Abstract
In this study, we analyzed the concentration distributions of 20 polycyclic aromatic hydrocarbons (PAHs) in 41 water samples which were collected from the northern part of Taihu Lake during 4 field campaigns (201511, 201606, 201702 and 201709). The concentrations were determined with GC–MS, and their spatial and seasonal distribution characteristics were interpreted. The results show that 2-ring PAHs present considerably higher concentrations in warm seasons than cold seasons, but the concentrations of the other higher-ring PAHs are rather stable in warm and cold seasons. The distribution patterns of these PAHs might be mainly attributed to ambient temperature effects on the PAH solubility in the water body. Meanwhile, the spatial distributions of the PAH concentrations in cold seasons were rather various in the sampling area, while the distributions in the warm seasons were homogeneous. The different distributions could result from the water recharge from the Yangtze River during cold seasons, which diluted PAH concentrations in the northeastern part of the lake. Furthermore, via literature review on PAH concentrations in water body, PAHs are in a wide range of levels and their patterns are different among the studies, which should be more effected by local factors instead of general PAH properties. The results from this study also present special characteristics of PAHs in Taihu Lake, which exhibit more insight on PAHs existence in water bodies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Taihu Lake catchment is one of the areas with the highest level of urbanization and rural industrialization in China (Shen & Ma, 2005; Yeh et al., 2011), and it has a population of around 40 million accounting for more than 3% of the Chinese population. The lake is located in the center of the Yangtze Delta, covering an area of about 2,340 km2 with an average water depth of 1.9 m. It is the third-largest freshwater lake in China and is an important role in flood control and irrigation in the region, also for tourism, shipping, aquaculture and especially as a drinking water source for its neighboring cities (Qin et al., 2007; Tao et al., 2010). There are more than 110 inflows and outflows around the lake. The inflows are mostly located around the northern and western part of the lake, while the outflows are in the eastern and southern part, partly connecting Taihu Lake with the Yangtze River (Qin, 2008). The inflows around Taihu Lake discharge a large amount of industrial effluents, improperly treated municipal sewage, diffused pollutants from agriculture and aquaculture, and accumulated atmospheric depositions into the lake (Li et al., 2021b; Shen et al., 2005; Li et al., 2021a). These discharges attaching complex pollutants gradually lead to serious ecological and water quality deterioration in the lake ecosystems, especially in the northern part of the lake surrounded by more inflows and developed areas (Jiang et al., 2012; Tao et al., 2018; Wang et al., 2003; Wilhelm et al., 2011; Xu et al., 2014). In addition, the water retention time in Taihu Lake is even more than 10 months, which is rather longer than many other lakes and results in more potential for water deterioration (Qin, 2008).
PAHs are ubiquitously distributed organic pollutants in environmental compartments. They consist of two or more aromatic rings and the simplest PAH structure is two-ring naphthalene (naph). The 2-ring and 3-ring PAHs are more volatile and hydrophilic, while with molecular mass increase they become hydrophobic and lipophilic and more resistant to environmental degradation. PAHs can be emitted from both petrogenic and pyrogenic processes, while nowadays, their sources are mainly from pyrolytic processes such as biomass and fossil fuel combustions. US EPA listed 16 PAHs as priority pollutants, and some of PAHs such as benzo[a]pyrene (BaP), dibenzo[a,h]anthracene (DahA), benzo[a]anthracene (BaA) are classified as mutagenic, carcinogenic and genotoxic contaminants (Boffetta et al., 1997; Kim et al., 2013; US EPA, 1993; Arey et al., 2010). It has been reported that bioconcentration factors of hydrophobic organic pollutants including PAHs are positively correlated with their partitioning coefficient Kow (Axelman et al., 1999; Geyer et al., 1984), so heavier and more toxic PAHs have higher potentials to be bioaccumulated in organisms.
Combining the significant roles of Taihu Lake and the ubiquitous source and toxicity of PAHs, it is necessary to investigate the PAH distribution characteristics to assist the mitigation strategies for PAH pollution in surface water. In this study, we analyzed 41 water samples collected from the northern part of Taihu Lake to explain the spatial and seasonal variations of PAH concentrations, and the results were further interpreted to illustrate the effects of ambient conditions and anthropogenic activities on PAH distributions in the water body. Furthermore, previous studies on PAHs in water bodies were reviewed to conclude the general distribution characteristics of PAHs in surface water.
