Abstract
Gray whales utilizing their foraging grounds off northeastern Sakhalin Island, Russia, have been increasingly exposed to anthropogenic activities related to oil and gas development over the past two decades. In 2015, four seismic vessels, contracted by two operators, conducted surveys near and within the gray whale feeding grounds. Mitigation and monitoring plans were developed prior to the survey and implemented in the field, with real-time data transfers to assist the implementation of measures aimed at minimizing impacts of acoustic exposure. This study examined the behavioral response of gray whales relative to vessel proximities and sounds generated during seismic exploration. Five shore-based teams monitored gray whale behavior from 1 June to 30 September using theodolite tracking and focal follow methodologies. Behavioral data were combined with acoustic and benthic information from studies conducted during the same period. A total of 1270 tracks (mean duration = 0.9 h) and 401 focal follows (1.1 h) were collected with gray whales exposed to sounds ranging from 59 to 172 dB re 1 μPa2 SPL. Mixed models were used to examine 13 movement and 10 respiration response variables relative to “natural,” acoustic, and non-acoustic explanatory variables. Water depth and behavioral state were the largest predictors of gray whale movement and respiration patterns. As vessels approached whales with increasing seismic/vessel sound exposure levels and decreasing distances, several gray whale movement and respiration response variables significantly changed (increasing speed, directionality, surface time, respiration intervals, etc.). Although the mitigation measures employed could have reduced larger/long-term responses and sensitization to the seismic activities, this study illustrates that mitigation measures did not eliminate behavioral responses, at least in the short-term, of feeding gray whales to the activities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gray whales (Eschrichtius robustus) have been described as both ecosystem sentinels and engineers (Moore, 2008; Moore & Reeves, 2018; Nelson & Johnson, 1987). Their benthic foraging strategy makes them unique among cetaceans, particularly among baleen whale species. Their mode of feeding can re-suspend large amounts of nutrients and sediments into the environment that can be equated to geological processes (Nelson & Johnson, 1987). Globally, gray whales exist in the northern hemisphere of the Pacific Ocean. Historically, gray whales were present in the northern hemisphere of the Atlantic Ocean, but whaling in the seventeenth century drove the Atlantic population into extinction (Mead & Mitchell, 1984). The Pacific eastern gray whale population also suffered severely from whaling primarily due to their coastal affinity and concentration on their breeding and foraging habitats. After post-modern whaling, eastern gray whales were listed as endangered by the International Union for Conservation of Nature (IUCN). Due to a number of conservation measures, eastern gray whales rebounded to pre-whaling numbers in what amounts to one of the most successful conservation stories today (Jones & Swartz, 2009; Reilly et al., 1983; Rugh et al., 2005).
On the western side of the Pacific Ocean, another population of gray whales, the western gray whale, was previously thought to have been extinct due to whaling (Bowen, 1974; Brownell & Chun, 1977). However, studies in Russia discovered a remnant and genetically distinct population foraging in a relatively small spatial area off northeastern Sakhalin Island, Russia (Lang et al., 2011; LeDuc et al., 2002; Weller et al., 2002a). After years of research in the late 1990s to obtain sufficient population and genetic data, studies indicated the population was small (< 150 individuals) with fewer than 25 reproductive females (Weller et al., 2002a). Based on these data, the IUCN initially classified the western gray whale population as critically endangered. More recently, based on population growth rates and inclusion of data from Sakhalin and Kamchatka, Russia, IUCN changed the population status to endangered (Cooke et al., 2018). Cooke et al. (2018) noted, however, that the population would still be considered critically endangered based on individuals that were only observed off Sakhalin due to the low number of reproductive females.
One of the major concerns towards the future survival of the population was the discovery of large oil and gas reserves that existed in close proximity to the western gray whales’ feeding grounds. Since the mid-1990s, the level of anthropogenic pressure on the Sakhalin gray whale feeding grounds has increased; what was once a relatively pristine area has been subjected to an ever-growing amount of vessel traffic, dredging, pipeline placement, platform installations, pile driving, seismic exploration activity, and, more recently, large-scale salmon fishery operations (Gailey, 2013; Gailey et al., 2007, 2016, 2022; Lowry et al., 2018; Muir et al., 2015a, b, 2016; Yazvenko et al., 2007). The amount of activity within a single summer foraging period culminated in 2015 with two operators conducting four seismic surveys that spanned the majority of the gray whale foraging season and habitat off Sakhalin Island.
When the oil and gas industry began working off Sakhalin in the late 1990s, little was known about gray whale responses to anthropogenic activities. A few studies have suggested gray whales abandoned entire breeding lagoons in response to increasing vessel activity (Bryant et al., 1984; Gard, 1978). Behavioral response studies on the eastern gray whale feeding grounds suggested that 10% of gray whales would respond to received root mean square seismic sound pressure levels (SPL) above 163 dB re 1 μPa2 (Malme et al., 1986, 1988). Behavioral response studies on western gray whales found that gray whales significantly changed their movement, respiration, abundance, and distribution despite employing mitigation approaches to minimize acoustic exposure levels (Gailey et al., 2007; Weller et al., 2002b; Yazvenko et al., 2007). More recent impact studies on gray whale distribution and behavior during a seismic survey in 2010, however, found little to no responses to the activity (Gailey et al., 2016; Muir et al., 2015a, b, 2016). It remained unclear if gray whales tolerated or habituated to the activities or if, as suggested by the authors, the impact studies were too limited in sample sizes to detect responses given the small number of whales observed and the short duration (3 weeks) of the survey. In fact, Gailey et al. (2016) provided power analyses of their dataset that documented their inability to detect subtle to moderate levels of potential changes in movement and respiration given their sample sizes.
Due to the potential sensitivities to the presence of anthropogenic activity in close proximity to the western gray whale feeding grounds, two oil and gas companies undertaking seismic operations (Exxon Neftegas Limited (ENL) and Sakhalin Energy Investment Company (SEIC) developed mitigation measures to limit sound levels received by gray whales to less than 163 dB re 1 μPa2 SPL. Comprehensive monitoring programs were developed to better understand the gray whale population, their habitat, and the potential impacts of industrial activities as well as to examine the effectiveness of the applied mitigation strategies (Bröker et al., 2015; Johnson et al., 2007).
In 2015, both SEIC and ENL conducted seismic surveys within four of their licensed blocks. The surveys involved up to four seismic source vessels that spanned both the duration and spatial extent of the western gray whale feeding season and habitat. The number of seismic vessels and areas of exploration varied throughout the feeding season. In addition, mitigation measures taken by both companies deviated from previous mitigation strategies by not actively implementing the 163 dB re 1μPa2 airgun shutdowns aimed at reducing behavioral disturbance of gray whales during the early part of their respective surveys, when few whales would be present, to facilitate completion of activities as soon as possible.
