolipid biosurfactants, main classes, functional properties and related potential applications in environmental biotechnology. Journal of Polymers and the Environment, 26(5), 2192–2206. https://doi.org/10.1007/s10924-017-1076-4
Mishra, M., Muthuprasanna, P., Prabha, K. S., Rani, P. S., Satish, I. A., Ch, I. S., & Shalini, S. (2009). Basics and potential applications of surfactants-A review. International Journal of PharmTech Research, 1(4), 1354–1365.
Montero-Rodríguez, D., Andrade, R. F., Ribeiro, D. L. R., Rubio-Ribeaux, D., Lima, R. A., Araújo, H. W., & Campos-Takaki, G. M. (2015). Bioremediation of petroleum derivative using biosurfactant produced by Serratia marcescens UCP/WFCC 1549 in low-cost medium. International Journal of Current Microbiology and Applied Sciences, 4, 550–562.
Mukherjee, S., Das, P., Sivapathasekaran, C., & Sen, R. (2009). Antimicrobial biosurfactants from marine Bacillus circulans: Extracellular synthesis and purification. Letters in Applied Microbiology, 48(3), 281–288. https://doi.org/10.1111/j.1472-765X.2008.02485.x
Naeem, A. H., Mumtaz, S., Haleem, A., Qazi, M. A., Malik, Z. A., Dasti, J. I., & Ahmed, S. (2017). Isolation and molecular characterization of biosurfactant-producing bacterial diversity of Fimkassar oil field, Pakistan. Arabian Journal for Science and Engineering, 42(6), 2349–2359. https://doi.org/10.1007/s13369-017-2527-x
Nikolova, C., & Gutierrez, T. (2021). Biosurfactants and their applications in the oil and gas industry: Current state of knowledge and future perspectives. Frontiers in Bioengineering and Biotechnology. https://doi.org/10.3389/fbioe.2021.626639
Obafemi, Y. D., Taiwo, O. S., Omodara, O. J., Dahunsi, O. S., & Oranusi, S. (2018). Biodegradation of crude petroleum by bacterial consortia from oil-contaminated soils in Ota, Ogun State, South-Western, Nigeria. Environmental technology & innovation, 12, 230–242. https://doi.org/10.1016/j.eti.2018.09.006
Patel, D., Solanki, J., Ingale, S., & Nataraj, M. (2022). Optimization of production parameter and application of glycoprotein produced from Sphingobacterium thalpophilum DP9. Vegetos, 35(1), 26–37. https://doi.org/10.1007/s42535-021-00296-w
Pornsunthorntawee, O., Chavadej, S., & Rujiravanit, R. (2009). Solution properties and vesicle formation of rhamnolipid biosurfactants produced by Pseudomonas aeruginosa SP4. Colloids and Surfaces b: Biointerfaces, 72(1), 6–15. https://doi.org/10.1016/j.colsurfb.2009.03.006
Rahman, P. K., Pasirayi, G., Auger, V., & Ali, Z. (2010). Production of rhamnolipid biosurfactants by Pseudomonas aeruginosa DS10-129 in a microfluidic bioreactor. Biotechnology and Applied Biochemistry, 55(1), 45–52. https://doi.org/10.1042/BA20090277
Reysenbach, A.L., Pace, N.R. (1995) In: Robb FT, Place AR (eds) Archaea: a laboratory. Manual – thermophiles. Cold Spring Harbour Laboratory Press, New York, pp 101–107
Roy, A. (2014). Production and characterization of biosurfactant from bacterial isolates (Doctoral dissertation, Netaji Subhas Institute of Technology).