Materials and methods
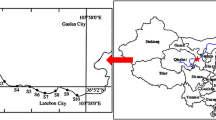
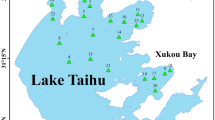
During 4 field campaigns (2015 Nov., 2016 June, 2017 Feb. and 2017 Sep.), 41 water samples in total were collected from the northern part of Taihu Lake covering Gonghu bay, Meiliang bay and Zhushan bay (Fig. 1). In each location, 2 L of water samples were taken at 0.5 m depth and kept in 2 1-L amber glass bottles for PAH measurement and backup, respectively. After sampling, the samples were immediately transported to the laboratory for PAH extraction.
Around 1 L of each sample was filtered with 0.45-µm pore size filters, then 1 mL (400.2 ng PAH standards) of stock solution was injected as an internal standard to each sample before PAH extraction. PAHs were extracted with ISOLUTE® ENV + 200 mg/3 mL SPE cartridge which was activated with 3 × 3 mL ethyl acetate before PAH loading, and the extraction speed was around 1 L/h. After extraction, the loaded cartridges were dried naturally and then kept in individual sealed polyethylene bags. They were transported to Germany by flight for further PAH analyses.
The cartridges were eluted with 3 × 3 mL of acetone, and the eluates were immediately concentrated to around 1 mL with nitrogen stream. Then 1 µL of the concentrated eluate was injected into a GC–MS (Agilent 7890A/5975C) for PAH concentration measurement. PAHs in GC–MS were carried by helium in pulsed splitless mode and detected in the selected ion monitoring (SIM) mode of the MS. The column is HP- 5MS 5% Phenyl Methyl Silox with 30 m length, 250 µm i.d. and 0.25-µm film thickness. The running time of one complete sequence is 43.333 min. The oven temperature is 75 °C for 3 min and is further programmed to 235 °C at 20 °C/min for 18 min and then to 300 °C at 15 °C/min for 8 min, finally to 320 °C at 10 °C/min.
For quantification, PAH standards (PAH-mix 14, PAH-mix 45 and deuterated PAH-mix 31) were obtained from Dr. Ehrenstorfer Augsburg, Germany. The three standards were diluted together in cyclohexane to four different concentrations for external calibration and response factors calculation. The internal standard (dilution of deuterated PAH-mix 31) with 5 deuterated PAHs was then used to quantify the analytes. All solvents and cleanup chemicals were purchased from Carl Roth GmbH + Co.KG, Germany.
In total, there are 20 PAHs analyzed in this study, including 2-ring: naphthalene (naph), 2-methylnaphthalene (2methylnaph), 1-methylnaphthalene (1methylnaph); 3-ring: acenaphthylene (acenaphthy), acenaphthene (acenaphthe), fluorene, phenanthrene (phen), anthracene (anthra); 4-ring: fluoranthene (fluor), pyrene, benzo[a]anthracene (BaA), chrysene (chry); 5-ring: benzo[b]fluoranthene (BbF), benzo[k]fluoranthene (BkF), benzo[e]pyrene (BeP), benzo[a]pyrene (BaP), perylene, dibenzo[a,h]anthracene (DahA), 6-ring: benzo[g,h,i]perylene (BghiP), indeno[1,2,3-c,d]pyrene (INcdP). The quantification limit for the GC–MS analysis was between 10 and 25 pg of injected mass in a standard, depending on the PAH compound. Since the water volume of each sample is 1 L, this limit corresponds to 10–25 ng/L of individual PAHs in the water samples.
Results and discussion
Seasonal variation of PAH concentrations
The detectable PAHs in the water samples are dominated by naph, 2methylnaph and 1methylnaph, and then fluorene and phen are the relatively abundant compounds (The concentration data are attached in the data supplementary S. Table 2). Comparing the four campaigns, the concentrations of naph, 2methylnaph and 1methylnaph in the samples collected in warm seasons of 2016 June (201606) and 2017 Sep. (201709) are up to one order of magnitude higher than that in the samples collected in cold season (2015 Nov. (201511) and 2017 Feb. (201702)) (Fig. 2). Particularly, the concentrations of 2methylnaph in the campaign 201709 are even up to 20 folds higher compared to that in the samples collected in cold season. However, the concentrations of fluorene and phen are rather low and do not show noticeable seasonal variations, and the concentrations of the other PAHs are typically below the quantification limit in both warm and cold seasons.