Later in the season, airgun shutdowns were implemented to avoid exposure of animals to sound levels > 163 dB re 1μPa2 SPL, but the two companies applied this mitigation measure differently, with ENL employing behavioral mitigation criteria for all individuals while SEIC restricted the criteria to only mother-calf pairs (Aerts et al., 2022; Rutenko et al., 2022; SEIC, 2015). This was arguably a less stringent approach than previous mitigation measures developed for western gray whales (Bröker et al., 2015; Johnson et al., 2007). The monitoring studies undertaken in 2015 did provide an opportunity to collect sufficient samples and to observe gray whales being exposed to higher sound levels than during previous impact studies. As such, one operator (ENL) implemented a comprehensive and extensive monitoring program (Aerts et al., 2022). The research design monitored not only ENL but SEIC activities as well.
In this study, we examine if western gray whale movement and respiration patterns were influenced by sound (seismic, onshore pile driving, and vessel sound) and/or proximity of the activities that occurred. We sought to include environmental, temporal, and spatial related variables that have previously been shown to account for variability in gray whale movement and respiration patterns (Gailey, 2013; Gailey et al., 2007, 2016). One crucial consideration that was not considered or available in previous studies was prey availability, and we provide an initial examination of these data in this study.
Methods
Study site, observation platforms, and seismic surveys
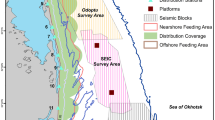
During the summer of 2015, a total of four seismic source vessels operated off northeastern Sakhalin Island, Russia, to capture data about oil and gas reservoirs within four license blocks near or within the western gray whale feeding grounds (Fig. 1). The seismic operations were spatially and temporally coordinated by the two operators with seismic lines closest to the feeding areas being acquired as early in the feeding season as possible. Aerts et al. (2022) summarizes the seismic operations as well as the mitigation and monitoring approaches taken in 2015.
Five shore-based behavioral teams monitored the movements, behavior state, and respiration patterns of western gray whales from eight separate locations during the seismic operations. The locations of behavioral observations were dependent on the seismic survey activity that occurred at the time. Two teams observed gray whales from stations 4 and 6 throughout the study period (1 June–30 Sept). The three other teams occupied stations 5, 12, and 13 before and during the Odoptu and Chaivo seismic surveys, but moved to stations 8, 9, and 10 when the Piltun-Astokh seismic survey started and remained there until 30 Sept (Fig. 1). Due to the relatively low elevations of the onshore stations, wooden towers (4-m height) were custom built at each station, with the exception of station 6, to increase the observation range of the stations. The towers had an independent structure in the center to provide a stable platform for the theodolite. Observation height from the stations ranged from 10.8 to 23.7 m. These elevations were considered to be sufficiently high to monitor the nearshore feeding ground of the western gray whales that generally extends less than 15 km from shore with gray whales occurring on average approximately 1.5 km from shore (Gailey, 2013; Gailey et al., 2016; Muir et al., 2015a, b, 2016) (Fig. 1).
Movement and respiration data collection
Gray whales were monitored from five of the eight stations during each good-weather day (good visibility with Beaufort Sea State < 5). An individual or group of gray whales was tracked from shore using theodolite tracking techniques (Gailey & Ortega-Ortiz, 2002; Würsig et al., 1991). At each station, a Sokkia DT5A or DT540 theodolite was used that had a 30 × monocular magnification and 5 arc-sec level of angular precision and tilt compensation. The maximum distance from a station that a gray whale was tracked depended on the observation height of that station. At lower elevation stations, gray whales were tracked up to approximately 6 km from the station, while at higher elevation stations, whales were tracked to distances up to 12 km. Whales were continuously tracked until the whales were either no longer visible or environmental conditions hampered reliable tracking. Gray whales observed closest to either the nearest vessel or seismic activity were preferentially tracked throughout the study with single or recognizable individuals being favored to avoid measurement errors within the track. The geographic positions of the whales were estimated in real-time and visually displayed in a custom version of the Pythagoras theodolite data collection system (Gailey & Ortega-Ortiz, 2002).
Focal follow observation techniques were used to record respiration patterns of gray whales in the field (Altmann, 1974; Martin et al., 1993). Focal follows were conducted only on single or individual recognizable whales to ensure respiration events were not missed during data collection. A focal follow included at least one observer visually focusing on the whale’s location aided by 7 × 50 Fujinon FMTRC-SX binoculars. The focal observer verbally stated a behavioral event (respiration, peduncle arch, etc.), which was immediately recorded by a computer operator using a programmable keyboard connected to the Pythagoras behavioral software (Gailey & Ortega-Ortiz, 2002). Focal follow sessions were recorded in conjunction with theodolite tracking of the same whale, which provided the ability to link respiration information to the spatial location of the animal. A focal follow session ceased if the whale moved out of the observation area or environmental conditions were unacceptable (visibility < 5 km, Beaufort Sea State > 3 or wind gust speeds > 20 km h−1).
Behavioral response and explanatory variables
Definitions of all response and explanatory variables are provided in Table 1 of Gailey et al. (2016). Data processing and response variables were consistent with previous behavioral studies of western gray whales (Gailey, 2013; Gailey et al., 2007, 2016). All movement data derived from theodolite tracking were resampled based on a 90-s criterion to avoid over- or under-sampling issues and to standardize step lengths (Turchin, 1998). For every resampled track, movement response variables (whale speed, directionality, reorientation rate, etc.) were calculated at 10.5-min intervals (hereafter referred to as a “bin”). This bin duration was chosen to examine short-term changes in movement patterns. Observations of gray whale respirations at the surface during focal follows were processed to measure the animal’s respiration interval, dive time, surface time, number of blows per surfacing, percent time at surface, surface blow rate, and dive surface blow rate. These respiration variables were also averaged over 10.5 min to be consistent with the movement variables.
To account for parameters that may alter gray whale movement and respiration patterns, we included two types of explanatory variables: natural and impact variables. In this study, we selectively chose only those natural variables that in previous analyses explained a significant amount of variation in gray whale movement and respiration response variables (Gailey, 2013; Gailey et al., 2007, 2016). This simplified the behavior response models by reducing the number of variables considered. Natural explanatory variables considered in the behavioral response models were the observation station, date of the observation, time of day, Beaufort Sea State, visibility, water depth, tide height, and behavioral state. Behavioral state was classified as feeding, feeding/traveling, traveling, or mixed. Mixed behavior denoted any combination of unknown, transitional, or unrecognized behavior as well as other seldom observed behavioral states, such as resting, milling, and social.
Anthropogenic variables (e.g., “impact” explanatory variables) that were hypothesized to change gray whale behavior, such as sound exposure and/or vessel distance, were also included in the analyses. Acoustic monitoring data (Rutenko et al., 2022) were used to calculate received levels for several acoustic metrics, which included (1) sound exposure level (SEL), (2) RMS sound pressure level (SPL), and (3) peak sound pressure level (PK). All acoustic metrics were estimated for pulse (pile driving/seismic) sounds, while only SEL and SPL were estimated for continuous (vessel) sounds in the study area. Two explanatory variables based on the SEL metric were computed for every 30-s increment along a whale track: the SEL over the current time step (SEL30s) and the SEL accumulated from the beginning of the track to the current time step (cSEL). Vessel activity was recorded in the field using a combination of four separate automatic identification system (AIS) receivers, satellite AIS, and vessel GPS logs. Non-acoustic variables considered in the response models were the closest vessel approach (km), number of vessels, and the relative orientation of the vessel to the whale (ROW).