Samanta, A., Pal, P., Mandal, A., Sinha, C., Lalee, A., Das, M., ... & Mitra, D. (2012). Estimation of biosurfactant activity of an alkaline protease producing bacteria isolated from municipal solid waste. Central European Journal of Experimental Biology, 1, 26–35. http://scholarsresearchlibrary.com/archive.html
Santos, D. K. F., Rufino, R. D., Luna, J. M., Santos, V. A., & Sarubbo, L. A. (2016). Biosurfactants: Multifunctional biomolecules of the 21st century. International Journal of Molecular Sciences, 17(3), 401. https://doi.org/10.3390/ijms17030401
Semai, A., Plewniak, F., Charrié-Duhaut, A., Sayeh, A., Gil, L., Vandecasteele, C., ... & Bertin, P. N. (2021). Characterisation of hydrocarbon degradation, biosurfactant production, and biofilm formation in Serratia sp. Tan611: A new strain isolated from industrially contaminated environment in Algeria. Antonie van Leeuwenhoek, 114(4), 411–424. https://doi.org/10.1007/s10482-021-01527-5
Sharma, J., Kapley, A., Sundar, D., & Srivastava, P. (2022). Characterization of a potent biosurfactant produced from Franconibacter sp. IITDAS19 and its application in enhanced oil recovery. Colloids and Surfaces B: Biointerfaces, 214, 112453. https://doi.org/10.1016/j.colsurfb.2022.112453
Singh, P., & Tiwary, B. N. (2016). Isolation and characterization of glycolipid biosurfactant produced by a Pseudomonas otitidis strain isolated from Chirimiri coal mines, India. Bioresources and Bioprocessing, 3(1), 1–16. https://doi.org/10.1186/s40643-016-0119-3
Sittisart, P., & Gasaluck, P. (2022). Biosurfactant production by Lactobacillus plantarum MGL-8 from mango waste. Journal of Applied Microbiology. https://doi.org/10.1111/jam.15452
Tambekar, D. H., Dose, P. N., Gunjakar, S. R., & Gadakh, P. V. (2012). Studies on biosurfactant production from Lonar Lake’s Achromobacter xylosoxidans bacterium. International Journal of Advances in Pharmacy, Biology and Chemistry, 1(3), 415–419.
Thavasi, R., Nambaru, V. S., Jayalakshmi, S., Balasubramanian, T., & Banat, I. M. (2011). Biosurfactant production by Pseudomonas aeruginosa from renewable resources. Indian Journal of Microbiology, 51(1), 30–36. https://doi.org/10.1007/s12088-011-0076-7
Tuleva, B. K., Ivanov, G. R., & Christova, N. E. (2002). Biosurfactant production by a new Pseudomonas putida strain. Zeitschrift Für Naturforschung C, 57(3–4), 356–360. https://doi.org/10.1515/znc-2002-3-426
Udoh, T., & Vinogradov, J. (2019). Experimental investigations of behaviour of biosurfactants in brine solutions relevant to hydrocarbon reservoirs. Colloids and Interfaces, 3(1), 24. https://doi.org/10.3390/colloids3010024
Vatsa, P., Sanchez, L., Clement, C., Baillieul, F., & Dorey, S. (2010). Rhamnolipid biosurfactants as new players in animal and plant defense against microbes. International Journal of Molecular Sciences, 11(12), 5095–5108. https://doi.org/10.3390/ijms11125095
Vijayalekshmi, V., Muthukumaran, P., Jeyaseelan, A. (2021). Screening, isolation and molecular characterization of biosurfactants producing Serratia marcescens from petrochemical exposed site. In: Marimuthu, P.D., Sundaram, R., Jeyaseelan, A., Kaliannan, T. (eds) Bioremediation and Green Technologies. Environmental Science and Engineering. Springer, Cham. pp 243–255. https://doi.org/10.1007/978-3-030-64122-1_17
Vipra, A., Desai, S. N., Junjappa, R. P., Roy, P., Poonacha, N., Ravinder, P., ... & Padmanabhan, S. (2013). Determining the minimum inhibitory concentration of bacteriophages: Potential advantages. Advances in Microbiology, 3 (2),1–10. https://doi.org/10.4236/aim.2013.32028
Zeng, F., Zhou, H., Lin, X., Li, Y., Liang, Y., Xie, Q., ... & Zhang, C. (2022). Enhanced remediation of fracturing flowback fluids by the combined application of a bioflocculant/biosurfactant-producing Bacillus sp. SS15 and its metabolites. Chemosphere, 134870. https://doi.org/10.1016/j.chemosphere.2022.134870
Acknowledgements
The authors thank the Addis Ababa University and Addis Ababa Science and Technology University, Ethiopia, for providing a facility for experimental analysis.
Funding
This work is supported by the funding of the Addis Ababa Science and Technology University, Ethiopia, under the scheme of post-graduate student research.
Author information
Authors and Affiliations
Contributions
Kidist Mulugeta: Formal analysis, methodology, writing—original draft preparation, funding acquisition. Murugesan Kamaraj: Supervision, writing—review and editing, project administration. Mesfin Tafesse: Project administration, resources, supervision. Gessesse Kebede: Conceptualization, project administration. Getachew Gemechu: Investigation, formal analysis. Masi Chandran: Validation, visualization. All authors have approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mulugeta, K., Kamaraj, M., Tafesse, M. et al. Biomolecules from Serratia sp. CS1 indigenous to Ethiopian natural alkaline lakes: biosurfactant characteristics and assessment of compatibility in a laundry detergent. Environ Monit Assess 194, 873 (2022). https://doi.org/10.1007/s10661-022-10533-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-022-10533-7