a Taihu Lake catchment with main rivers and big cities; b water sampling locations (10 samples labeled with 11- were sampled in 2015 Nov.; 11 samples labeled 6- were sampled in 2016 June; 10 samples labeled 2- were sampled in 2017 Feb.; 10 samples labeled 9- were sampled in 2017 Sep.). The coordination of the sampling locations is attached in data supplementary S. Table 1
The water temperature in cold and warm seasons was around 10 °C and 30 °C, respectively. It has been known that the water solubility of some PAHs including naph increase with the increase of temperature (Pérez-tejeda et al., 1990; Whitehouse, 1984), and also, the water partitioning of PAHs is temperature-dependent (Jenkins et al., 1996; Slmonich & Hites, 1994; Tsapakis & Stephanou, 2005). For example, the partitioning of naph and methylnaph from diesel, gasoline and coal tar to water is significantly higher than the partitioning of the other heavier PAHs in ambient temperature, and the temperature effect on partitioning of the other PAHs cannot be detected until 100 ºC or even higher (Lee et al., 1992a, b; Yang et al., 1997). Therefore, the noticeably high concentrations of naph and methylnaph in warm seasons should be mainly attributed to temperature effects. In addition, the concentrations of 1methylnaph and 2methylnaph are similar or higher than that of naph in individual sampling campaign. However, the combustion emission of methylnaph is generally severalfolds lower than that of naph (Herrington et al., 2012; Jenkins et al., 1996; Mcdonald et al., 2000), so it can be suspected that the high concentrations of methylnaph in the warm season samples (201709 and 201606) should result from other sources besides combustion.
Spatial distribution of PAH concentrations
It is rather consistent in the spatial distributions of the concentrations of naph, methylnaph (1mehtylnaph + 2methylnaph) and the sum of the other PAHs (acenaphthy + acenaphthe + fluorene + phen + anthra + fluor + pyrene) in cold seasons, the higher concentrations are located in Zhushan bay and the lower concentrations are located in Gonghu bay and the eastern part of Meiliang bay (Fig. 3a, c, e). The spatial distributions of the concentrations of naph and the other PAHs in warm season, however, are somewhat homogeneous (Fig. 3b, f). However, the spatial distribution of methylnaph concentrations in warm season does not show specific spatial patterns, and they present marked differences between the two campaigns, that is, significantly high concentrations in samples 201709 and relatively low concentrations in samples 201606 (Fig. 3d). The inconsistent distributions of methylnaph with the other PAHs further support the suspicion that there are additional origins of methylnaph particularly in the sampling period of Sep. 2017.
Seasonal variations of PAH concentrations in the lake water (the samples collected in 2015 Nov. are numbered 201511 with labels of filled diamond; the samples collected in 2017 Feb. are numbered 201702 with labels of filled circle; the samples collected in 2017 Sep. are numbered 201709 with labels of unfilled diamond; the samples collected in 2016 June are numbered 201606 with labels of unfilled circle. Samples 201511 and 201702 were collected in cold seasons, 201909 and 201606 were collected in warm seasons)
Spatial variation of PAH concentrations in the water body. The figures a and b are the concentration distributions of naph, c and d are the concentrations of methylnaph (1methylnaph + 2methylnaph), e and f are the sum concentrations of the other PAHs (acenaphthy + acenaphthe + fluorene + phen + anthra + fluor + pyrene). a, c and e are the results from collected in 2015 Nov. and 2017 Feb. in cold seasons, b, d and f are the results from the samples collected in 2016 June and 2017 Sep. in warm seasons. The concentration scale in the figures is different. QL: quantification limit
Generally, from Nov. to the following April is the dry season in this area and the water level in the lake decreases significantly, while there is rather more precipitation in the other months in the catchment, especially monsoon occurs almost in every summer resulting in heavy precipitation and flood (Chen et al., 2001; Hu et al., 2021; Tao et al., 2012; Xu et al., 2007). The general inflows are located around the northern and the southwestern part of the lake and the outflows are around the eastern and southeastern part of the lake (Qin, 2008), which result in the water flow direction in the lake typically from the western part to the southeastern part. The water level in Taihu Lake is somehow adjusted by water recharge from the Yangtze River through the Wangyu River connecting the northeastern part of Taihu Lake (Gonghu bay) (Fig. 1a) (Zhai et al., 2010). In dry season, there is less water input from the inflow rivers in the northern and western part of the lake, so the water recharge from the Yangtze River to the northeastern part of Taihu Lake can somewhat change the water flow directions in the northern part of the lake. The time allocation of the water recharge mainly depends on the lake water level, which can be obtained in the documents of monthly and yearly water regime downloaded from Taihu Basin Authority of Ministry of Water Resources (http://www.tba.gov.cn/channels/48.html).
The samples 201511 and 201702 were collected in dry seasons with water recharge from the Yangtze River, but the samples 201606 and 201709 were collected during rainy seasons with more water inflow from the rivers around the northern and northwestern part of the lake and also more water discharge to the Yangtze River. The PAH concentration distributions in samples 201511 and 201702 present that higher concentrations were located in Zhushan bay and in the western of Meiliang bay close to the inflow river outlets, and lower concentrations were located in the eastern area of Meiliang bay and Gonghu bay (Fig. 3a, c, e). These spatial distributions suggest that PAH abundance in the Yangtze River was lower than that in the northern part of Taihu Lake, and PAH concentrations in Gonghu bay and the eastern Meiliang were diluted by the water recharge from the Yangtze River. However, the concentration distributions in samples 201606 and 201709 were rather homogeneous, which can be attributed to that in rainy season, there is more water input from the other inflow rivers and high water dynamics in the lake homogenize PAH concentration distributions in the water body.