Acoustic monitoring and sound level estimation
Vessel, pile driving, and seismic survey sounds were recorded with 40 automatic underwater acoustic recorders (AUARs) with frequency range of 2 to 15,000 Hz. Nine of these AUARs (denoted as RI-AUARs) were equipped with both an iridium and a digital radio-telemetric channel that provided real-time acoustic waveform data within the 2- to 2000-Hz frequency band with a potential dynamic range of 145 dB (Rutenko et al., 2022). The RI-AUARs were equally spaced along the offshore boundary of the gray whale feeding area (Fig. 1) and provided real-time data to a shore-based acoustic monitoring team. Thirty-one monitoring sites equipped with non-telemetric AUARs were located closer to shore in water depths of 10 m as well as in deeper waters to provide detailed information on sound propagation for post hoc analyses. Rutenko et al. (2022) provide further details of acoustic data acquisition during the seismic surveys.
Prey availability
As recommended by Gailey et al. (2007, 2016), prey biomass and availability could be important variables to consider while examining gray whale movement and respiration patterns. We explored the maximum prey biomass observed at the animal’s location over the course of each bin. Blanchard et al. (2022a, b) provides a summary of the benthic prey biomass data and data collection methods used in 2015. Besides repeat sampling of the 2002–2014 benthic grid covering the Sakhalin gray whale feeding areas described in Blanchard et al. (2022a, b), the 2015 benthic study mainly focused on examining the spatial and within season changes of prey availability. A finer scale sampling grid, covering waters of < 20 m within the nearshore feeding area, was sampled three different times within the gray whale foraging season (Blanchard et al., 2022a, b). Interpolation methods were required to estimate the biomass of each group at the gray whale location. To estimate benthic biomass at the gray whale location, we applied both kriging and inverse distance weighted (IDW) interpolation approaches. The kriging approach considered spatial variables and water depth in the interpolation, while IDW simply uses distance to other neighboring data points. Behavior and prey analyses were conducted separately from the behavioral models examining potential disturbances to whales, since the interpolated area of prey availability did not cover whales observed outside the 20-m isobath. The prey groups that were examined here were (1) amphipods, (2) isopods, (3) Cumaceans, (4) Polychaetes, (5) bivalves, and (6) total biomass (summation of groups 1–5). ANOVAs were used to examine differences in prey biomass at the whales’ locations among the different behavioral states.
Behavioral response models
The behavioral response models were used to determine associations of the response variables (e.g., whale speed, dive time) to the natural (e.g., water depth, time of day, behavioral state) and impact (e.g., SEL30, closest vessel distance) explanatory variables. The model approach assumed the response variables had an approximately normal distribution. To meet this assumption, transformation methods were applied. Logit transformations were applied to linearity and mean direction indices, while natural log transformations were applied to speed, distance from shore, and respiration variables. Collinearity among covariates was examined using pair-wise Pearson correlation coefficients for all continuous variables, and box-plots were used to assess non-continuous against continuous variables. A correlation coefficient larger than 0.60 warranted concern that one covariate could mask the effects of another.
Associations among the variables of interest were examined using mixed linear models (Pinheiro & Bates, 2000). This modeling approach was chosen because autocorrelation was potentially present due to the time series nature of the sequential bins of observation. Autocorrelation within tracks was accounted for by estimating mixed linear models that assumed unstructured, constant, or autoregressive dependencies in model residuals. Another analytical challenge was the potential issue of pseudo-replication with a single track or focal follow having more or less representative bins within the dataset compared to others. To adjust for this bias, we weighted each observation by a value inversely proportional to the probability of obtaining that observation (Horvitz & Thompson, 1952; Overton & Stehman, 1995). Model effects were estimated with generalized estimating equations using the R function “lme” available in the “nlme” package (Pinheiro et al., 2018). Model selection was based on a stepwise selection procedure that relied on the Bayesian information criterion (BIC). BIC was chosen as the measure of variable utility because it generally yields a more parsimonious model. Both forward and backward step selections were used to include natural and/or impact effects. Standardized residual plots were inspected to assess model fit.
Results
Effort
Seismic surveys by the two operators were carried out from 11 Jun to 23 Sept 2015. The Odoptu survey was acquired from 11 Jun to 7 July, the Piltun-Astokh survey from 8 to 25 Jul, and the Chaivo/Arkutun-Dagi survey from 7 Jul to 23 Sept (see Aerts et al., 2022) for further details about the seismic operations). The movement and respiration patterns of gray whales were monitored from 1 Jun to 30 Sept 2015 by five shore-based behavior teams (Fig. 1). The total number of observation days at each station ranged from 24 to 85 days with an average of 480 h (cumulatively 3843 h) of effort being conducted among the behavioral teams. Station 5 had the least amount of effort. The mean number of observation days per team was 88 (range 70–101). A total of 1270 Gy whale tracks and 401 focal follows were collected during the field season. The average duration of a gray whale track was 0.9 h (range = 0.2–13.1 h) with a total of 44,634 geographic positions of gray whales recorded. The mean focal follow session lasted 1.1 h (0.2–12.2 h) with 30,735 respiration events collected. A total of 7403 movement and 2328 respiration bins were derived for the analyses. Gray whales were observed feeding in a localized area 26% of the time, while feeding/traveling (or searching) behavior was observed 33% of the time. Approximately 30% of the gray whale activity budget in 2015 was spent on traveling, while the remaining 11% of the behaviors observed were a mixture of milling, socializing, resting, nursing, etc. The combined foraging behavior (59%) was similar to those previously reported for western gray whales (Gailey, 2013; Sychenko, 2011; Villegas-Amtmann et al., 2015), but slightly less time was spent on concentrated feeding compared to the observed feeding/traveling behaviors.
Response variables
Gray whale movement and respiration response variables were highly associated with one another and with behavioral states (feeding, traveling, etc.). Traveling gray whales exhibited higher speeds with increased spatial range and more directional movement than non-traveling gray whales, while feeding gray whales’ reorientation rate was more variable with less geographical movement than non-feeding gray whales. While gray whales were feeding, they typically increased their dive time, decreased the interval between subsequent respirations, shortened their surface time, and had a higher surface blow rate (number of blows per surface time). In general, gray whales were minimizing their surface time and maximizing their time underwater while engaged in feeding compared to traveling.
Principal component analyses (PCA) were applied to the response variables of movement and respiration to examine if a combination of response variables (speed, linearity, reorientation rate, etc.) would be a better indicator of response as opposed to examining each response variable separately. There are a number of inter-relationships among the response variables. For example, traveling animals tend to do so more linearly at higher speeds and increased range as opposed to foraging animals. When gray whales are feeding, they tend to increase their dive time and decrease the time at the surface with shorter respiration intervals. The principal component scores (e.g., PC1, PC2, PC3) were used as response variables in separate models. The first three component scores for movement (69% of the variation) and respiration (92% of the variation) were used in the analyses (Fig. 2). The first two components were interpreted to explain variation in the relationships of the different movement and respiration among the different behavioral states, while the third component was interpreted to account for the remaining variation that could be related to other natural or impact related variables.