Review other studies on PAHs in water
As this research is focused on dissolved PAHs in the lake water, the comparison and discussion from other publications are also based on dissolved PAHs in surface water. Most researches only measured the 16 PAHs listed by US EPA, and few researches also measured other PAHs such as 1mehtylnaph and 2methylnaph (Guo et al., 2011; Tongo et al., 2017). There are variable PAH patterns and orders of magnitude variations in their concentration levels in different studies. The detected PAHs are generally dominated by 2-ring and 3-ring PAHs, especially 2-ring naph and 3-ring phen (Bhutto et al., 2021; Li et al., 2006, 2010; Oyo-Ita et al., 2021; Sun et al., 2009), which is similar with the PAH patterns in this study. Meanwhile, the concentrations of 3-ring acenaphthy and acenaphthe in some studies are found as high as the concentration of naph (Kabziński et al., 2002; Tongo et al., 2017; Sarria-villa et al., 2016; Kafilzadeh, 2015; Ambade et al., 2021). The 4-ring fluor and pyrene are generally the heaviest PAHs detected in water samples (Li et al., 2006, 2010; Sun et al., 2009), but in some researches 5-ring and 6-ring PAHs also show relatively high concentrations (Dhananjayan et al., 2012; Edori & Odoemelam, 2022; Guo et al., 2011). However, markedly high concentrations of 1methylnaph and 2methylnaph as that found in the current study have not been investigated and/or discovered in previous studies. The concentrations of individual PAHs range from several ng/L to thousands ng/L in different studies, and the variations mostly occur in the 2-ring and 3-ring PAHs (Dhananjayan et al., 2012; Kafilzadeh, 2015; Patrolecco et al., 2010; Sarria-villa et al., 2016; Tongo et al., 2017; Yang et al., 2013).
Seasonal variations of PAH concentrations in water are also not consistent: higher PAH concentrations in warm season than in cold season (Deng et al., 2006; Matsunaka et al., 2022; Montuori et al., 2016; Qin et al., 2014), which is similar to this study; higher concentrations in cold season than in warm season (Guigue et al., 2014; Liu et al., 2016a, b; Mzoughi & Chouba, 2011); no obvious differences between cold and warm seasons (Guigue et al., 2011; Kafilzadeh, 2015; Liu et al., 2016a, b). In addition, it was also found that naph was the dominant in cold seasons, but the 3-ring PAHs were the dominant in warm seasons (Li et al., 2015a, b). Combing the seasonal variations of PAH concentrations, it indicates that PAH distributions in water are specifically related to local situation rather than temperature-affected partitioning. Therefore, even though there are significant amount of researches on PAHs in water phase, it is difficult to conclude general characteristics of PAH distribution and existence in water bodies.
Conclusion
In Taihu Lake water, there are higher concentrations of PAHs in warm seasons than cold seasons, which is mainly attributed to temperature effects rather than the other factors. In cold seasons, the higher PAH concentrations were located in the northwestern part of the lake, while the relatively concentrations were distributed in the northeastern part. This distribution patterns mainly resulted from the water recharge from the Yangtze River through the outlet located in the northeastern part of the lake. In warm seasons, the PAH concentration distributions were more homogeneous, which is attributed to more precipitation and other inflows increasing the water dynamics and homogenizing PAH concentration distributions. According to the literature review on PAH concentrations in surface water bodies, different studies present different PAH patterns and seasonal variations, suggesting that specific local situations have more effects on PAH existence than its general properties.
References
Ambade, B., Sethi, S. S., Kumar, A., Sankar, T. K., & Kurwadkar, S. (2021). Health risk assessment, composition, and distribution of Polycyclic Aromatic Hydrocarbons (PAHs) in drinking water of Southern Jharkhand, East India. Archives of Environmental Contamination and Toxicology, 80, 120–133. https://doi.org/10.1007/s00244-020-00779-y
Arey, J., Beland, F. A., & Borlak, J. (2010). IARC monographs on the evaluation of carcinogenic risks to humans. US Environmnetal Protection Agency, (1993), 1–868.