Principle component analysis (PCA) of western gray whale movement (A) and respiration (B) response variables. Movement variables were defined as SPD (speed), Range (range index), TRK_R (directionality index), LIN (linearity), RR (reorientation rate), MDIR (mean direction), ROW_SV (relative orientation to closest seismic vessel), ROW_CV (relative orientation to closest vessel), and Direction (spatial direction of movement). Respiration variables were defined as SRate (surface blow rate), Dives (dive time), NumSurfs (number of blows per surfacing), Surfs (surface time), TimeAtSurface (percent time at surface), and RI (respiration interval)
Acoustic exposure
Western gray whales were exposed to pile driving, seismic, and vessel sounds in 2015 with a few whales (< 1.2% of the dataset) exposed to all three at the same time. The onshore pile driving activity was spatially localized and of short duration. Gray whale exposure to pile driving sounds occurred on 14 different days with a mean SEL30s of 110 dB re 1 μPa2s (range 97–123). Vessel activity occurred throughout the foraging season but varied spatially and in intensity. Gray whale exposure to vessel sounds had a mean SEL30s of 113 dB re 1 μPa2s (59–156). Exposure to vessel sounds was observed in 68% of the behavioral dataset in this study. Gray whale exposure to seismic pulses had a mean SEL30s of 120 dB re 1 μPa2s (53–172). Over 55% of the behavioral dataset showed exposure to some degree of seismic sounds. However, gray whale exposure to particularly high sound levels (SEL30s > 156 dB re 1 μPa2s) for vessel or seismic activity were infrequent (Fig. 3). Due to high collinearity (Pearson’s correlation > 0.95) among the acoustic metrics, only SEL30s and cSEL were included in the behavioral response models. Due to the low levels and small sample size of pile driving sounds, pile driving was not further considered in the behavioral response models.
Prey availability
The median prey biomass values at gray whale locations from the kriging and IDW interpolations varied (Table 1), with IDW approach generally yielding higher values. Amphipod biomass was significantly lower while feeding/traveling (df = 3, F = 9.88, P < 0.001) for kriging, while biomasses were significantly higher during feeding and feeding/traveling (3, 11.46, < 0.001) compared to traveling or mixed behaviors. A similar pattern was observed for isopods for kriging (3, 9.06, < 0.001) and IDW (3, 7.45, < 0.001) approaches. Bivalve median biomasses were observed to be significantly lower during mixed behaviors for kriging (3, 35.93, < 0.001), but higher during traveling (3, 22.47, < 0.001) with IDW. Polychaete and cumacean median biomass were slightly lower during mixed behaviors for kriging and IDW. Total biomass was lowest, while animals were feeding with the kriging (3, 50.00, < 0.001) interpolation but slightly higher with IDW (3, 12.34, < 0.001).
Inclusion of prey biomass in the behavioral response models yielded mixed results. For example, amphipod significantly entered into the dive time model for kriging but not for IDW. As the duration of the dive time significantly increases with water depth (below) and the kriging interpolation used water depth to adjust the biomass, it is likely that these results reflected covariance with water depth. By including behavioral state in the movement and respiration models, it largely accounted for differences while engaged in foraging compared to prey types and biomass values. In other words, while engaged in feeding behaviors, gray whales were not demonstrably responding by moving slower, changing directions more, or breathing quicker where biomass was higher or in association with prey type. For this reason and due to the number of extraneous variables, we excluded prey biomass as a potential explanatory variable in the behavioral response models.
Behavioral response to anthropogenic sound and proximity
The behavioral state and water depth were the largest predictors of the respiration response variables. As gray whales observed off Sakhalin feed, they decrease their respiration interval and time at the surface, but increase their dive time, number of blows per surfacing, and surface blow rate. When gray whales were observed in deeper waters, they slightly increased their respiration interval, surface time, blows per surfacing, and dive time. There was also a decrease in the surface blow rate and dive-surface blow rate associated with increased water depth. Tide height entered into several of the respiration response models but was non-significant. With increased tide height, the surface time and dive time increased with lower dive-surface blow rate.
The impact variables accounting for variation in respiration responses were mainly associated with vessel distances as opposed to acoustic metrics. However, accumulated SEL from vessels was significant for several respiration variables. As vessels approached a gray whale, their respiration intervals significantly decreased with a higher surface blow rate and decreased dive time. With an increasing amount of accumulated SEL, whales spent less time at the surface with a decreased dive-surface blow rate. The distance of the closest seismic vessel also appeared to have altered the gray whales dive-surface blow rate, surface blow rate, and time at the surface (Table 2).
Similar to the respiration models, behavioral state and water depth were the largest predictors for the movement response variables. Traveling gray whales exhibited higher speeds, longer ranges, and more directionality than feeding or feeding/traveling whales (Table 3). Gray whales were noted to have higher speeds and directionality in deeper water depths. Time of day was a predictor for a few response variables by BIC selection, but the coefficient was particularly small (< 0.00001) and p values not significant. Both seismic and vessel sounds and distances explained a significant amount of the variation in a number of response variables. As a seismic vessel approached a gray whale, the gray whale’s speed, range, and distance from shore increased. Whales also moved more perpendicular to vessels as vessels approached whales at closer distances. As seismic sound exposure levels increased, whales appeared to move closer to shore. With increasing continuous sound exposure levels from vessels, gray whales increased their reorientation rate and decreased their directionality. As a vessel approached closer to whales, whales altered their direction of movement. This was similar to the whale’s relative orientation to the vessel that was influenced by the proximity and direction the vessel approaching the whale. Principal component score responses reflected the results of the individual responses. Behavioral state was the largest predictor of the first two principal components. The remaining variation in the third component was significantly associated with water depth, vessel distance, and accumulated sound energy level.
Individual responses to activities
Moderate to subtle changes in marine mammal behavior in response to anthropogenic activity are likely to be more frequent compared to larger responses. In addition, the responses can be context dependent on the activity that occurred at the time. However, examining obvious responses provides insight to developing an appropriate analytical framework for future analyses. As such, we provide a case example of a gray whale feeding as a seismic vessel approached (Fig. 4). The gray whale altered its feeding behavior to traveling towards shore as the seismic source vessel turned towards the whale’s direction. However, as the seismic vessel moved farther away from the whale, the whale resumed feeding albeit in an area different from the whale’s original feeding area.
Individual gray whale movement (13.1-h track) while exposed to seismic, pile driving, and vessel sounds. The accumulated sound exposure level (cSEL; orange), 30-s sound exposure level (SEL30s; red), sound pressure level (SPL; green), peak sound pressure level (SPK; blue), closest vessel distance (purple), gray whale speed (km/h, dark blue), and gray whale displacement (the squared distance (km2) an animal moved from the point of origin, dark orange) are represented in the right graph for the timeline. The color scheme in the map of the seismic vessel and whale tracks represents the SEL30s the whale received at that time, while the purple arrow in the map indicates the direction of vessel movement
Discussion
Sample size
The amount of seismic activity that occurred near western gray whales in 2015 provided a unique opportunity to advance our understanding of behavioral responses at the population level for western gray whales to anthropogenic activity. Gailey et al. (2016) illustrated the statistical sample sizes needed to detect moderate to subtle behavioral changes in western gray whale movements and respirations in relation to sound exposure. Relatively small sample sizes (< 60) were required to detect large (50% change in the response variable) changes in gray whale movements and respiration, while sample sizes of 300–1700 were required to detect subtle changes (10% change) among the response variables analyzed in this study. Previous behavioral response studies on western gray whales during seismic activity (Gailey et al., 2007, 2016) were limited in duration (3 weeks) and behavioral monitoring teams (1–2 teams). The five behavioral teams deployed in 2015 coupled with the duration of the surveys (4 months) yielded sample sizes four times greater than all the previous combined behavioral response studies on western gray whales to seismic activity. As such, this study had a higher probability to detect more moderate to subtle (< 50%) changes in movement and respiration variables and in fact documented clear evidence of responses to industrial sounds.
Prey availability
Due to the extent of the feeding area, detailed documentation of the benthic communities is challenging, which in turn presents challenges to understanding how the benthic community changes within the gray whales foraging grounds. Point samples were taken from certain grid areas during three separate periods (Blanchard et al., 2022a, b). To estimate biomass values where whales were observed to be feeding, we attempted both kriging and IDW interpolations. It is arguable that the more complex kriging approach provided an advantage over the IDW approach from an estimation perspective, but was limited in scope by the narrow data requirements for kriging. Gray whales were observed to be feeding in areas with higher Amphipoda biomass, a preferential prey (Blanchard et al., 2019; Demchenko, 2010; Demchenko et al., 2016), compared to traveling or other behavioral states observed.
Although prey availability has been previously suggested as an explanatory variable within behavioral response models, this study found that prey biomass does not particularly alter gray whale movement or respiration patterns. This suggests gray whale movement and respirations patterns while engaged in feeding or traveling activities were similar regardless of prey biomass. It is possible that prey availability would alter the gray whale’s foraging strategy (feeding/travel versus feeding), spatial distribution, and abundance rather than the gray whale’s behavioral movement or respiration behaviors.
Other natural influences on gray whale behavior
Natural spatial and temporal variables were found to have little association with gray whale behavior, suggesting that western gray whales move and respire similarly throughout the nearshore feeding habitat and their foraging season. The spatial variable “Station” was not found to explain the variability in any of the response variables and time of day entered into a few models, but the coefficients were particularly small and found to be inconclusive as explanatory variables. Interestingly, water depth was one of the largest predictors of gray whale movement and respiration patterns, as noted in previous studies, and was particularly important for dive time (Gailey, 2013; Gailey et al., 2007, 2016). Studies on eastern gray whales have also reported changes in dive time, surface time, and surface-blow rate associated with increasing water depth (Guerrero, 1989; Mallonée, 1991; Stelle et al., 2008; Würsig et al., 1986). However, this study had not only a strong association with water depth for respiration, but also for gray whale movement patterns. This could be partially related to sample sizes, anthropogenic activity, or a different foraging strategy. Gailey et al. (2022) reported that gray whales in 2015 were unusually far from shore in the northern part of the study area during the middle part of the feeding season. Blanchard et al. (2022a, b) also observed a seasonal high abundance of sand lance in that area, which could indicate that the gray whales observed farther from shore were feeding on a seasonal more mobile prey, which could have influenced the response variables analyzed.
The behavioral state of gray whales significantly influenced the majority of their movement and respiration patterns. Gray whales feeding in a centralized location had slower speeds and more variable directionality compared to traveling whales. Gray whales also increase their amount of time below the surface while feeding compared to traveling. It was unsurprising, however, that the dive-surface blow rate was not significantly associated with behavioral state since this variable is more or less a physiological constant. However, Gailey et al. (2016) found few associations with behavioral state in a number of the respiration models that conducted similar analyses, albeit with a drastically reduced sample size (36 focal follows, 395 bins). Behavioral states were classified based primarily on observations of the animal’s movement and respiration behaviors while on the surface; one could expect, therefore, the vast majority of the variation to be explained by these categorical definitions. However, despite inclusion of behavioral states, significant variation remained in the models.
Sound and vessel influence on gray whale behavior
Vessel distances and sound exposure significantly changed gray whale movement and respiration patterns. Whales were breathing faster and moving at higher speeds when vessels were closer to whales or when sound exposures were higher. Similar to Gailey et al. (2016), the gray whale direction of movement was significantly related to the direction and approach distance of the closest vessel. The association between the relative orientation of the whale and vessel was intended to examine impacts from a context-based approach (Ellison et al., 2012). Whales would likely behave differently if the source was moving toward or away from the whale. Gray whales could also respond differently depending on the location of the source relative to the whale’s current direction. For example, despite receiving similar sound exposures, migrating gray whales notably responded to a sound source directly in their migration route, but little behavioral response was observed when the same source was placed outside of their migratory pathway (Tyack & Clark, 1998). Gailey et al. (2016) and the case study illustrated here present similar context-based responses. Whales farther from shore were more likely to be closer to the vessels. The relationship of gray whale distance from shore and vessel proximity indicated that whales were moving closer to shore to increase their distance from the vessels.
Chronic exposure to disrupting activities, even with low levels of exposure, can lead to displacement out of critically important foraging habitats. With an increasing amount of anthropogenic activity, there could be a potential for increased tolerance or habituation to sound and/or vessel activity. Alternatively, gray whales could become sensitized to anthropogenic activity, especially following previous negative experiences. It is unknown in this study how frequently a single individual or group of individuals (e.g., pregnant females) repeatedly responded to anthropogenic activities. Schwarz et al. (2022) demonstrate that a portion of the population remained in the offshore feeding area and younger gray whales, which could be more naïve to previous exposures, are more likely to occur in the nearshore feeding area. It is possible that some individuals who are sensitized to anthropogenic activity could be over-represented in the analyses. However, concurrent shore-based photo-identification with tracking suggests that greater than 60% of the individuals identified by all five photo-identification teams were represented to some degree within the analyses.
Repetitive disruption of gray whale foraging activities accumulates over time and could ultimately result in biologically significant impacts to individuals and thus the population. For example, bioenergetic studies estimated that a loss of 10 feeding days or 3% reduction of energy intake due to disturbance could lead to an unsuccessful pregnancy (McHuron et al., 2021; Villegas-Amtmann et al., 2015, 2017). Other studies have also hypothesized that industrial activities off Sakhalin could be one contributing factor towards the observed slow recovery of this endangered population (Weller et al., 2002a). Simulation studies further suggested that calf survival could be impacted in the future when pregnant females arrived on the foraging grounds in poorer body condition during a year of disturbance activity (McHuron et al., 2021). Although gray whales have been documented to abandon entire lagoons for years and up to a decade as a result of anthropogenic activity (Bryant et al., 1984; Jones et al., 1994), western gray whales have a high site fidelity and annual return to the Sakhalin foraging area despite the increasing amount of human related activity occurring on their foraging habitat. This could suggest that they may have no alternative or comparable foraging habitat other than the foraging areas off Sakhalin Island. While eastern gray whales have been shown to forage over larger ranges (Lagerquist et al., 2019), the western gray whale known foraging range is relatively small (Mate et al., 2015; Muir et al., 2015a, b), which may make this population more susceptible to anthropogenic or environmental perturbations (Gailey et al., 2020; Lowry et al., 2018).