Axelman, J., Naes, K., Naf, C., & Broman, D. (1999). Accumulation of polycyclic aromatic hydrocarbons in semipermeable membrane devices and caged mussles (Mytilus Edulis L.) in relation to water column phase distribution. Environmental Toxicology, 18, 2454–2461. https://doi.org/10.1002/etc.5620181111
Bhutto, S. U. A., Xing, X., Shi, M., Mao, Y., Hu, T., Tian, Q., Cheng, C., Liu, W., Chen, Z., & Qi, S. (2021). Occurrence and distribution of OCPs and PAHs in water, soil and sediment of Daye lake. Journal of Geochemical Exploration, 226, 1–14. https://doi.org/10.1016/j.gexplo.2021.106769
Boffetta, P., Jourenkova, N., & Gustavsson, P. (1997). Cancer risk from occupational and environmental exposure to polycyclic aromatic hydrocarbons. Cancer Causes and Control, 8, 444–472. https://doi.org/10.1023/A:1018465507029
Chen, X., Zong, Y., Zhang, E., Xu, J., & Li, S. (2001). Human impacts on the Changjiang Yangtze River basin, China, with special reference to the impacts on the dry season water discharges into the sea. Geomorphology, 41, 111–123. https://doi.org/10.1016/S0169-555X(01)00109-X
Deng, H., Peng, P., Huang, W., & Song, J. (2006). Distribution and loadings of polycyclic aromatic hydrocarbons in the Xijiang River in Guangdong, South China. Chemosphere, 64, 1401–1411. https://doi.org/10.1016/j.chemosphere.2005.12.027
Dhananjayan, V., Muralidharan, S., & Peter, V. R. (2012). Occurrence and distribution of polycyclic aromatic hydrocarbons in water and sediment collected along the harbour line, Mumbai, India. International Journal of Oceanography, 2012, 1–7. https://doi.org/10.1155/2012/403615
Edori, O. S., & Odoemelam, S. (2022). Concentration, source apportionment and ring size analysis of Polycyclic Aromatic Hydrocarbons (PAHs) in water from Kolo Creek, Niger Delta, Nigeria. International Journal of Chemical Research, 4, 36–41.
Geyer, H., Politzki, G., & Freitag, D. (1984). Prediction of ecotoxicological behaviour of chemicals: Relationship between n-octanol/water partition coefficient and bioaccumulation of organic chemicals by alga chlorella. Chemosphere, 13, 269–284. https://doi.org/10.1016/0045-6535(84)90134-6
Guigue, C., Tedetti, M., Ferretto, N., Garcia, N., Méjanelle, L., & Goutx, M. (2014). Spatial and seasonal variabilities of dissolved hydrocarbons in surface waters from the Northwestern Mediterranean Sea: Results from one year intensive sampling. Science of the Total Environment, 466–467, 650–662. https://doi.org/10.1016/j.scitotenv.2013.07.082
Guigue, C., Tedetti, M., Giorgi, S., & Goutx, M. (2011). Occurrence and distribution of hydrocarbons in the surface microlayer and subsurface water from the urban coastal marine area off Marseilles, Northwestern Mediterranean Sea. Marine Pollution Bulletin, 62, 2741–2752. https://doi.org/10.1016/j.marpolbul.2011.09.013
Guo, W., He, M., Yang, Z., Lin, C., & Quan, X. (2011). Aliphatic and polycyclic aromatic hydrocarbons in the Xihe River, an urban river in China’ s Shenyang City: Distribution and risk assessment. Journal of Hazardous Materials, 186, 1193–1199. https://doi.org/10.1016/j.jhazmat.2010.11.122
Herrington, J. S., Hays, M. D., George, B. J., & Baldauf, R. W. (2012). The effects of operating conditions on semivolatile organic compounds emitted from light-duty, gasoline-powered motor vehicles. Atmospheric Environment, 54, 53–59. https://doi.org/10.1016/j.atmosenv.2012.02.043
Hu, J., Liu, Y., Sang, Y. F., Liu, C., & Singh, V. P. (2021). Precipitation variability and its response to urbanization in the Taihu Lake Basin, China. Theoretical and Applied Climatology, 144, 1–15. https://doi.org/10.1007/s00704-021-03597-x
Jenkins, B. M., Jones, D. A., Turn, S. Q., & Williams, R. B. (1996). Particle concentrations, gas-particle partitioning, and species intercorrelations for polycyclic aromatic hydrocarbons (PAH) emitted during biomass burning. Atmospheric Environment, 30, 3825–3835. https://doi.org/10.1016/1352-2310(96)00084-2
Jiang, X., Wang, W., Wang, S., Zhang, B., & Hu, J. (2012). Initial identification of heavy metals contamination in Taihu Lake, a eutrophic lake in China. Journal of Environmental Sciences, 24, 1539–1548. https://doi.org/10.1016/S1001-0742(11)60986-8
Kabziński, A. K. M., Cyran, J., & Juszczak, R. (2002). Determination of polycyclic aromatic hydrocarbons in water (including drinking water) of Łódź. Polish Journal of Environmental Studies, 11, 695–706.