Conclusion
The results of this study found multiple indicators of behavioral response to seismic and vessel sounds and proximity. These results are similar to those found in a study associated with a seismic survey conducted in 2001 in the same study area (Gailey et al., 2007; Weller et al., 2002b; Yazvenko et al., 2007) but differ from those found in a study associated with a seismic survey in 2010 (Gailey et al., 2016; Muir et al., 2015a, b, 2016), for which Gailey et al. (2016) concluded that samples sizes had insufficient statistical power to detect moderate to subtle changes in behavior. This suggests that anthropogenic activities off Sakhalin Island impacted gray whale foraging activities, at least in the short-term. Although gray whales responded on a population level to the activities, it is unknown if the responses resulted in biologically significant (i.e., decreased growth, survival, reproductive success) impacts to individuals or the population. Mitigation measures applied during 2015 could have reduced larger/long-term responses and sensitization to activities, but this study found that it did not eliminate all behavioral responses of gray whales relative to the anthropogenic activities that occurred off Sakhalin Island. Future studies should investigate the longevity of behavioral response in western gray whales to the activities as well as examine responses within components of the population (e.g., mother-calf pairs, pregnant females, immature adults) to better understand the energetic loss as a result of the activities. In addition, individual heterogeneity and repetitive responses should be further explored relative to natural covariates and anthropogenic exposure as a substantial amount of variability existed in the movement and respiration datasets that required large sample sizes to identify responses. Some of the components of the population, such as pregnant females and mother/calves, could be more sensitive to anthropogenic activity but their sensitivity was not directly examined in this study due to the inability to identify all individuals from shore. A behavioral dose–response model (Dunlop et al., 2018) should also be developed to re-examine the 163 dB re 1 μPa2 SPL criteria that have historically been used as a mitigation measure to reduce disruption to gray whale feeding activities. Disruption to feeding activities as a result of seismic and vessels on their foraging ground should also not be viewed in isolation. For example, (Gailey et al., 2020) found a correlation between the number of foraging days (as determined by sea-ice conditions off Sakhalin) and reproductive success and calf survival. Of particular concern are the few reproductive females in this small population that have significantly higher energetic costs due to their pregnancy and calf rearing (Christiansen et al., 2018; Villegas-Amtmann et al., 2015, 2017). Local fisheries and the possibility of entanglement could also result in further disturbance and survival of individuals within this population (Lowry et al., 2018). As gray whales have a number of other factors impacting their future survival throughout their home range, it is important to continue to monitor and examine the effectiveness of mitigation strategies employed off Sakhalin to rapidly assess potential impacts and address these management issues in the future.
References
Aerts, L. A., Jenkerson, M. R., Nechayuk, V. E., Gailey, G., Racca, R., Blanchard, A. L., et al. (2022). Seismic surveys near gray whale feeding areas off Sakhalin Island, Russia: assessing impact and mitigation effectiveness. Environmental Monitoring and Assessment, 194. https://doi.org/10.1007/s10661-022-10016-9.
Altmann, J. (1974). Observational Study of Behavior: Sampling Methods. Behaviour, 49(3–4), 227–266. https://doi.org/10.1163/156853974X00534
Blanchard, A. L., Ainsworth, L., Gailey, G., Demchenko, N. L., & Shcherbakov, I. (2022a). Benthic studies adjacent to Sakhalin Island, Russia 2015a III: benthic energy density spatial models in the nearshore gray whale feeding area. Environmental Monitoring and Assessment, 194. https://doi.org/10.1007/s10661-022-10018-7
Blanchard, A. L., Demchenko, N. L., Aerts, L. A. M., Yazvenko, S. B., Ivin, V. V., & Shcherbakov, I. (2022b). Benthic studies adjacent to Sakhalin Island, Russia, 2015b I: benthic biomass and community structure in the nearshore gray whale feeding area. Environmental Monitoring and Assessment, 194. https://doi.org/10.1007/s10661-022-10017-8
Blanchard, A. L., Demchenko, N. L., Aerts, L. A., Yazvenko, S., Ivin, V., Shcherbakov, I., & Melton, H. (2019). Prey biomass dynamics in gray whale feeding areas adjacent to Northeastern Sakhalin (the Sea of Okhotsk), Russia, 2001–2015. Marine Environmental Research, 145, 123-136
Bowen, S. L. (1974). Probable extinction of the Korean stock of the gray whale (Eschrichtius robustus). Journal of Mammalogy, 55(1), 208–209.
Bröker, K., Gailey, G., Muir, J., & Racca, R. (2015). Monitoring and impact mitigation during a 4D seismic survey near a population of gray whales off Sakhalin Island, Russia. Endangered Species Research, 28(3), 187–208. https://doi.org/10.3354/esr00670
Brownell, R. L., & Chun, C. I. (1977). Probable Existence of the Korean Stock of the Gray Whale (Eschrichtius robustus). Journal of Mammalogy, 58(2), 237–239. https://doi.org/10.2307/1379584
Bryant, P. J., Lafferty, C. M., & Lafferty, S. K. (1984). Reoccupation of Laguna Guerrero Negro, Baja California, Mexico, by gray whales. The gray whale, Eschrichtius robustus, 375–387.
Christiansen, F., Vivier, F., Charlton, C., Ward, R., Amerson, A., Burnell, S., & Bejder, L. (2018). Maternal body size and condition determine calf growth rates in southern right whales. Marine Ecology Progress Series, 592, 267–281. https://doi.org/10.3354/meps12522
Cooke, J. G., Taylor, B. L., Reeves, R. R., & Brownell Jr, R. (2018). Eschrichtius robustus western subpopulation. The IUCN Red List of Threatened Species 2018: e.T8099A50345475. https://www.iucnredlist.org/species/8099/50345475#population. Accessed 27 November 2018
Demchenko, N. L. (2010). Ecological aspects of the dominant amphipod Monoporeia affinis (Amphipoda: Pontoporeiidae) in the infralittoral zone on the northeastern coast of the Sakhalin Island (Sea of Okhotsk). Zool. Baetica, 21, 143–149.
Demchenko, N. L., Chapman, J. W., Durkina, V. B., & Fadeev, V. I. (2016). Life History and Production of the Western Gray Whale’s Prey, Ampelisca eschrichtii Krøyer, 1842 (Amphipoda, Ampeliscidae). PLoS ONE, 11(1), e0147304. https://doi.org/10.1371/journal.pone.0147304
Dunlop, R. A., Noad, M. J., McCauley, R. D., Kniest, E., Slade, R., Paton, D., & Cato, D. H. (2018). A behavioural dose-response model for migrating humpback whales and seismic air gun noise. Marine Pollution Bulletin, 133, 506–516. https://doi.org/10.1016/j.marpolbul.2018.06.009
Ellison, W. T., Southall, B. L., Clark, C. W., & Frankel, A. S. (2012). A new context-based approach to assess marine mammal behavioral responses to anthropogenic sounds. Conservation Biology, 26(1), 21–28.