Kafilzadeh, F. (2015). Distribution and sources of polycyclic aromatic hydrocarbons in water and sediments of the Soltan Abad River, Iran. The Egyptian Journal of Aquatic Research, 41, 227–231. https://doi.org/10.1016/j.ejar.2015.06.004
Kim, K.-H., Jahan, S. A., Kabir, E., & Brown, R. J. C. (2013). A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environment International, 60, 71–80. https://doi.org/10.1016/J.ENVINT.2013.07.019
Lee, L. S., Hagwall, M., Delflno, J. J., & Raot, P. S. C. (1992a). Partitioning of polycyclic aromatic hydrocarbons from diesel fuel into water. Environmental Science & Technology, 26, 2104–2110. https://doi.org/10.1021/es00035a005
Lee, L. S., Rao, P. S. C., & Okuda, I. (1992b). Equilibrium partitioning of polycyclic aromatic hydrocarbons from coal tar into water. Environmental Science & Technology, 2115, 2110–2115. https://doi.org/10.1021/es00035a006
Li, G., Xia, X., Yang, Z., Wang, R., & Voulvoulis, N. (2006). Distribution and sources of polycyclic aromatic hydrocarbons in the middle and lower reaches of the Yellow River, China. Environmental Pollution, 144, 985–993. https://doi.org/10.1016/j.envpol.2006.01.047
Li, J., Shang, X., Zhao, Z., Tanguay, R. L., Dong, Q., & Huang, C. (2010). Polycyclic aromatic hydrocarbons in water, sediment, soil, and plants of the Aojiang River waterway in Wenzhou, China. Journal of Hazardous Materials, 173, 75–81. https://doi.org/10.1016/j.jhazmat.2009.08.050
Li, L., Zhao, X., Liu, D., Song, K., Liu, Q., & He, Y. (2021a). Occurrence and ecological risk assessment of PPCPs in typical inflow rivers of Taihu lake, China. Journal of Environmental Management, 285, 1–8. https://doi.org/10.1016/j.jenvman.2021.112176
Li, P., Cao, J., Diao, X., Wang, B., Zhou, H., Han, Q., & Zheng, P. (2015a). Spatial distribution, sources and ecological risk assessment of polycyclic aromatic hydrocarbons in surface seawater from Yangpu Bay, China. Marine Pollution Bulletin, 93, 53–60. https://doi.org/10.1016/j.marpolbul.2015.02.015
Li, Y., Li, P., Ma, W., Song, Q., Zhou, H., Han, Q., & Diao, X. (2015b). Spatial and temporal distribution and risk assessment of polycyclic aromatic hydrocarbons in surface seawater from the Haikou Bay, China. Marine Pollution Bulletin, 92, 244–251. https://doi.org/10.1016/j.marpolbul.2014.12.014
Li, Y., Zhou, S., Jia, Z., Liu, K., & Wang, G. (2021b). Temporal and spatial distributions and sources of heavy metals in atmospheric deposition in western Taihu Lake, China. Environmental Pollution, 284, 1–8. https://doi.org/10.1016/j.envpol.2021.117465
Liu, M., Feng, J., Hu, P., Tan, L., Zhang, X., & Sun, J. (2016a). Spatial-temporal distributions, sources of polycyclic aromatic hydrocarbons (PAHs) in surface water and suspended particular matter from the upper reach of Huaihe River, China. Ecological Engineering, 95, 143–151. https://doi.org/10.1016/j.ecoleng.2016.06.045
Liu, S., Liu, X., Liu, M., Yang, B., Cheng, L., Li, Y., & Qadeer, A. (2016b). Levels, sources and risk assessment of PAHs in multi-phases from urbanized river network system in Shanghai. Environmental Pollution, 219, 555–567. https://doi.org/10.1016/j.envpol.2016.06.010
Matsunaka, T., Nagao, S., Inoue, M., Mundo, R., Tanaka, S., Tang, N., Yoshida, M., Nishizaki, M., Morita, M., Takikawa, T., Suzuki, N., Ogiso, S., Hayakawa, K., & Hayakawa, K. (2022). Seasonal variations in marine polycyclic aromatic hydrocarbons off Oki Island, Sea of Japan, during 2015–2019. Marine Pollution Bulletin, 180, 1–10. https://doi.org/10.1016/j.marpolbul.2022.113749
Mcdonald, J. D., Zielinska, B., Fujita, E. M., Sagebiel, J. C., Chow, J. C., & Watson, J. G. (2000). Fine particle and gaseous emission rates from residential wood combustion. Environmental Science and Technology, 34, 2080–2091. https://doi.org/10.1021/es9909632
Montuori, P., Aurino, S., Garzonio, F., Sarnacchiaro, P., Nardone, A., & Triassi, M. (2016). Distribution, sources and ecological risk assessment of polycyclic aromatic hydrocarbons in water and sediments from Tiber River and estuary, Italy. Science of the Total Environment, 566–567, 1254–1267. https://doi.org/10.1016/j.scitotenv.2016.05.183
Mzoughi, N., & Chouba, L. (2011). Distribution and partitioning of aliphatic hydrocarbons and polycyclic aromatic hydrocarbons between water, suspended particulate matter, and sediment in harbours of the west coastal of the gulf of Tunis (Tunisia). Journal of Environmental Monitoring, 13, 689–698. https://doi.org/10.1039/c0em00616e
Oyo-Ita, I., Nkom, P. Y., Ugim, S. U., Bassey, F. I., & Oyo-Ita, O. E. (2021). Seasonal changes of PAHs in water and suspended particulate matter from cross river estuary, SE Nigeria in response to human-induced activity and hydrological cycle. Polycyclic Aromatic Compounds, 1–18. https://doi.org/10.1080/10406638.2021.1939070
Patrolecco, L., Ademollo, N., Capri, S., Pagnotta, R., & Polesello, S. (2010). Occurrence of priority hazardous PAHs in water, suspended particulate matter, sediment and common eels (Anguilla anguilla) in the urban stretch of the River Tiber (Italy). Chemosphere, 81, 1386–1392. https://doi.org/10.1016/j.chemosphere.2010.09.027
Pérez-tejeda, P., Vanes, C., & Maestre, A. (1990). Solubility of naphthalene in water + alcohol solutions at various temperatures. Journal of Chemical and Engineering Data, 35, 244–246. https://doi.org/10.1021/je00061a007
Qin, B. (2008). Lake Taihu, China: dynamics and environmental change. Springer Science & Business Media.
Qin, B., Xu, P., Wu, Q., Luo, L., & Zhang, Y. (2007). Environmental issues of Lake Taihu. Kluwer Academic Publishers. https://doi.org/10.1007/s10750-006-0521-5
Qin, N., He, W., Kong, X. Z., Liu, W. X., He, Q. S., Yang, B., Wang, Q. M., Yang, C., Jiang, Y. J., Jorgensen, S. E., Xu, F. L., & Zhao, X. L. (2014). Distribution, partitioning and sources of polycyclic aromatic hydrocarbons in the water-SPM-sediment system of Lake Chaohu, China. Science of the Total Environment, 496, 414–423. https://doi.org/10.1016/j.scitotenv.2014.07.045
Sarria-villa, R., Ocampo-duque, W., Páez, M., & Schuhmacher, M. (2016). Presence of PAHs in water and sediments of the Colombian Cauca River during heavy rain episodes, and implications for risk assessment. Science of the Total Environment, 540, 455–465. https://doi.org/10.1016/j.scitotenv.2015.07.020
Shen, G., Lu, Y., Wang, M., & Sun, Y. (2005). Status and fuzzy comprehensive assessment of combined heavy metal and organo-chlorine pesticide pollution in the Taihu Lake region of China. Journal of Environmental Management, 76, 355–362. https://doi.org/10.1016/j.jenvman.2005.02.011
Shen, X., & Ma, L. J. C. (2005). Privatization of rural industry and de facto urbanization from below in southern Jiangsu, China. Geoforum, 36, 761–777. https://doi.org/10.1016/j.geoforum.2005.01.005
Slmonich, S. L., & Hites, R. A. (1994). Vegetation-atmosphere partitioning of polycyclic aromatic hydrocarbons. Environmental Science & Technology, 28, 939–943. https://doi.org/10.1021/es00054a028
Sun, J. H., Wang, G. L., Chai, Y., Zhang, G., Li, J., & Feng, J. (2009). Distribution of polycyclic aromatic hydrocarbons (PAHs) in Henan Reach of the Yellow River, Middle China. Ecotoxicology and Environmental Safety, 72, 1614–1624. https://doi.org/10.1016/j.ecoenv.2008.05.010
Tao, H., Gemmer, M., Jiang, J., & Lai, X. (2012). Assessment of CMIP3 climate models and projected changes of precipitation and temperature in the Yangtze River Basin, China. Climatic Change, 111, 737–751. https://doi.org/10.1007/s10584-011-0144-3
Tao, Y., Dan, D., Kun, L., Chengda, H., Haibing, C., Guo, F., & Qiujin, X. (2018). δ15N and nutrient stoichiometry of water, aquatic organisms and environmental implications in Taihu lake, China*. Environmental Pollution, 237, 166–173. https://doi.org/10.1016/j.envpol.2018.02.048