Gailey, G. (2013). Anthropogenic disturbance of western gray whale behavior off Sakhalin Island, Russia (Dissertation). Texas A&M University.
Gailey, G., & Ortega-Ortiz, J. (2002). A note on a computer-based system for theodolite tracking of cetaceans. Journal of Cetacean Research and Management, 4(2), 213–218.
Gailey, G., Sychenko, O., McDonald, T., Racca, R., Rutenko, A., & Bröker, K. (2016). Behavioural responses of western gray whales to a 4-D seismic survey off northeastern Sakhalin Island, Russia. Endangered Species Research, 30, 53–71. https://doi.org/10.3354/esr00713
Gailey, G., Sychenko, O., Tyurneva, O., Yakovlev, Y., Vertyankin, V., van der Wolf, P., et al. (2020). Effects of sea ice on growth rates of an endangered population of gray whales. Scientific Reports, 10(1), 1–8. https://doi.org/10.1038/s41598-020-58435-3
Gailey, G., Würsig, B., & McDonald, T. L. (2007). Abundance, behavior, and movement patterns of western gray whales in relation to a 3-D seismic survey, Northeast Sakhalin Island, Russia. Environmental Monitoring and Assessment, 134(1–3), 75–91. https://doi.org/10.1007/s10661-007-9812-1
Gailey, G., Zykov, M., Sychenko, O., Rutenko, A., Blanchard, A. L., Aerts, L. A., et al. (2022). Gray whale density during seismic surveys near their Sakhalin feeding ground. Environmental Monitoring and Assessment, 194. https://doi.org/10.1007/s10661-022-10025-8
Gard, R. (1978). Aerial census and a population dynamics study of gray whales in Baja California during the 1976 calving and mating season. NTIS.
Guerrero, J. A. (1989). Feeding behavior of gray whales in relation to patch dynamics of their benthic prey (Thesis). San Jose State University.
Horvitz, D. G., & Thompson, D. J. (1952). A generalization of sampling without replacement from a finite universe. Journal of the American Statistical Association, 47(260), 663–685.
Johnson, S. R., Richardson, W. J., Yazvenko, S. B., Blokhin, S. A., Gailey, G., Jenkerson, M. R., et al. (2007). A western gray whale mitigation and monitoring program for a 3-D seismic survey, Sakhalin Island, Russia. Environmental Monitoring and Assessment, 134(1–3), 1–19. https://doi.org/10.1007/s10661-007-9813-0
Jones, M. L., & Swartz, S. L. (2009). Gray Whale. In Encyclopedia of Marine Mammals (pp. 503–511). Elsevier. https://doi.org/10.1016/B978-0-12-373553-9.00119-X
Jones, M. L., Swartz, S. L., & Dahlheim, M. E. (1994). Census of gray whale abundance in San Ignacio Lagoon: a follow-up study in response to low whale counts recorded during an acoustic playback study of noise effects on gray whales. Rep. No. NTIS PB94195062 to the US Marine Mammal Commission, Washington, DC.
Lagerquist, B. A., Palacios, D. M., Winsor, M. H., Irvine, L. M., Follett, T. M., & Mate, B. R. (2019). Feeding home ranges of pacific coast feeding group gray whales: Gray Whale Home Ranges. The Journal of Wildlife Management, 83(4), 925–937. https://doi.org/10.1002/jwmg.21642
Lang, A. R., Weller, D. W., LeDuc, R., Burdin, A. M., Pease, V. L., Litovka, D., et al. (2011). Genetic analysis of stock structure and movements of gray whales in the eastern and western North Pacific. In 19th Biennial Conference on the Biology of Marine Mammals. Tampa, Florida. https://swfsc.noaa.gov/publications/CR/2011/2011Lang.pdf. Accessed 1 January 2017
LeDuc, R. G., Weller, D. W., Hyde, J., Burdin, A. M., Rosel, P. E., & Brownell, R. L., Jr. (2002). Genetic differences between western and eastern gray whales. Journal of Cetacean Research and Management, 4(1), 1–5.
Lowry, L. F., Burkanov, V. N., Altukhov, A., Weller, D. W., & Reeves, R. R. (2018). Entanglement risk to western gray whales from commercial fisheries in the Russian Far East. Endangered Species Research, 37, 133–148. https://doi.org/10.3354/esr00914
Mallonée, J. S. (1991). Behaviour of gray whales (Eschrichtius robustus) summering off the northern California coast, from Patrick’s Point to Crescent City. Canadian Journal of Zoology, 69(3), 681–690. http://www.nrcresearchpress.com/doi/abs/10.1139/z91-100. Accessed 1 January 2017
Malme, C. I., Würsig, B., Bird, J. E., & Tyack, P. (1988). Observations of feeding gray whale responses to controlled industrial noise exposure. Port and Ocean Engineering under Arctic Conditions, 2, 55–73.
Malme, C. I., Würsig, B., Bird, J. E., & Tyack, P. L. (1986). Behavioral Responses of Gray Whales to Industrial Noise: Feeding Observations and Predictive Modeling (Vol. 6265). Bolt, Beranek, and Newman. https://books.google.com/books?id=U8yxtgAACAAJ
Martin, P., Bateson, P. P. G., & Bateson, P. (1993). Measuring behaviour: An introductory guide. Cambridge University Press.
Mate, B. R., Ilyashenko, V. Y., Bradford, A. L., Vertyankin, V. V., Tsidulko, G. A., Rozhnov, V. V., & Irvine, L. M. (2015). Critically endangered western gray whales migrate to the eastern North Pacific. Biology Letters, 11(4), 20150071–20150071. https://doi.org/10.1098/rsbl.2015.0071
McHuron, E. A., Aerts, L. A., Gailey, G., Sychenko, O., Costa, D. P., Mangel, M., & Schwarz, L. K. (2021). Predicting the population consequences of acoustic disturbance, with application to an endangered gray whale population. Ecological Applications, 31(8), e02440.
Mead, J. G., & Mitchell, E. D. (1984). Atlantic gray whales. The gray whale, Eschrichtius robustus, 33–53.
Moore, S. E. (2008). Marine mammals as ecosystem sentinels. Journal of Mammalogy, 89(3), 534–540. https://doi.org/10.1644/07-MAMM-S-312R1.1
Moore, S. E., & Reeves, R. R. (2018). Tracking arctic marine mammal resilience in an era of rapid ecosystem alteration. PLOS Biology, 16(10), e2006708. https://doi.org/10.1371/journal.pbio.2006708
Muir, J. E., Ainsworth, L., Joy, R., Racca, R. R., Bychkov, Y., Gailey, G., et al. (2015a). Distance from shore as an indicator of disturbance of gray whales during a seismic survey off Sakhalin Island, Russia. Endangered Species Research, 29(2), 161–178. https://doi.org/10.3354/esr00701
Muir, J. E., Ainsworth, L., Racca, R. R., Bychkov, Y., Gailey, G., Vladimirov, V., et al. (2016). Gray whale densities during a seismic survey off Sakhalin Island, Russia. Endangered Species Research, 29(3), 211–227. https://doi.org/10.3354/esr00709
Muir, J., Joy, R., Bychkov, Y., Bröker, K., Gailey, G., Vladmirov, V., et al. (2015b). Delineation of a coastal gray whale feeding area using opportunistic and systematic survey effort. Endangered Species Research, 29(2), 147–160. https://doi.org/10.3354/esr00705
Nelson, C. H., & Johnson, K. R. (1987). Whales and walruses as tillers of the sea floor. Scientific American, 256(2), 112–118.