Tao, Y., Yao, S., Xue, B., Deng, J., Wang, X., Feng, M., & Hu, W. (2010). Polycyclic aromatic hydrocarbons in surface sediments from drinking water sources of Taihu Lake, China: Sources, partitioning and toxicological risk. Journal of Environmental Monitoring, 12, 2282–2289. https://doi.org/10.1039/c0em00144a
Tongo, I., Ezemonye, L., & Akpeh, K. (2017). Levels, distribution and characterization of polycyclic aromatic hHydrocarbons (PAHs) in Ovia river, Southern Nigeria. Journal of Environmental Chemical Engineering, 5, 504–512. https://doi.org/10.1016/j.jece.2016.12.035
Tsapakis, M., & Stephanou, E. G. (2005). Occurrence of gaseous and particulate polycyclic aromatic hydrocarbons in the urban atmosphere: Study of sources and ambient temperature effect on the gas/particle concentration and distribution. Environmental Pollution, 133, 147–156. https://doi.org/10.1016/j.envpol.2004.05.012
US Environmnetal Protection Agency. (1993). Provisional guidance for quantitative risk assessment of polycyclic aromatic hydrocarbons.
Wang, H., Wang, C., Wu, W., Mo, Z., & Wang, Z. (2003). Persistent organic pollutants in water and surface sediments of Taihu Lake, China and risk assessment. Chemosphere, 50, 557–562. https://doi.org/10.1016/S0045-6535(02)00484-8
Whitehouse, B. G. (1984). The effects of temperature and salinity on the aqueous solubility of polynuclear aromatic hydrocarbons. Marine Chemistry, 14, 319–332. https://doi.org/10.1016/0304-4203(84)90028-8
Wilhelm, S. W., Farnsley, S. E., LeCleir, G. R., Layton, A. C., Satchwell, M. F., DeBruyn, J. M., Boyer, G. L., Zhu, G., & Paerl, H. W. (2011). The relationships between nutrients, cyanobacterial toxins and the microbial community in Taihu (Lake Tai), China. Harmful Algae, 10, 207–215. https://doi.org/10.1016/J.HAL.2010.10.001
Xu, J., Zhang, Y., Zhou, C., Guo, C., Wang, D., Du, P., Luo, Y., Wan, J., & Meng, W. (2014). Distribution, sources and composition of antibiotics in sediment, overlying water and pore water from Taihu Lake, China. Science of the Total Environment, 497–498, 267–273. https://doi.org/10.1016/J.SCITOTENV.2014.07.114
Xu, L., Zhang, Q., Li, H., & Viney, N. R. (2007). Modeling of surface runoff in Xitiaoxi Catchment, China. Water Resources Management, 21, 1313–1323. https://doi.org/10.1007/s11269-006-9083-6
Yang, D., Qi, S., Zhang, Y., Xing, X., Liu, H., Qu, C., Liu, J., & Li, F. (2013). Levels, sources and potential risks of polycyclic aromatic hydrocarbons (PAHs) in multimedia environment along the Jinjiang River mainstream to Quanzhou Bay, China. Marine Pollution Bulletin, 76, 298–306. https://doi.org/10.1016/j.marpolbul.2013.08.016
Yang, Y., Miller, D. J., & Hawthorne, S. B. (1997). Toluene solubility in water and organic partitioning from gasoline and diesel fuel into water at elevated temperatures and pressures. Journal of Chemical and Engineering Data, 9568, 908–913. https://doi.org/10.1021/je960395v
Yeh, A. G. O., Xu, J., & Liu, K. (2011). China’s post-reform urbanization: retrospect, policies and trends. IIED.
Zhai, S., Hu, W., & Zhu, Z. (2010). Ecological impacts of water transfers on Lake Taihu from the Yangtze River, China. Ecological Engineering, 36, 406–420. https://doi.org/10.1016/j.ecoleng.2009.11.007
Acknowledgements
This work was supported by BMBF-SIGN 02WCL1336C and by the International Science & Technology Cooperation Program of China (No. 2016YFE0123700). The author Aili Li sincerely thanks the Chinese Scholarship Council.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is non-financial interests to be disclosed in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, A., der Beek, T.a., Zhang, J. et al. Characterizing spatiotemporal variations of polycyclic aromatic hydrocarbons in Taihu Lake, China. Environ Monit Assess 194, 713 (2022). https://doi.org/10.1007/s10661-022-10358-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-022-10358-4