Overton, W. S., & Stehman, S. V. (1995). The Horvitz-Thompson theorem as a unifying perspective for probability sampling: With examples from natural resource sampling. The American Statistician, 49(3), 261–268.
Pinheiro, J. C., & Bates, D. M. (2000). Linear mixed-effects models: basic concepts and examples. In Mixed-effects models in S and S-Plus (pp. 3–56). Springer.
Pinheiro, J. C., Bates, D. M., DebRoy, S., Sarkar, D., & R Core Team. (2018). nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1–137. https://CRAN.R-project.org/package=nlme
Reilly, S. B., Rice, D. W., & Woleman, A. A. (1983). Population assessment of the gray whale, Eschrichtius robustus, from California shore censuses, 1967–80. Fish. Bull. U.S., (81), 267–268.
Rugh, D. J., Hobbs, R. C., Lerczak, J. A., & Breiwick, J. M. (2005). Estimates of abundance of the eastern North Pacific stock of gray whales (Eschrichtius robustus) 1997–2002. Journal of Cetacean Research and Management, 7(1), 1–12.
Rutenko, A., Zykov, M., Gritsenko, V. A., Fershalov, M. Y., Jenkerson, M. R., Manulchev, D., et al. (2022). Real-time acoustic monitoring with telemetry to mitigate potential effects of seismic survey sounds on marine mammals: a case study offshore Sakhalin Island. Environmental Monitoring and Assessment, 194. https://doi.org/10.1007/s10661-022-10021-y
Schwarz, L. K., Gailey, G., Tyurneva, O., Yakovlev, Y., Sychenko, O., Van der Wolf, P., & Vertyankin, V. V. (2022). Western gray whales on their summer feeding grounds off Sakhalin Island in 2015: Who is foraging where? Environmental Monitoring and Assessment, 194. https://doi.org/10.1007/s10661-022-10022-x
SEIC. (2015). Monitoring and mitigation plan for 2015 Piltun-Astokh 4D seismic survey (Report to the Western Gray Whale Advisory Panel) (pp. 1–5). Gland, Switzerland: Sakhalin Energy Investment Company and Exxon Neftegas Limited. https://www.iucn.org/sites/dev/files/content/documents/mmp_2015_piltun_astokh_4d_seismic_en.pdf. Accessed 6 February 2019
Stelle, L. L., Megill, W. M., & Kinzel, M. R. (2008). Activity budget and diving behavior of gray whales (Eschrichtius robustus) in feeding grounds off coastal British Columbia. Marine Mammal Science, 24(3), 462–478. https://doi.org/10.1111/j.1748-7692.2008.00205.x
Sychenko, O. (2011). Western gray whale (Eschrictius robustus) mother and calf ecology off Sakhalin Island (Thesis). Texas A&M University.
Turchin, P. (1998). Quantitative Analysis of Movement: Measuring and modeling population redistribution in plants and animals. Sinauer Associates.
Tyack, P. L., & Clark, C. W. (1998). QUICKLOOK – Playback of low frequency sounds to gray whales migrating past the central California coast – January, 1998. Phase II Report. US Navy, Rep. from Woods Hole Oceanographic Institute, Woods Hole, MA; and Bioacoustics Res. Prog., Cornell Univ., Ithaca, NY. http://www.whoi.edu/fileserver.do?id=57468&pt=10&p=40212
Villegas-Amtmann, S., Schwarz, L., Gailey, G., Sychenko, O., & Costa, D. (2017). East or west: The energetic cost of being a gray whale and the consequence of losing energy to disturbance. Endangered Species Research, 34, 167–183. https://doi.org/10.3354/esr00843
Villegas-Amtmann, S., Schwarz, L. K., Sumich, J. L., & Costa, D. P. (2015). A bioenergetics model to evaluate demographic consequences of disturbance in marine mammals applied to gray whales. Ecosphere, 6(10), art183. https://doi.org/10.1890/ES15-00146.1
Weller, D. W., Burdin, A., Würsig, B., Taylor, B., & Brownell, R. L., Jr. (2002a). The western gray whale: A review of past exploitation, current status and potential threats. Journal of Cetacean Research and Management, 4(1), 7–12.
Weller, D. W., Ivashchenko, Y. V., Tsidulko, G. A., Burdin, A. M., & Brownell Jr, R. L. (2002b). Influence of seismic surveys on western gray whales off Sakhalin Island, Russia in 2001. Paper SC/54/BRG14 presented to the International Whaling Commission Scientific Committee. http://digitalcommons.unl.edu/usdeptcommercepub/73/. Accessed 1 January 2017
Würsig, B., Cipriano, F., & Würsig, M. (1991). Dolphin movement patterns: information from radio and theodolite tracking studies. In Dolphin societies: discoveries and puzzles (pp. 79–111).
Würsig, B., Wells, R. S., & Croll, D. A. (1986). Behavior of gray whales summering near St. Lawrence Island, Bering Sea. Canadian Journal of Zoology, 64(3), 611–621.
Yazvenko, S. B., McDonald, T. L., Blokhin, S. A., Johnson, S. R., Meier, S. R., Melton, H. R., et al. (2007). Distribution and abundance of western gray whales during a seismic survey near Sakhalin Island, Russia. Environmental Monitoring and Assessment, 134(1–3), 45–73. https://doi.org/10.1007/s10661-007-9809-9
Acknowledgements
We thank our team leads, Sarah Piwetz, Ophélie Sagnol, and Wai Ho Simon Wong, and appreciate the valuable data assistance from Vladimir Chernitsyn, Andrei Doroshenko, Alexei Filipiechev, Kiril Galenko, Aleksandr Kvashnin, Dmitrii Pelageev, Mikhail Pushilin, Aleksandr Omeliyanenko, Andrei Shostak, and Andrei Sychenko. Lisanne Aerts, Ervin Kalinin, Vladimir Nechayuk, Roberto Racca, Mike Scott, Mike Swindoll, and Sergei Yazvenko provided excellent advice and assistance throughout the project. We also appreciate the financial, logistical, and safety management support provided by Exxon Neftegas Limited. We thank Bernd Würsig and one anonymous reviewer as well as the guest editor, Bill Streever, for providing comments and suggestions that improved an earlier draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Western Gray Whales and Industry Seismic Operations
Our coauthor Rodger Melton passed away in 2017, but without his leadership, counsel, and technical knowledge, this work would not have been possible. Our coauthor Alexander Rutenko passed away in 2020. His expertise, compassion, and friendship will be missed.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gailey, G., Sychenko, O., Zykov, M. et al. Western gray whale behavioral response to seismic surveys during their foraging season. Environ Monit Assess 194 (Suppl 1), 740 (2022). https://doi.org/10.1007/s10661-022-10023-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-022-10023-w